Abstract
Free full text

NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma
Abstract
Hepatic cirrhosis and hepatocellular carcinoma (HCC) are the most common causes of death in patients with chronic liver disease. Chronic liver injury of virtually any etiology triggers inflammatory and wound healing responses that in the long run promote the development of hepatic fibrosis and HCC. Here, we review the role of the transcription factor nuclear factor-κB (NF-κB), a master regulator of inflammation and cell death, in the development of hepatocellular injury, liver fibrosis and HCC, with a particular focus on the role of NF-κB in different cellular compartments of the liver. We propose that NF-κB acts as a central link between hepatic injury, fibrosis and HCC, and that it may represent a target for the prevention or treatment of liver fibrosis and HCC. However, NF-κB acts as a two-edged sword and inhibition of NF-κB may not only exert beneficial effects but also negatively impact hepatocyte viability, especially when NF-κB inhibition is pronounced. Finding appropriate targets or identifying drugs that either exert only a moderate effect on NFκB activity or that can be specifically delivered to nonparenchymal cells will be essential to avoid the increase in liver injury associated with complete NF-κB blockade in hepatocytes.
Introduction
Inflammation is an integral part of the hepatic wound-healing response to injury induced by alcohol, hepatitis viruses, excess fat and cholestasis. While inflammation may be beneficial in the short term (for example by promoting regeneration after massive liver injury or inducing immune responses that lead to the eradication of pathogens), chronic inflammation and the associated regenerative wound-healing response are strongly linked to the development of fibrosis, cirrhosis and hepatocellular carcinoma (HCC). As >80% of HCCs arise in patients with hepatic fibrosis or cirrhosis,1 it appears that the chronic wound-healing process in the liver is an essential contributor to hepatocarcinogenesis, and that HCC as the final stage of this process truly represents ‘a wound that never heals’. In the progression through the various stages of liver disease, inflammation seems to be the key promoter that drives this maladaptive wound-healing response.
It is commonly believed that during carcinogenesis genetic alterations are the ‘match that lights the fire’ and inflammation the ‘fuel that feeds the flames’. However, chronic hepatic injury is sufficient to induce both tumor initiation and promotion, via replication-induced mutations and chronic inflammation. In the past 10 years, a number of inflammatory mediators have been shown to contribute to the progression of chronic liver disease,2–7 many of which are either targets or activators of nuclear factor-κB (NF-κB). NF-κB is a key transcriptional regulator of the inflammatory response,8–10 and plays an essential role in the regulation of inflammatory signaling pathways in the liver. First, NF-κB is activated in virtually every chronic liver disease, including alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), viral hepatitis and biliary liver disease.11–17 Second, NF-κB regulates multiple essential functions in hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) as outlined below. Third, genetic inactivation of different NFκB signaling components results in liver phenotypes that include spontaneous injury, fibrosis and carcinogenesis suggesting that NF-κB makes an essential contribution to liver homeostasis and wound-healing processes.18–21
Here we review the contribution of NF-κB to chronic liver disease, with a particular focus on the role of NF-κB in different hepatic cell compartments and its effects on chronic inflammation and fibrosis as events that set the stage for the development of HCC. We support the hypothesis that the effects of NF-κB on hepatocarcinogenesis strongly depend on the cell type and the degree of NF-κB activation or inhibition. Activation of NF-κB in non-parenchymal cells generally promotes inflammation, fibrosis and hepatocarcinogenesis, whereas suppression of NF-κB activation in parenchymal cells promotes hepatocarcinogenesis in some settings and inhibits hepatocarcinogenesis in others.
The NF-κB transcription factor family
The NF-κB signaling pathway is a highly conserved evolutionary pathway, with key functions in the regulation of immune and inflammatory responses, as first demonstrated in Drosophila melanogaster and subsequently in mammals.10 NF-κB is composed as a heterodimer or homodimer of various members of the Rel family of DNA-binding proteins, and regulates the transcription of genes that contain κB binding sites. The human NF-κB family consists of five cellular DNA-binding subunits: p50, p52, cRel, p65 (also called RelA) and RelB, encoded by NFKB1, NFKB2, REL, RELA and RELB, respectively (Table 1).10 The DNA-binding subunits of NF-κB share an N-terminal Rel homology domain (RHD), which mediates dimerization, interaction with the inhibitor of κB (IκB) proteins, nuclear translocation and DNA binding.22 In addition, p65, RelB and cRel contain C-terminal transactivation domains that trigger gene transcription. p65 contains two potent transactivation domains within its C-terminus and is probably the strongest activator of most genes with κB sites.23 The two other members of the human NF-κB family, p52 and p50, become active DNA-binding proteins after cleavage of larger precursors (inactive p105 is cleaved to p50 and p100 to p52).24 They activate transcription when they form heterodimers with subunits that contain transactivation domains (p65, RelB or cRel), but may also repress gene transcription when forming dimers without a transactivation domain (for example, p50–p50 homodimers).25
Table 1
Structure of the NFκB and IκB proteins and the IKK complex.129
| Protein | Amino acid length | Domains |
|---|---|---|
| NF-κB | ||
| p65 (RelA) | 551 | RHD, TAD |
| cRel | 619 | RHD, TAD |
| RelB | 579 | LZ, RHD, TAD |
| NF-κB and IκB | ||
| p100 (p52) | 899 | RHD, 7 ankyrin repeats |
| p105 (p50) | 969 | RHD, 7 ankyrin repeats |
| IκB | ||
| IκBα | 317 | 6 ankyrin repeats |
| IκBβ | 356 | 6 ankyrin repeats |
| IκBγ | 607 | 7 ankyrin repeats |
| IκBε | 361 | 7 ankyrin repeats |
| Bcl3 | 446 | 7 ankyrin repeats |
| IκBζ | 718 | 5 ankyrin repeats |
| IKK complex | ||
| IKK1 (IKKα) | 745 | Kinase, LZ, HLH, NBD |
| IKK2 (IKKβ) | 756 | Kinase, LZ, HLH, NBD |
| NEMO (IKKγ) | 419 | CC1, CC2, LZ, Z |
Abbreviations: CC, coiled-coil domain; HLH, helix-loop-helix; IκB, inhibitor of κB; IKK inhibitory κB kinase; LZ, leucine zipper; NBD, NEMO, NF-κB essential modulator binding domain; NF-κB, nuclear factor κB; RHD, rel homology domain; TAD, transactivation domain; Z, zinc finger.
Regulation of NF-κB activation
NF-κB is activated by various stimuli that present a potential danger to the host, resulting in the initiation of inflammatory, immune and wound-healing responses and the clearance of pathogens. Among the most potent activators of NF-κB are pathogen-derived molecules (such as lipopolysaccharide [LPS], viral and bacterial DNA and RNA) that stimulate Toll-like receptors (TLRs), as well as inflammatory cytokines such as tumor necrosis factor (TNF) or interleukin (IL)-1.8, 26 The activation of NF-κB leads to the transcription of hundreds of genes with κB binding sites, most of which are involved in the regulation of inflammation, immune responses and cell survival.27
As uncontrolled inflammation may be deleterious, NF-κB activity is tightly regulated at several levels. In an unstimulated cell, the activity of NF-κB is to a large degree regulated by binding to inhibitory units of the group of IκB proteins, such as IκBα, IκBβ, IκBε, IκBγ, IκBζ and the related Bcl3 protein (Table 1). Binding of IκB to NF-κB masks the nuclear localization site of NF-κB and effectively sequesters NF-κB in the cytoplasm.28, 29 In order to activate NF-κB in response to specific stimuli, NF-κB needs to first be liberated from its inhibitory IκB partner. NF-κB can then translocate to the nucleus and initiate gene transcription (Figure 2). This activation can be achieved by two different pathways: the canonical pathway, which is activated in response to cytokines such as TNF, IL-1 or TLR agonists; and the noncanonical NF-κB pathway, which is particularly important in B cells30, 31 and is not discussed further here.
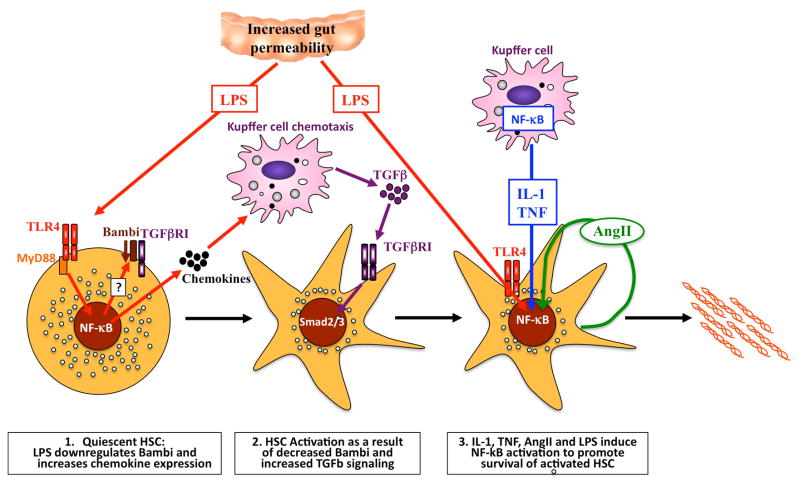
The contribution of NF-κB to hepatic stellate cell activation and survival. LPS activates TLR4 on quiescent HSCs to downregulate the inhibitory TGFβ pseudoreceptor BAMBI, and to increase chemotaxis of Kupffer cells in an NF-κB-dependent manner. Recruited Kupffer cells then release TGFβ, which activates HSCs in an unrestricted manner owing to low levels of BAMBI. Once HSCs have become activated, NF-κB plays an additional important role by enhancing their survival. The NF-κB activation in activated hepatic stellate cells is mediated by LPS, mediators released from Kupffer cells (such as IL-1β and TNF) and angiotensin II, which is released and acts on HSCs in an autocrine manner. Together, the increased activation and survival of HSCs result in increased numbers of activated HSCs in the liver and increased deposition of extracellular matrix. Abbreviations: AngII, angiotensin II; HSC, hepatic stellate cell; IL-1, interleukin 1; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; TLR4, Toll-like receptor 4; TGFβ, transforming growth factor β, TGFβR1, transforming growth factor β receptor 1; TNF, tumor necrosis factor.
In the canonical pathway, IκB is phosphorylated by a high-molecular-weight kinase complex consisting of two different catalytic IκB kinase (IKK)-subunits and the structural protein NF-κB essential modulator (NEMO; also called IKKγ). IκBα, the most prominent IκB family member, is phosphorylated by this complex at two different serine residues (Ser32 and Ser36). Phosphorylation marks IκBα for subsequent degradation through an ubiquitin-dependent pathway,32 allowing nuclear entry, DNA-binding and transcriptional activity of the now-liberated NF-κB (Figure 2). While IκB proteins such as IκBα generally act as inhibitors of NF-κB activation, some IκB family members (such as nonphosphorylated IκBβ) may also upregulate NF-κB-mediated gene expression by binding p65–cRel complexes.33
As outlined above, IKK1 (also named IKKα) and IKK2 (also named IKKβ) are the catalytic subunits of the IKK complex and are both able to phosphorylate IκB, but IKK2 exerts a stronger kinase activity in vitro than IKK1.34–37 Despite fulfilling a key role in the regulation of NF-κB, the IKK complex also exerts functions independently of NF-κB. For example, IKK1 is recruited to NF-κB target genes, where it phosphorylates histone H3 and promotes cytokine-induced κB gene transcription38 as well as to retinoic-acid-dependent genes to regulate their transcription.39 Moreover, NEMO and IKKs appear to have NF-κB-independent functions that are likely to play a role in the regulation of cell death, inflammation and carcinogenesis.20,40,41 For example, IKKs phosphorylate and inhibit the transcription factor FOXO3 to promote carcinogenesis.40 NEMO is also involved in the regulation of interferon signaling41 and exerts NF-κB-independent functions in hepatocarcinogenesis.20
A second level of regulation of NF-κB activity is mediated by post-transcriptional modification of NF-κB subunits. In addition to phosphorylating IκBα, IKKs also directly phosphorylate p65 at residues Ser468 and Ser536 and cRel at Ser484, Ser494 and Ser525.22 A number of other kinases, such as the cAMP-dependent protein kinase A, glycogen synthase kinase 3β, protein kinase Cζ, serine threonine protein kinase 1 and ribosomal protein S6 kinase, are involved in the post-transcriptional modification of NF-κB subunits. 22 Additional post-translational modification of NF-κB include acetylation, ubiquitination or prolyl isomerization.22 Numerous studies suggest that post-translational modifications enhance NF-κB activity by modulating nuclear translocation, DNA binding, transactivation and the interaction with Cbp/p300-interacting transactivator 1. However, there is a lack of in vivo studies using knockin strategies to validate the role of most of the post-transcriptional modifications. Ser276 is the only phosphorylation site of p65 whose role has been validated in vivo: a Ser276Ala mutation causes premature death of knockin mice,42 and a Ser276Asp mutation leads to constitutive activation and systemic inflammation.43 Thus, phosphorylation of Ser276 in p65 is absolutely required for NF-κB activity, whereas the contribution of other sites remains to be proven in vivo. It should also be mentioned that the gene encoding IκBα is itself an NF-κB target gene and part of a negative-feedback loop—IκBα mediates nuclear export of the DNA-binding subunits, thus adding a third level of regulation.44 Finally, there is also feedback via NF-κB-dependent regulation of microRNAs; miR-146a expression is dependent upon NF-κB and is upregulated in response to TLR agonists and inflammatory cytokines.45 miR-146a targets signaling molecules that have a crucial function in the NF-κB pathway, such as IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6), thus providing a negative feedback loop in the NF-κB signaling pathway.45
NF-κB links injury, fibrosis and HCC
A number of studies have substantiated the hypothesis that inflammation is not merely associated with chronic liver disease, but that it actively promotes disease progression 17, 46–48. >80% of HCCs occur in patients with hepatic cirrhosis or fibrosis and thus develop in a setting of chronic hepatocyte injury, regeneration, infiltration of inflammatory cells and an abundance of activated myofibroblasts.49 NF-κB has a wide range of functions in different cellular compartments influencing the survival of hepatocytes, inflammation in Kupffer cells, and survival, inflammation and activation in HSCs. This broad functionality means that NF-κB takes center stage in the regulation of central aspects of chronic liver disease and the resulting wound-healing responses that ultimately determine outcome (i.e. resolution of the disease, organ fibrosis and/or HCC).
The crucial role of NF-κB in the liver is underlined by the fact that genetic ablation of NF-κB regulators in mouse models leads to spontaneous liver injury, fibrosis and HCC,18, 20, 21 thus modeling the clinically most important complications of chronic liver disease in patients. Surprisingly, chronic injury per se is sufficient to initiate and promote hepatocarcinogenesis in these models, and does not require a priori mutations in oncogenes or tumor-suppressor genes18, 20, 21 suggesting that HCC indeed is a ‘wound that never heals’. These genetic HCC models not only emphasize the key role of NF-κB in the progression of hepatic disease processes, but also underline the mechanistic links between liver injury, inflammation, fibrosis and HCC.18, 20, 21 Notably, these links also play a role in patients, since more than 80% of HCCs arise in patients with hepatic cirrhosis, i.e. in a setting of chronic inflammation and fibrosis1. Altogether, the intimate links between different cellular compartments in chronic liver disease suggest that the role of inflammatory and anti-apoptotic pathways such as NF-κB in the development of HCC can only be fully understood using animal models with chronic injury, inflammation and fibrosis.
NF-κB and hepatocellular injury
Owing to its anatomical location, the liver is the primary target of gut-derived pathogens and their products and is frequently involved in systemic infections.50 Many of these bacterial products (such as LPS) and LPS-induced cytokines (such as TNF) are toxic to hepatocytes, which means the hepatic response to these inflammatory mediators needs to be tightly regulated to avoid liver injury.51 Interestingly, NF-κB mediates both proinflammatory and antiapoptotic responses to these mediators, ensuring that hepatocytes are protected from cell death while appropriate inflammatory and immune responses are initiated. The dual function of the NF-κB pathway requires a delicate balance, since too little or too much NF-κB activation may have a negative impact on the liver owing to increased inflammation or insufficient protection from cell death. Accordingly, the NF-κB pathway is causally involved in many aspects of chronic liver disease, such as hepatitis and hepatocarcinogenesis.
Despite elevated levels of TNF in response to infection and other triggers of inflammation 52, TNF usually does not induce cell death in the liver. Accordingly, treatment of mice or primary hepatocyte cultures with TNF or LPS results in NF-κB activation, transcription of cytoprotective genes and virtually no cell death 53 unless transcription or translation is inhibited by cycloheximide, actinomycin D or D-galactosamine,54–56. Genetic mouse models, such as the Rela−/− knockout mouse, have provided evidence that NF-κB is the main transcriptional pathway for protecting hepatocytes from TNF-induced and LPS-induced cell death. Rela−/− knockout mice die in utero 15–16 days after conception as a result of hepatocyte apoptosis.57 This effect is caused by increased sensitivity to TNF, since mice that lack both Tnf and Rela are rescued from embryonic lethality.58 Blocking NF-κB by other approaches, such as using an IκB super-repressor, have confirmed these results 59, 60. While p65 is the main cytoprotective NF-κB subunit in hepatocytes, cRel provides additional protection; Rel−/−Rela−/− double-knockout mice undergo liver degeneration and embryonic death ~1.5 days earlier than Rela−/− single-knockout mice.61
Genetic experiments have also highlighted the crucial functions of the IKK subunits in TNF-mediated liver apoptosis.56–61 Ikk2−/− mice die in utero at embryonic day 12.5 as a result of massive apoptosis in the liver; fibroblasts from these mice show no activation of NF-κB in response to TNF and IL-1.62–64 A similar phenotype was noted in Nemo−/− mice, which also show massive hepatocyte apoptosis and a defect in NF-κB activation upon TNF stimulation in primary mouse embryonic fibroblasts.65 By contrast, Ikk1−/− mice die shortly after birth and have a phenotype marked by skeletal defects, thickening of the skin and impaired limb outgrowth, but normal liver development.66, 67
These results were further strengthened by the findings of conditional gene targeting experiments.18–21,62,63 Conditional deletion of Nemo or the IKK-activating kinase TGF-β-activated kinase 1 (TAK1) in hepatocytes resulted in complete blockage of TNF-induced NF-κB activation.18–21 These deletions also caused massive hepatocyte apoptosis in isolated hepatocytes and acute liver failure in vivo in response to low-dose LPS injections.18–21 Surprisingly, hepatocyte-specific deletion of Ikk2 resulted in substantial remnant NF-κB activation in response to TNF in hepatocytes and did not sensitize the livers of adult mice to TNF-induced or LPS-induced liver failure.68, 69 These findings suggest that IKK1 and IKK2 withhold redundant functions in canonical NF-κB signaling in the adult liver. Indeed, mice with combined hepatocyte-specific deletions of both Ikk1 and Ikk2 exhibited acute liver failure in response to LPS injection comparable with the response in Nemo−/− mutant mice.19 Mice deficient in Nemo or Tak1 even display spontaneous hepatocyte death, demonstrating that complete absence of NF-κB activity results in induction of cell death. This effect is most likely mediated by low circulating levels of TNF induced by the commensal gut microbiota, or ligand-independent triggering of TNF receptor I as suggested by the decreased hepatocyte death seen in Nemo−/−/Fadd−/−18 and Tak1−/−Tnrf−/− double knockout mice.21
It should be emphasized that a situation similar to the Nemo−/− or Tak1−/− mouse models, with virtually no NF-κB activity in hepatocytes, is unlikely to occur in patients. Moreover, it was demonstrated that TAK1 and NEMO control additional, as yet undefined, pathways that regulate hepatocyte injury in an NF-κB-independent manner.20 This finding highlights that the results of knockout studies should be interpreted carefully with regards the function of NF-κB in hepatocytes. However, it is likely that NF-κB activation in the liver is not homogeneous and that a proportion of hepatocytes with insufficient NF-κB activation at a given moment might be susceptible to injury, and contribute to a vicious cycle of chronic injury, regeneration and inflammation similar to that seen in the Nemo−/− and Tak1−/− mice. Accordingly, decreased NF-κB activation in patients with chronic hepatitis C is correlated with increased apoptosis and accelerated disease progression.15
The mechanism how NF-κB exerts its antiapoptotic function in TNF-induced apoptosis remained unclear for a long time. NF-κB induces transcription of a variety of target genes with potentially antiapoptotic functions, including the cellular inhibitors of apoptosis (cIAPs), such as cIAP1, cIAP2, XIAP, antiapoptotic Bcl2 family members A1 and BclxL, cFLIP and TRAF1 or TRAF2.27, 70 Genetic studies published in the past decade have unraveled a complex network regulated by NF-κB. This network involves interactions with other stress-related pathways, namely the c-Jun-(N)-terminal kinase (JNK) and the p38 mitogen-activated protein kinase (MAPK) signaling cascades, and ultimately regulates cell death (Figure 2).
The induction of cell death in the absence of NF-κB activation largely depends on a prolonged activation of JNK.71, 72,73 This prolonged JNK activation induced by TNF depends on the production of reactive oxygen species (ROS), whose generation is usually blocked by NF-κB.68, 74 In NF-κB-defective cells, ROS accumulate because of reduced expression of the NF-κB target gene SOD2, which encodes the ROS-metabolizing enzyme superoxide dismutase 2 (SOD2).18, 75 ROS oxidize and inhibit JNK-inactivating phosphatases, thus causing prolonged JNK activity upon TNF stimulation.75 Prolonged JNK activation in turn accelerates the turnover of the NF-κB-induced antiapoptotic protein cFLIP (an inhibitor of caspase 8), through JNK-mediated phosphorylation and activation of the E3 ubiquitin ligase Itchy homolog.76 It should be pointed out that in another study, ablation of JNK1 and JNK2 in hepatocytes had little effect on hepatitis induced by LPS or concanavalin A.77 By contrast, ablation of JNK1 and JNK2 in both hematopoietic cells and hepatocytes reduced TNF production and hepatitis after administration of concanavalin A and LPS,77 providing evidence for a predominant role of JNK in the regulation of TNF production rather than in regulating hepatoycte death in a cell-autonomous manner.
NF-κB also collaborates with the p38 MAPK signaling cascade to protect hepatocytes from liver injury induced by TNF or LPS. Conditional deletion of p38α, the main p38 isoform, results in excessive JNK activation in response to LPS injection via hyperactivation of the upstream MAPK kinases MEKK4 and MEKK7.78 While this effect on JNK activation is not sufficient to sensitize hepatocytes to LPS-mediated or TNF-mediated apoptosis, an additional (slight) inhibition of NF-κB via co-deletion of Ikk2 results in massive apoptosis and liver failure in mice with liver-specific mutations in p38α and Ikk2 genes.78 NF-κB and p38α, therefore, have a synergistic effect in suppressing hyperactivation of JNK in response to TNF activation. The NF-κB–p38–JNK pathway represents a crucial network for the transmission of TNF-dependent signals and the activation of the caspase cascade in hepatocytes.
In addition, NF-κB target genes, such as IL6, activate additional cell signaling pathways including Stat3, which exerts a crucial role in inflammation, wound healing and hepatocarcinogenesis, and may be an essential link between inflammation and cancer 79, 80. In fact, data published in 2010 suggest that IL-6 is part of a procarcinogenic positive-feedback loop in which Stat3 activation upregulates miR-21 and miR-181b1, leading to downregulation of PTEN, upregulation of NF-κB and promotion of cellular transformation.81
While these data clearly point towards a cell-death-preventive function of NF-κB in hepatocyte apoptosis, some studies have indicated that a proinflammatory role of NF-κB might also promote hepatocyte cell death: Ischemia–reperfusion injury in rodents represents a complex model that reflects liver damage after organ transplantation or hemorrhagic shock and leads to an excessive inflammatory response followed by necrotic cell death. Mice with conditional deletion of Ikk2 in hepatocytes have a defect in NF-κB activation upon ischemia–reperfusion.69 Surprisingly, this partial NF-κB inhibition did not increase cell death, but instead resulted in dramatic attenuation of cellular necrosis along with considerably lower levels of aminotransferases and a decreased inflammatory infiltrate.69 At present, it is not clear how this cell-death-promoting action of NF-κB in the ischemia–reperfusion model is mediated, but findings in TAK1-deficient hepatocytes suggest that IKK subunits might even exert NF-κB-independent functions in cellular necrosis.20 Collectively, the effect of NF-κB on hepatocyte death seems to depend on the damaging agent and may vary among different models of liver injury.
NF-κB in liver fibrosis
Liver fibrosis is the result of chronic liver injury of different etiologies. While liver fibrosis usually reverses after cessation of injury,82–84 it may progress to cirrhosis if the underlying disease is not treated successfully, at which point it is generally irreversible.85 Moreover, liver fibrosis and liver cirrhosis are accompanied by an increased risk of developing HCC, as >80% of HCCs develop in patients with hepatic fibrosis or cirrhosis.1 At present, the most effective way to treat liver fibrosis is to heal the underlying disease,85 emphasizing the need to study the molecular mechanisms underlying this process. The principal cells responsible for promoting the hepatic deposition of cross-linked fibrillar collagen are hepatic myofibroblasts (HMF), which are believed to be largely derived from HSCs.86 In addition to promoting fibrosis, activated HSCs/HMF are also a source and a target of inflammatory mediators, which suggests that they are the crossroad of inflammation and fibrosis.86
NF-κB modulates hepatic fibrogenesis predominantly in three different cellular compartments: (A) by regulating hepatocyte injury, the primary trigger of fibrogenic responses in the liver; (B) by regulating inflammatory signals elicited in macrophages and other inflammatory cells in the liver; and (C) by regulating fibrogenic responses in HSCs. As described above, decreased or absent NF-κB activity in hepatocytes can lead to increased hepatocellular injury and subsequent fibrosis. The effect of increased NF-κB activation in hepatocytes on liver fibrosis is not well known. It is likely that normal or slightly increased NF-κB activity in hepatocytes protects against liver fibrosis by preventing hepatocyte death. However, there is probably a threshold beyond which NF-κB activation promotes the secretion of inflammatory and chemotactic factors in hepatocytes, and thereby worsens hepatic inflammation and fibrosis.
Kupffer cells are an important contributor to HSC/HMF activation and liver fibrosis.3, 87 Inhibition of NF-κB in Kupffer cells results in decreased liver fibrosis, but the underlying mechanisms remain largely elusive.88 While the role of NF-κB activation in hepatocytes and Kupffer cells that leads to liver fibrosis is only incompletely understood, there is growing evidence that NF-κB acts as a key mediator of fibrosis in HSCs and/or hepatic myofibroblasts. A wide range of proinflammatory mediators activate NF-κB in HSC/HMF including LPS, TNF, IL-1β, angiotensin II and CD40L.3, 89–92 Moreover, HSC/HMF show activation of NF-κB during culture activation 93, and in human and mouse models of liver fibrosis as demonstrated by the presence of Ser536-phosphylated p65.91 Notably, NF-κB activation is almost exclusively found in HSCs/HMF whereas there is little NF-κB activation in hepatocytes.91 This observation is quite similar to the c-Jun/JNK pathway, which is also predominantly activated in HSC/HMF,94 suggesting that these cells are an important site of inflammation in the chronically injured and fibrotic liver. Notably, HSCs are a direct target of mediators that activate NF-κB, such as LPS in vivo.3 Accordingly, several NF-κB-dependent genes including IL-6, CXCL5, Ch25h and SAA3 are upregulated in HSCs isolated from bile-duct-ligated livers treated with CCl4.95
NF-κB has been suggested to regulate three key aspects of HSC and/or hepatic myofibroblast biology: (A) activation, (B) survival and (C) inflammatory responses (Figure 3). NF-κB may act as both a profibrogenic and an antifibrogenic signaling pathway: NF-κB exerts antifibrogenic effects by suppressing transcription of the Col1a1 gene.96 On the other hand, Crel deficient mice display decreased fibrosis and decreased activation of HSCs in vitro and in vivo.97 Conversely, deficiency of the NF-κB subunit p50, which largely represses NF-κB activation, increases hepatic fibrosis and inflammatory gene expression in HSCs.98, 99 NF-κB activation also plays a role in profibrogenic signaling pathways in response to LPS-mediated TLR4 activation such as the downregulation of the TGFβ pseudoreceptor BAMBI, leading to enhanced TGFβ signaling and HSC activation (Figure 3).3
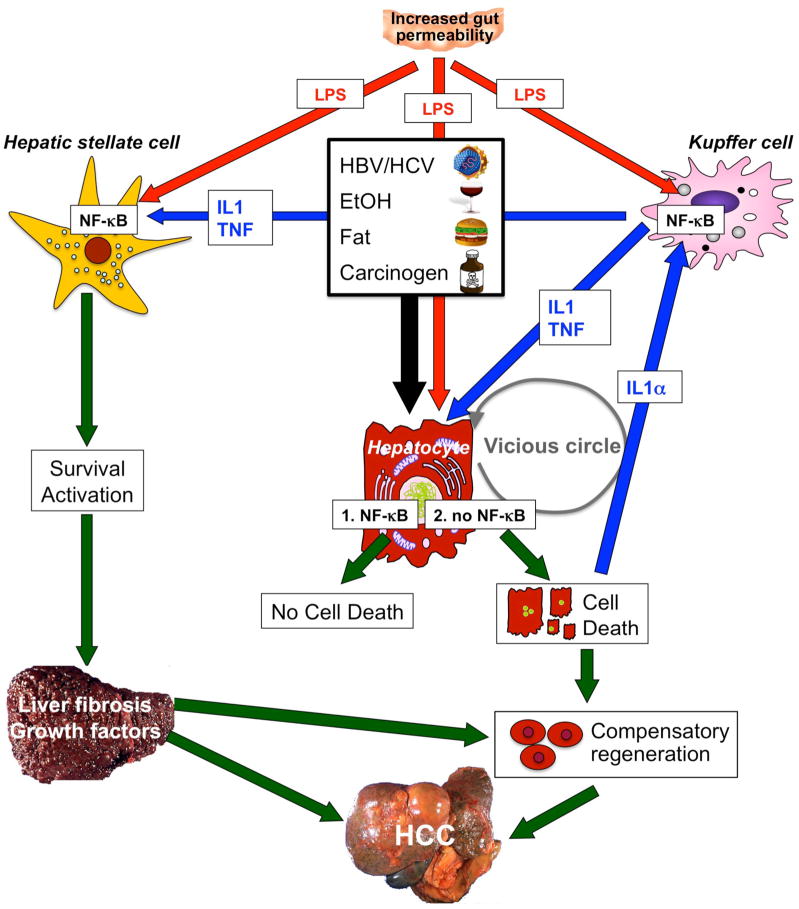
NF-κB contributes to hepatocarcinogenesis in the setting of chronic injury, inflammation and fibrosis. Low levels of NF-κB exacerbate injury induced by hepatitis viruses, alcohol, fat, LPS or carcinogens. However, complete absence of NF-κB (achieved by deletion of Tak1 or Nemo in mouse models) is sufficient to initiate apoptosis of hepatocytes even without these triggers, probably because low levels of LPS still reach the liver. Increased hepatic injury leads to stimulation of regenerative responses in progenitor cells and activation of Kupffer cells by IL-1α released from dying hepatocytes. In turn, these processes stimulate NF-κB activation in Kupffer cells and the release of mediators such as IL-1β and TNF. These mediators may induce further hepatocyte injury, IL-1α release and regenerative responses, leading to a vicious circle of injury, inflammation and regeneration. At the same time, LPS from the intestinal microbiota and TNF and IL-1β from Kupffer cells act on HSCs to promote their activation and survival. Activated HSCs and/or hepatic myofibroblasts produce extracellular matrix, which changes the hepatic microenvironment. Abbreviations: HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; IL, interleukin; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; TNF, tumor necrosis factor.
The profibrogenic role of TLR4 in the liver was confirmed in three different experimental models of liver fibrosis.3 This role has been confirmed in humans; a single nucleotide polymorphism of TLR4 that results in decreased TLR4 signaling is associated with reduced disease progression.4 Similarly, TLR9-mediated activation of NF-κB has been associated with HSC activation and liver fibrosis.7, 100, 101 TLR4 and TLR9 activation appear to predominantly result from increased translocation of bacterial TLR agonists from the gut, as sterilizing the gut with antibiotics exerts a strong antifibrotic effect.3, 102, 103
In patients with chronic liver disease, survival of HSC/HMF is required for mounting an efficient fibrogenic response. Conversely, increased apoptosis of HSC/HMF promotes the reversal of fibrosis.104 NF-κB is a key mediator of HSC/HMF survival during fibrogenic responses (Figure 3), as demonstrated by the ability of NF-κB inhibitors such as sulfasalazine and/or an NF-κB inhibitory NEMO-binding peptide to promote HSC/HMF apoptosis both in vitro and in vivo.91, 105 Moreover, the application of sulfasalazine or angiotensin II inhibitors also blocked hepatic fibrogenesis in experimental models of liver fibrosis, suggesting that the antiapoptotic effects of NF-κB directly translate into decreased fibrogenic responses.105 Moreover, NF-κB is involved in downregulation of miR-29 family members in HSCs, leading to reduced suppression of a whole family of collagens and other extracellular matrix proteins in HSCs.106 In addition to affecting fibrogenesis in HSC/HMF, activation of NF-κB also promotes the secretion of various inflammatory mediators from these cells, including chemokines such as CCL2, CCL3, CXCL2 and CXCL5.3, 95 The increased secretion of chemokines leads to an influx of inflammatory cells including macrophages, which are known to interact with HSCs and to affect HSC activation and wound healing.3, 87
In summary, the activation of NF-κB in HSCs and/or hepatic myofibroblasts appears to promote hepatic fibrosis via multiple mechanisms, including direct fibrogenic effects, antiapoptotic effects and the secretion of macrophage-recruiting chemokines. The increased activation of NF-κB in HSC/HMF may also contribute to a tumor-friendly microenvironment in the fibrotic liver, for example by providing inflammatory and chemotactic cytokines and chemokines as well as quantitative and qualitative changes of the extracellular matrix. However, no functional investigations have been performed to establish a role of NF-κB in HSC/HMF in tumor initiation or promotion in the setting of liver fibrosis.
NF-κB in HCC
In the great majority of cases, HCC arises in a setting of chronic inflammation and subsequent liver fibrosis and cirrhosis.1 In addition to infection with HBV or HCV, alcohol consumption, NAFLD and the uptake of liver toxins (e.g. aflatoxin B1) represent the main risk factors for hepatocarcinogenesis.49
NF-κB activation is a frequent and early event in human liver cancers of viral or nonviral etiologies and has been associated with the acquisition of a transformed phenotype during hepatocarcinogenesis.107 A number of different factors may trigger activation of NF-κB during hepatocarcinogenesis. In HBV-induced HCC, it has been suggested that the oncogenic HBV-X protein activates the NF-κB signaling pathway through the upregulation of TANK-binding kinase 1 (TBK1).108 This upregulation might promote progression of HCC via proliferative and antiapoptotic effects, as well as the spreading of liver tumor cells.109, 110 NF-κB activation is also detected in HCV-infected livers and in cells transfected with HCV core protein.16 Moreover, many patients with advanced liver disease display increased levels of LPS, resulting in activation of NF-κB in the liver.50 Fatty acids may also activate NF-κB,111 and are likely to contribute to the increased NF-κB activation seen in patients with NAFLD.11, 13 As emphasized in the previous sections, NF-κB activation in the setting of chronic injury is not restricted to hepatocytes but may also occur in other cellular compartments including macrophages, HSCs and HMF (Figure 4).
Genetically modified mouse models of HCC have been helpful for investigating the roles of NF-κB in different cell populations. A procarcinogenic role of NF-κB in hepatocytes was first demonstrated in the Mdr2−/− knockout mouse, which develops HCC [in response to chronic biliary hepatitis.112 In this model, hepatocyte-specific overexpression of the IκBα-super-repressor—a nondegradable mutant form of IκBα—leads to stable inhibition of inducible NF-κB activation and decreased liver tumor development compared with mice with intact NF-κB signaling. Importantly, NF-κB inhibition did not alter tumor initiation in this model, but was able to efficiently block later steps of the progression of transformed hepatocytes to HCC.103 This finding provides essential proof of the principle that NF-κB is a potential target for cancer prevention in chronic inflammatory liver disease. The findings from the Mdr2−/− mouse were supported by a study in another inflammatory HCC model—a transgenic mouse overexpressing the cytokines lymphotoxin α and/or lymphotoxin β in the liver.113 In this mouse model, inhibition of NF-κB by hepatocyte-specific deletion of IKK2 almost completely abolished hepatocarcinogenesis.113 This finding again argues for a pro-carcinogenic function of NF-κB in a setting in which hepatocarcinogenesis is predominantly driven by inflammatory mediators secreted from hepatocytes.
In contrast to the studies discussed above, NF-κB inhibition by deletion of IKK2 in hepatocytes resulted in increased liver tumor formation in the genotoxic diethylnitrosamine (DEN) tumor model.114–116 In another study, inhibition of NF-κB by deletion of NEMO (the regulatory IKK subunit) in parenchymal liver cells led to spontaneous hepatocarcinogenesis in mice.18 In both situations, hepatocellular injury appears to be an important mechanism that contributes to (in the DEN model) or initiates (in the NEMO-knockout model) HCC formation. Interestingly, IKK2 deletion also accelerates the development of HCC in the later stages of hepatocarcinogenesis by increasing proliferation of DEN-initiated hepatocytes and increasing Stat3 activation.116 This observation suggests that IKK2 prevents HCC by suppressing both liver injury and hepatocyte proliferation. Procarcinogenic effects of IKK2 deletion are, at least in part, mediated by increased JNK activation, as shown by the reduced HCC development in Jnk1 Ikk2 double knockout mice.115 Additionally, data from mice with deletion of Tak1 suggest that NEMO, and possibly other IKK subunits, might not only play a role in NF-κB activation but might also control other pathways to liver cancer.20
Obesity has been identified as an important risk factor for HCC 117, and it has been suggested that NAFLD will become a leading cause of the development of HCC 118. Moreover, the presence of diabetes nearly doubles the risk for HCC 119, 120. Patients with NAFLD display increased hepatic NF-κB activation,121 which is believed to contribute to hepatic insulin resistance.122, 123 Several studies support the hypothesis that NF-κB also plays an important role in the promotion of obesity associated HCC through IL6 and TNF, two well-known NF-κB target genes, whose expression is also considerably increased in obese patients: There is a strong increase in HCC in both genetic and chemical models of HCC124, 125 and ablation of IL-6 and TNF-R1 strongly reduces HCC load in mice receiving a combination of DEN and a high-fat diet.125 These studies also emphasize the link between NF-κB and other procarcinogenic pathways, such as Stat3, mediated by the NF-κB target gene IL6.
When transferring the data on NF-κB from mouse models into the human situation, one not only has to differentiate between its role in different phases and cellular compartments, but also to consider the physiology of the employed mouse models as well as the degree of NF-κB inhibition that often can reach unphysiological levels in genetic mouse models. For example, the DEN model of HCC does not induce the chronic injury, inflammation and fibrosis that is typical of the great majority of human HCCs, and the role of NF-κB in nonparenchymal cells is underestimated in this model. NF-κB most likely exerts specific effects in different cellular compartments such as hepatocytes, Kupffer cells and HSCs and/or hepatic myofibroblasts; that NF-κB activation may affect hepatocarcinogenesis through cell-specific mechanisms (Figure 4). When considering all these variables, NF-κB appears to act as a promoter rather than a suppressor of HCC in relevant physiological settings.
The role of NF-κB in other cell populations, such as macrophages, sinusoidal endothelial cells and HSCs and/or hepatic myofibroblasts in hepatocarcinogenesis has not yet been studied in detail. However, a study published in 2005 indicates that NF-κB is likely to have a procarcinogenic role in Kupffer cells.105 In this study, deletion of IKK2 in all hepatic cell populations (including Kupffer cells) by Mx1-Cre resulted in a decrease of DEN-induced hepatocarcinogenesis, whereas deletion of IKK2 in hepatocytes by Alb-Cre resulted in increased hepatocarcinogenesis.114 This study not only indicates a role for Kupffer cell NF-κB, but even suggests that the absence of IKK2 in Kupffer cells can override the increased injury observed in hepatocytes that lack IKK2. However, it should be noted that the deletion of Mx1-Cre is not specific for Kupffer cells, and the HCC suppression in this model could have been mediated by other bone-marrow-derived cells such as neutrophils, B cells, T cells or dendritic cells, or even resident hepatic cells, such as HSCs.
The contribution of liver sinusoidal endothelial cells to hepatic NF-κB activation and HCC is largely unknown. In liver development, IKK and/or NF-κB is required for the development of the sinusoidal vasculature and the prevention of hepatocyte apoptosis, but not the development of vasculature in other organs at the same time point.126 However, there is little known about the role of NF-κB in liver sinusoidal cells in chronic injury and hepatocarcinogenesis.
In addition to exerting specific effects in different hepatic cell populations, such as hepatocytes, Kupffer cells, and HSCs and/or hepatic myofibroblasts, it is also possible that NF-κB functions are not homogenous within one cell population. In addition, decreased NF-κB activation in some hepatocytes may result in apoptosis and regenerative responses, whereas increases in other populations may be responsible for their survival and malignant transformation. Moreover, it should be considered that the parenchymal cells are quite heterogeneous in the setting of chronic injury and that there is an increase in immature progenitor cells. It is conceivable that NF-κB exerts specific functions in progenitor cells and that too much and too little NF-κB activation in the same liver but within different cellular compartments or differentiation stages, contributes to hepatocarcinogenesis.
Despite the differences in NF-κB inhibition and HCC models among various studies, the data on the hepatocyte-specific function of NF-κB should not be seen as contradictory, but rather as a reflection of different models and circumstances, and an opportunity to learn about different aspects of the role of NF-κB in the liver. Several important conclusions relevant for the design of pharmacologic therapies targeting NF-κB can be drawn from these different models and studies. First, increased liver injury after NF-κB inhibition promotes liver cancer, as reflected in the NEMO knockout mice and TAK1 knockout mice, as well as the DEN-treated IKK2 knockout mice. Second, inhibition of NF-κB can suppress HCC if cells that are responsible for producing tumor-promoting proinflammatory mediators are targeted, at the same time increased liver injury is avoided. Third, it is possible that there are differences between targeting IKK (which may have functions not related to NF-κB) and targeting IκB (which does not appear to have functions outside of the NF-κB pathway).
Conclusions
Since its discovery more than 20 years ago, the NF-κB pathway has emerged as one of the best-characterized signaling pathways. The central role of NF-κB in liver homeostasis, and the regulation of inflammation, fibrosis and carcinogenesis is of high clinical relevance for chronic liver diseases. However, despite the considerable number of basic research studies and the growing number of chemical compounds targeting this pathway, there remains a gap in the translation of these findings into clinical hepatology. The low degree of enthusiasm for clinical applications is probably attributable to the complex consequences of IKK and NF-κB inhibition in mouse models of liver disease. This complexity reflects the diverse and often cell-specific functions of this pathway, as well as the current emphasis on the injury-promoting and HCC-promoting effects of hepatocyte-specific NF-κB inhibition. However, careful interpretation of data from mouse knockout models and further research of the role of NF-κB in nonparenchymal cells, such as HSC/HMF and Kupffer cells, in the development of fibrosis and their contribution to the tumor microenvironment may be helpful to translate anti-inflammatory treatment strategies based on NF-κB into clinical hepatology.
At present, there are no treatment options to prevent the development of cirrhosis and HCC in patients in whom the underlying disease cannot be cured. As outlined in this Review, NF-κB seems to be crucially involved both in fibrogenesis and in the initiation and promotion of HCC in the chronically inflamed liver. This involvement suggests that pharmacological targeting of this pathway might represent a promising strategy to intercept the potentially fatal fibrosis–carcinogenesis sequence. While new treatment approaches, such as the tyrosine kinase inhibitor sorafenib, might target both profibrogenic and procarcinogenic pathways,127, 128 the inflammatory components of chronic liver diseases that lead to fibrosis and HCC remain largely unaffected by this and other treatments.
A careful interpretation of results from mouse studies and additional preclinical studies will be helpful to establish promising candidates for anti-inflammatory interventions in patients with chronic liver disease. These interventions could be at the level of upstream activators of NF-κB such as LPS, TLR4, IL-1 or TNF, at the level of NF-κB subunits or NF-κB activating kinases, at the level of genes regulated by NF-κB such as IL6, or at the level of pathways that are modulated by NF-κB, such as JNK. The choice of molecular target, the degree of inhibition and the ability to target specific cell types in the liver will be crucial to develop regimens with low toxicity that would justify taking the step towards the first clinical studies in patients with chronic liver disease.
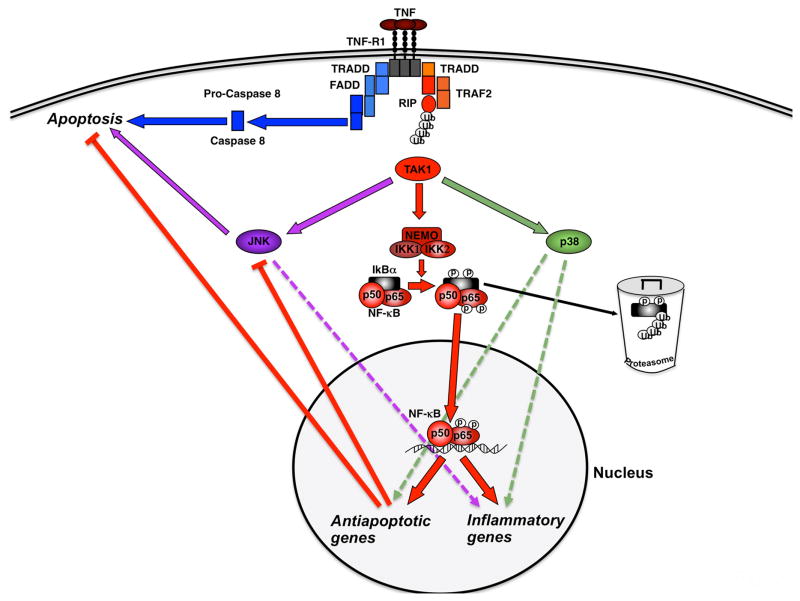
Regulation of NF-κB activation, inflammation and apoptosis after TNF stimulation. Upon TNF binding to its receptor (TNFR1), the IKK complex (IKK1, IKK2 and NEMO) becomes activated via adapter proteins like TRAF2 and RIP and mediates phosphorylation, ubiquitination and proteasome-mediated degradation of the inhibitory protein IκBα. This action liberates the p50–p65 NF-κB dimer and unmasks its nuclear localization site, resulting in NF-κB translocation to the nucleus, subsequent transcription of antiapoptotic and inflammatory genes and repression of JNK activation. These NF-κB-dependent signals are “counterbalancing” the TNF-induced cleavage of caspases like caspase-8 and the induction of apoptosis. Abbreviations: FADD, Fas-associated death domain; IKK, inhibitory κB kinase; IκB, inhibitor of κB; JNK, c-Jun-(N)-terminal kinase; NEMO, NF-κB essential modulator; NF-κB, nuclear factor κB; p, phosphorylation; TAK1, TGF-β-activated kinase 1; TNF, tumor necrosis factor; TNF-R1, TNF-receptor 1; TRADD, TNF-receptor-associated death domain; TRAF2, TNF-receptor-associated factor 2; RIP, receptor-interacting protein; Ub, ubiquitination
Acknowledgments
The authors acknowledge the support of NIH grants U54CA126513, R01DK076920 and R21AT003878 (R. F. Schwabe), European Research Council Starting grant ERC-2007-Stg/208237 (T. Luedde), the German Research Foundation grant SFB/TRR57/P06 (T. Luedde) and an Ernst-Jung-Foundation (Hamburg) grant (T. Luedde).
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nrgastro.2010.213
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3295539?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/nrgastro.2010.213
Article citations
A new opportunity for N-acetylcysteine. An outline of its classic antioxidant effects and its pharmacological potential as an epigenetic modulator in liver diseases treatment.
Naunyn Schmiedebergs Arch Pharmacol, 22 Oct 2024
Cited by: 0 articles | PMID: 39436429
Review
Evaluation of the therapeutic potential of novel nanoparticle formulations of glutathione and virgin coconut oil in an experimental model of carbon tetrachloride-induced liver failure.
BMC Pharmacol Toxicol, 25(1):74, 08 Oct 2024
Cited by: 0 articles | PMID: 39380023 | PMCID: PMC11460069
Modulatory Effects of Chalcone Thio-Derivatives on NF-κB and STAT3 Signaling Pathways in Hepatocellular Carcinoma Cells: A Study on Selected Active Compounds.
Int J Mol Sci, 25(19):10739, 05 Oct 2024
Cited by: 0 articles | PMID: 39409068 | PMCID: PMC11476945
Signaling pathways that activate hepatic stellate cells during liver fibrosis.
Front Med (Lausanne), 11:1454980, 18 Sep 2024
Cited by: 0 articles | PMID: 39359922 | PMCID: PMC11445071
Review Free full text in Europe PMC
New insights into potential biomarkers and their roles in biological processes associated with hepatitis C-related liver cirrhosis by hepatic RNA-seq-based transcriptome profiling.
Virus Res, 349:199457, 10 Sep 2024
Cited by: 0 articles | PMID: 39216827 | PMCID: PMC11415974
Go to all (716) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Canonical NF-κB signaling in hepatocytes acts as a tumor-suppressor in hepatitis B virus surface antigen-driven hepatocellular carcinoma by controlling the unfolded protein response.
Hepatology, 63(5):1592-1607, 04 Mar 2016
Cited by: 30 articles | PMID: 26892811
SHP2 is induced by the HBx-NF-κB pathway and contributes to fibrosis during human early hepatocellular carcinoma development.
Oncotarget, 8(16):27263-27276, 01 Apr 2017
Cited by: 14 articles | PMID: 28460481 | PMCID: PMC5432333
Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating the NF-κB pathway.
J Pathol, 235(3):490-501, 01 Feb 2015
Cited by: 45 articles | PMID: 25385148
Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis.
Hepatology, 46(2):590-597, 01 Aug 2007
Cited by: 260 articles | PMID: 17661407
Review
Funding
Funders who supported this work.
European Research Council (1)
The Function of inflammatory signalling pathways in acute and chronic liver disease and liver cancer (Luedde-Med3-Aachen)
Dr Tom Luedde, University Hospital Aachen
Grant ID: 208237
NCCIH NIH HHS (3)
Grant ID: R21 AT003878
Grant ID: R21AT003878
Grant ID: R21 AT003878-02
NCI NIH HHS (3)
Grant ID: U54 CA126513-05
Grant ID: U54CA126513
Grant ID: U54 CA126513
NIAAA NIH HHS (1)
Grant ID: R01 AA020211
NIDDK NIH HHS (3)
Grant ID: R01 DK076920-04
Grant ID: R01DK076920
Grant ID: R01 DK076920





