Abstract
Background
Assessment of health-related quality of life (HRQL) is important in patients with chronic obstructive pulmonary disease (COPD). Despite the high prevalence of COPD in Germany, Switzerland and Austria there is no validated disease-specific instrument available. The objective of this study was to translate the Chronic Respiratory Questionnaire (CRQ), one of the most widely used respiratory HRQL questionnaires, into German, develop an interviewer- and self-administered version including both standardised and individualised dyspnoea questions, and validate these versions in two randomised studies.Methods
We recruited three groups of patients with COPD in Switzerland, Germany and Austria. The 44 patients of the first group completed the CRQ during pilot testing to adapt the CRQ to German-speaking patients. We then recruited 80 patients participating in pulmonary rehabilitation programs to assess internal consistency reliability and cross-sectional validity of the CRQ. The third group consisted of 38 patients with stable COPD without an intervention to assess test-retest reliability. To compare the interviewer- and self-administered versions, we randomised patients in groups 2 and 3 to the interviewer- or self-administered CRQ. Patients completed both the standardised and individualised dyspnoea questions.Results
For both administration formats and all domains, we found good internal consistency reliability (Crohnbach's alpha between 0.73 and 0.89). Cross-sectional validity tended to be better for the standardised compared to the individualised dyspnoea questions and cross-sectional validity was slightly better for the self-administered format. Test-retest reliability was good for both the interviewer-administered CRQ (intraclass correlation coefficients for different domains between 0.81 and 0.95) and the self-administered format (intraclass correlation coefficients between 0.78 and 0.86). Lower within-person variability was responsible for the higher test-retest reliability of the interviewer-administered format while between person variability was similar for both formats.Conclusions
Investigators in German-speaking countries can choose between valid and reliable self-and interviewer-administered CRQ formats.Free full text

Self-administration and interviewer-administration of the German Chronic Respiratory Questionnaire: instrument development and assessment of validity and reliability in two randomised studies
Abstract
Background
Assessment of health-related quality of life (HRQL) is important in patients with chronic obstructive pulmonary disease (COPD). Despite the high prevalence of COPD in Germany, Switzerland and Austria there is no validated disease-specific instrument available. The objective of this study was to translate the Chronic Respiratory Questionnaire (CRQ), one of the most widely used respiratory HRQL questionnaires, into German, develop an interviewer- and self-administered version including both standardised and individualised dyspnoea questions, and validate these versions in two randomised studies.
Methods
We recruited three groups of patients with COPD in Switzerland, Germany and Austria. The 44 patients of the first group completed the CRQ during pilot testing to adapt the CRQ to German-speaking patients. We then recruited 80 patients participating in pulmonary rehabilitation programs to assess internal consistency reliability and cross-sectional validity of the CRQ. The third group consisted of 38 patients with stable COPD without an intervention to assess test-retest reliability. To compare the interviewer- and self-administered versions, we randomised patients in groups 2 and 3 to the interviewer- or self-administered CRQ. Patients completed both the standardised and individualised dyspnoea questions.
Results
For both administration formats and all domains, we found good internal consistency reliability (Crohnbach's alpha between 0.73 and 0.89). Cross-sectional validity tended to be better for the standardised compared to the individualised dyspnoea questions and cross-sectional validity was slightly better for the self-administered format. Test-retest reliability was good for both the interviewer-administered CRQ (intraclass correlation coefficients for different domains between 0.81 and 0.95) and the self-administered format (intraclass correlation coefficients between 0.78 and 0.86). Lower within-person variability was responsible for the higher test-retest reliability of the interviewer-administered format while between person variability was similar for both formats.
Conclusions
Investigators in German-speaking countries can choose between valid and reliable self-and interviewer-administered CRQ formats.
Background
Clinicians and investigators are showing increasing agreement that measurement of health-related quality of life (HRQL) is important for patient management.[1] For patients with chronic diseases such as COPD, the aim of treatments is to reduce symptoms and to improve quality of life.[2] However, only translated[3,4] but no clinically validated German versions of COPD-specific quality of life instrument exist.[5]
The interviewer-administered "Chronic Respiratory Questionnaire" (CRQ)[6] is a valid, reliable and responsive instrument.[7,8] that has seen extensive use. [9-11] The CRQ is simple to use and there is a significant body of literature guiding their interpretation. [12-15] However, the requirement for an interviewer may be inefficient and some investigators suggested that the individualised dyspnoea questions increases the time needed for administration.[8,16] A self-administered version[17] of the CRQ as well as a standardised dyspnoea domain are both available[18]. These administration formats need evaluation before investigators can confidently use them in clinical trials.
In addition, there is a need for a validated COPD-specific instrument in German-speaking countries. Ideally there should be one culturally adapted version for all German-speaking countries to ensure comparability of CRQ scores across these countries in future clinical trials. Therefore, the aim of this study was to translate the English versions of the interviewer- and self-administered CRQ as well as the individualised and standardised dyspnoea domains into German and to validate these formats concurrently in Switzerland, Germany and Austria. We focus in this report on the instrument development, cross-sectional validity and reliability. We report the evaluative properties of the German CRQ including responsiveness and longitudinal validity elsewhere.[19]
Methods
Patients
We recruited three separate groups of patients with COPD (see table table1)1) with a FEV1/FVC < 70% predicted and postbronchodilator FEV1 < 80% predicted according to GOLD criteria COPD[2] and no restriction of disease severity. Inclusion criteria were further: German as the first or "daily" language, age > 40 years, and ability to complete the CRQ within one session. We excluded patients with inability to read or write, with cognitive difficulties, with cancer or lung diseases other than COPD.
Table 1
Groups of COPD patients recruited for the adaptation and validation of the German CRQ
| Number of patients | Recruitment sites | Objective | |
| Group 1 | 44 | 4 pulmonary rehabilitation centres and one University hospital in Switzerland, Germany and Austria | Pilot testing of CRQ formats during translation and adaptation process |
| Group 2 | 80 | 4 pulmonary rehabilitation centres in Switzerland, Germany and Austria | Internal consistency reliability and cross-sectional validity of CRQ formats |
| Group 3 | 38 | One outpatient service of a University hospital and three private practices in Switzerland and Germany | Test-retest reliability of CRQ formats |
The first group of patients consisted of 44 patients from four rehabilitation clinics and a University hospital in Switzerland (Zuercher Hoehenklinik Wald, Klinik Barmelweid and University Hospital of Zurich), Germany (Pulmoresearch Institute Hamburg) and Austria (Rehabilitationsklinik Weyer/Enns). In these patients we pilot tested the CRQ formats during the translation and adaptation process (table (table1).1). These patients did not participate in the subsequent validation study.
We recruited an additional 80 patients (group 2) from the same four rehabilitation centres. These patients followed an intense multidisciplinary pulmonary rehabilitation program that consisted mainly of physical exercise but also offered patient education, relaxation therapies and psychosocial support. In these patients we assessed the internal consistency reliability and the cross-sectional validity of the German CRQ.
Finally, we recruited a third group of patients (group 3, n = 38) who did not undergo any changes in the therapeutic management for at least six weeks and were in a stable pulmonary condition to assess test-retest reliability of the German CRQ. We recruited these patients from one University hospital (Zurich, Switzerland) and private offices of pulmonologists in Switzerland and Germany.
Chronic Respiratory Questionnaire
The CRQ is divided into the four domains of fatigue, emotional function, mastery and dyspnoea. Patients answer to each of the 20 questions on a seven points scale expressing the degree of disability from 1 (maximum impairment) to 7 (no impairment). The standardised dyspnoea domain comprises five items concerning activities that cause shortness of breath in some patients with COPD as previously described.[18,19] When applicable we trained all interviewers in the use of this instrument in identical fashion to ensure consistent application following the recommendations of the developer of the original CRQ (GHG).
Translation and instrument development
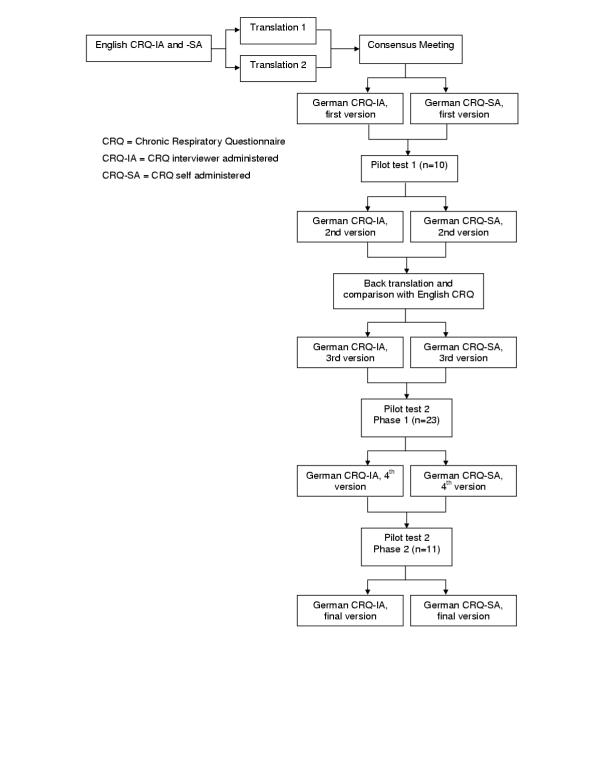
We followed a sequential forward and backward translation approach (applied in patient group 1) (see figure figure11).[20] Two translators independently translated the English interviewer administered CRQ (CRQ-IA) and self-administered CRQ (CRQ-SA) as well as the individual and standardised dyspnoea items into German. In a Consensus Meeting with the translators, two pulmonologists and a methodologist agreed on first German versions for these formats. We then pilot tested these versions in 10 patients of group 1 to identify difficulties in understanding. In addition, we tested various possible wordings of items, answer choices and instructions if the translation team considered more than one possible version. An English translator with experience in biomedical sciences but unaware of the original English CRQ performed a back translation of the German CRQ formats into the source language (English). A team of McMaster University investigators compared the back translation with the English CRQ to check for conceptual discrepancies.
After administration of the CRQ to an additional 23 patients in group 1, the translation team discussed the comments from these patients and decided in consensus on modifications. Finally, we recruited another 11 patients (belonging to group 1) to investigate whether these changes were appropriate.
Instrument testing
To allow comparisons between the different administration formats of the German CRQ, we validated them concurrently in two randomised studies. In the first study we randomly assigned 80 patients to either the CRQ-IA group (n = 40) or CRQ-SA group (n = 40). All patients completed the individualised and standardised dyspnoea items. To eliminate order effects we also randomised the order of administration (first individualised, then standardised or vice versa). We assessed all patients within 3 days after enrolment in any of the four rehabilitation programs.
In the second randomised study, we used the same randomisation procedure and included 38 stable patients (group 3). These patients completed the CRQ twice, ten days apart. These patients were blinded to their previous scores at the follow-up interview and did not undergo any therapy changes.
We generated two separate randomisation lists by computer (one for group 2 and one for group 3) in blocks of four per centre. Allocation of patients to either the CRQ-IA or CRQ-SA was concealed using a central telephone system. The site investigators, who were unaware of details on block randomisation, contacted the study coordinator (MP) by telephone for each patient who had given informed consent to receive the group assignment. The study coordinator registered the patient's initials, gender and date of birth to verify if all patients were allocated correctly. All local ethics committees approved the study protocol and patients provided informed consent prior to participation in the study.
Validation instruments to assess cross-sectional validity of the CRQ
Patients performed a six-minute walking test to assess functional exercise capacity at the beginning and end of the rehabilitation. In addition, we used a modified Borg scale in German[21] to assess the intensity of perceived dyspnoea at the end of the six-minute walking test. The Borg scale consisted of a scale labelled from 0 to 10 and with verbal descriptors. Zero represented "no dyspnoea at all" and 10 "very, very severe dyspnoea". We used two additional instruments to assess HRQL: The German self-administered SF-36 Health Survey[22] and the Feeling Thermometer (FT). The SF-36 is a generic instrument for assessment of HRQL and assesses 8 subscales of HRQL. Other investigators used the SF-36 in trials with COPD patients participating in respiratory rehabilitation.[23] The FT is an anchor based visual analogue scale from 0 to 100 where 0 (dead) represents the worst and 100 (full health) the best health state. Accumulating evidence suggests that the FT works well as a HRQL instrument in various groups of patients, including patients with COPD [24-26]. All these outcome measures were taken at the same time as the CRQ administration, i.e. at the beginning and end of the rehabilitation.
Statistical analysis
We calculated CRQ domain scores by summing the scores of the single items and then dividing the sum by the number of items in the respective domain. We used parametric tests because scores on the seven points Likert-type scale did not differ significantly from a normal distribution (Shapiro-Wilk test and analysis of normal quantile-quantile plots).
We assessed internal consistency for each domain by calculating Crohnbach's alpha for CRQ baseline scores. In addition, we calculated for each standardised item its corrected item-total correlation, which should exceed 0.2[27] and calculated Crohnbach's alpha again excluding the item under study. We did not include the individualised dyspnoea domain in this analysis because patients select different items so that the domain cannot be assessed across patients for internal consistency.
To assess cross-sectional validity (in patient group 2) we used Pearson correlation coefficients between the CRQ baseline scores and those of the validation measures.
Finally, we assessed test-retest reliability using intraclass correlation coefficients for the baseline and follow-up CRQ domain scores of the stable COPD patients (group 3) by taking the between person variance at baseline and follow-up as the signal and within person variance as well as between person variance at baseline and follow-up as the noise. All statistical analyses were performed with SPSS for Windows version 10.0 (SPSS Inc, Chicago, Ill).
Results
Translation and instrument development (group 1)
The wording of the questions and answer choices correspond to the original version. We did not add or remove items nor change the answer scales apart from adaptation to German. Modifications became necessary for the instructions of the individualised dyspnoea items of the CRQ-SA. The original translation of the instructions was too extensive and too complicated and patients were unable to complete this domain by themselves without difficulties. Therefore, we simplified the instructions omitting some of the instructions that added text without contributing substantially to the understanding. In addition, we listed each item of the individualised and standardised dyspnoea domain separately. This means that there are five separate questions for the dyspnoea domain.
Patients at times were surprised that the list of activities of the individualised dyspnoea domain did not begin with a physical activity ("being angry or upset"). Therefore we placed this item at position 5 of the list of 26 items. Accordingly, the standardised dyspnoea question 1 of the English CRQ ("Shortness of breath when being angry or upset") was unchanged but placed as question 3 in the German CRQ. We pilot tested the changes and patients were able to complete the German CRQ-SA without major difficulties and understood all items and answer choices.
Internal consistency and cross-sectional validity (group 2)
Nine patients did not complete the study for the following reasons: five withdrew for non-specified reasons (one patient in CRQ-IA group and four in CRQ-SA group) and two patients did not meet the a priori inclusion criteria upon review of their baseline data (one patient in each group with FEV1/FVC > 70%). In addition, two patients of the CRQ-SA group discontinued the rehabilitation program shortly after admission (one patient had an acute exacerbation requiring inpatient care and the other went home shortly after beginning of the rehabilitation). We excluded these two patients because we had decided a priori to include only patients with complete validation data in the analysis. The baseline characteristics of these two patients did not differ from the included patients. Thus, we analysed data from 38 patients of the CRQ-IA group and 33 patients of the CRQ-SA group. The patients of the two groups (CRQ-IA and CRQ-SA) were similar at baseline: Mean age was 67.4 years (SD 8.7) in the CRQ-IA and 67.7 (SD 8.3) in CRQ-SA group, FEV1/FVC predicted was 48.5% (SD 13.3) in the CRQ-IA and 49.9% (SD 10.5) in the CRQ-SA group and the average smoking history was 44.9 pack years (26.1) in the CRQ-IA and 46.8 (27.6) in the CRQ-SA group.
Crohnbach's alpha for baseline data were between 0.73 and 0.89 for both administration formats and met our a priori defined requirements for adequate internal consistency reliability (table (table2).2). For the CRQ-IA, corrected item-total correlations for baseline data were for all but one item above 0.32 (table (table3).3). Item 9 ("How often during the last two weeks have you felt embarrassed by your coughing or heavy breathing?") had a very low item-total correlation of -0.03. If this item was deleted internal consistency reliability would be markedly improved (0.86). Corrected item-total correlations tended to be higher for the CRQ-SA (0.37–0.85). As for the CRQ-IA item 9 showed the lowest corrected item-total correlation but was considerable higher compared with the CRQ-IA (0.37).
Table 2
Inter-item correlations† (internal consistency reliability) of the interviewer and self-administered format for baseline scores
| DOMAIN | GERMAN CRQ-IA (n = 33) | GERMAN CRQ-SA (n = 38) |
| Standardised Dyspnoea items | 0.73 | 0.78 |
| Fatigue items | 0. 81 | 0.83 |
| Emotional Function items | 0.77 | 0.89 |
| Mastery items | 0.76 | 0.86 |
† Crohnbach's alpha. CRQ-IA = Interviewer administered Chronic Respiratory Questionnaire. CRQ-SA = Self-administered Chronic Respiratory Questionnaire
Table 3
Corrected item-total correlations and internal consistency reliability if item was deleted for baseline scores of the interviewer and self-administered CRQ format
| Item | CRQ-IA | CRQ-SA | ||
| Corrected item-total correlation | Crohnbach alpha if item deleted | Corrected item-total correlation | Crohnbach alpha if item deleted | |
| Standardised dyspnoea domain | Standardised dyspnoea domain | |||
| 1 | 0.46 | 0.70 | 0.56 | 0.74 |
| 2 | 0.68 | 0.62 | 0.67 | 0.71 |
| 3 | 0.50 | 0.69 | 0.44 | 0.78 |
| 4 | 0.32 | 0.74 | 0.70 | 0.71 |
| 5 | 0.56 | 0.66 | 0.48 | 0.77 |
| Fatigue domain | Fatigue domain | |||
| 8 | 0.64 | 0.76 | 0.56 | 0.83 |
| 11 | 0.65 | 0.75 | 0.67 | 0.79 |
| 15 | 0.67 | 0.74 | 0.67 | 0.79 |
| 17 | 0.56 | 0.80 | 0.78 | 0.73 |
| Emotional function domain | Emotional function domain | |||
| 6 | 0.66 | 0.71 | 0.80 | 0.85 |
| 9 | -0.03 | 0.86 | 0.37 | 0.92 |
| 12 | 0.72 | 0.70 | 0.67 | 0.87 |
| 14 | 0.41 | 0.76 | 0.65 | 0.88 |
| 16 | 0.84 | 0.67 | 0.79 | 0.86 |
| 18 | 0.56 | 0.74 | 0.74 | 0.87 |
| 20 | 0.57 | 0.73 | 0.85 | 0.85 |
| Mastery domain | Mastery domain | |||
| 7 | 0.49 | 0.75 | 0.67 | 0.83 |
| 10 | 0.65 | 0.66 | 0.76 | 0.80 |
| 13 | 0.44 | 0.78 | 0.65 | 0.85 |
| 19 | 0.72 | 0.63 | 0.77 | 0.79 |
CRQ-IA = Interviewer administered Chronic Respiratory Questionnaire CRQ-SA = Self-administered Chronic Respiratory Questionnaire
Correlations with other validation measures were generally higher for the standardised dyspnoea questions compared to the individualised questions and for the self-administered compared to the interviewer-administered dyspnoea questions, respectively (table (table4).4). The correlations of the CRQ-SA dyspnoea domain with the FT, the SF-36 Mental Health and Vitality Index were higher than those of the CRQ-IA.
Table 4
Cross-sectional validity for the individualised and standardised dyspnoea domains: Correlations for baseline scores.
| Instrument and domain | CRQ-IA Dyspnoea Domains | CRQ-SA Dyspnoea Domains | ||
| Individualised† | Standardised† | Individualised† | Standardised† | |
| Feeling Thermometer | 0.04 (-0.13;0.21) | 0.12§ (-0.05;0.29) | 0.09* (-0.08;0.26) | 0.58* § (0.44;0.72) |
| SF-36-General Health Perception Index | 0.03 (-0.14;0.20) | 0.18 (0.01;0.35) | 0.28 (0.12;0.44) | 0.35 (0.19;0.51) |
| SF-36-Physical Functioning Index | 0.42 (0.26;0.58) | 0.54 (0.40;0.68) | 0.34* (0.18;0.50) | 0.68* (0.55;0.81) |
| Mental Health Index | 0.04 (-0.13;0.21) | 0.20§ (0.03;0.37) | 0.35 (0.19;0.51) | 0.54§ (0.40;0.68) |
| SF-36-Vitality Index | 0.17* § (0.00;0.34) | 0.59* (0.45;0.73) | 0.60§ (0.46;0.74) | 0.50 (0.35;0.65) |
| Six minutes walk test | 0.25 (0.08;0.42) | 0.30 (0.14;0.46) | 0.10 (-0.07;0.27) | 0.28 (0.12;0.44) |
| Borg Scale | -0.17 (-0.35;0.01) | -0.03 (-0.20;0.14) | -0.28 (-0.44;-0.12) | -0.34 (-0.50;-0.18) |
CRQ-IA = Interviewer administered German Chronic Respiratory Questionnaire. CRQ-SA = Self-administered German Chronic Respiratory Questionnaire. † Pearson Correlation Coefficient (95% confidence intervals); r > 0.28 significant at p < 0.05. * indicate significant differences between the individualised and standardised dyspnoea domains §indicate significant differences between the domains of the CRQ-IA and CRQ-SA.
For the fatigue domain, the correlations were similar for the CRQ-IA and CRQ-SA except for the correlations with the six-minute walking test, which was significant higher for the CRQ-IA (table (table5).5). We did not observe statistically significant differences between the CRQ-IA and CRQ-SA for the correlations of the emotional function and mastery domain.
Table 5
Cross-sectional validity for the fatigue, emotion and mastery domains. Correlations for baseline scores.†
| Instrument and domain | CRQ-IA Domains | CRQ-SA Domains | ||||
| Fatigue† | Emotion† | Mastery† | Fatigue† | Emotion† | Mastery† | |
| Feeling Thermometer | 0.10 (-0.07;0.27) | 0.08 (-0.09;0.25) | 0.17 (0.01;0.33) | 0.16 (0.00;0.32) | 0.19 (0.03;0.35) | 0.30 (0.14;0.46) |
| SF-36-General Health Perception Index | 0.46 (0.31;0.61) | 0.28 (0.12;0.44) | 0.36 (0.20;0.52) | 0.18 (0.01;0.35) | 0.12 (-0.05;0.29) | 0.38 (0.22;0.54) |
| SF-36-Physical Functioning Index | 0.45 (0.30;0.60) | -0.07 (-0.24;0.10) | 0.30 (0.14;0.46) | 0.21 (0.04;0.38) | -0.15 (-0.32;0.02) | 0.39 (0.23;0.55) |
| SF-36-Mental Health Index | 0.53 (0.38;0.68) | 0.72 (0.60;0.84) | 0.62 (0.49;0.75) | 0.63 (0.50;0.76) | 0.69 (0.57;0.81) | 0.42 (0.26;0.58) |
| SF-36-Vitality Index | 0.72 (0.60;0.84) | 0.63 (0.50;0.76) | 0.67 (0.54;0.80) | 0.66 (0.53;0.79) | 0.50 (0.35;0.65) | 0.49 (0.34;0.64) |
| Six minutes walk test | 0.35§ (0.18;0.52) | 0.24 (0.08;0.40) | 0.30 (0.14;0.46) | 0.00§ (-0.17;0.17) | -0.04 (-0.21;0.13) | 0.00 (-0.17;0.17) |
| Borg Scale | -0.16 (-0.34;0.02) | -0.11 (-0.28;0.06) | -0.11 (-0.28;0.08) | -0.11 (-0.28;0.06) | -0.18 (-0.35;-0.01) | -0.31 (-0.47;-0.15) |
CRQ-IA = Interviewer administered German Chronic Respiratory Questionnaire CRQ-SA = Self-administered German Chronic Respiratory Questionnaire † Pearson Correlation Coefficient (95% confidence intervals); r > 0.28 significant at p < 0.05 § indicate significant differences between the domains of the CRQ-IA and CRQ-SA
Test-retest reliability (group 3)
In patients randomised to the CRQ-IA (n = 16), mean age was 63.7 (SD 9.1), FEV1/FVC in percent-predicted 44.3 (SD 10.4) and patients had a mean smoking history of 52.4 pack years (SD 28.9). Mean age in the CRQ-SA group (n = 19) was 61.1 (SD 8.0), FEV1/FVC in percent-predicted 44.4 (SD 13.6) and a mean smoking history of 54.6 pack years (SD 33.8). Intraclass correlation coefficients were higher for the CRQ-IA but also well above 0.7 for all CRQ-SA domains (table (table6).6). Lower within-person variability was responsible for the higher test-retest reliability of the interviewer-administered format while between-person variability was similar for both formats.
Table 6
Test-retest reliability# of the German CRQ
| Domain | GERMAN CRQ-IA | GERMAN CRQ-SA |
| Individualised dyspnoea | 0.81 | 0.86 |
| Standardised dyspnoea | 0.92 | 0.78 |
| Fatigue | 0.95 | 0.79 |
| Emotional Function | 0.92 | 0.82 |
| Mastery | 0.92 | 0.80 |
# Intraclass correlation coefficient CRQ-IA = Interviewer administered Chronic Respiratory Questionnaire CRQ-SA = Self-administered Chronic Respiratory Questionnaire
Discussion
We developed different administration formats of the German CRQ and validated them in two randomised studies. We found good internal consistency reliability for the interviewer- and self-administered CRQ. Cross-sectional validity was higher for the standardised compared to individual dyspnoea questions. Test-retest reliability exceeded our preset threshold (intraclass correlation coefficient > 0.7) for both the CRQ-IA and the CRQ-SA.
The strengths of our study included the stepwise development of the German CRQ formats, which allowed us to reconsider and to test the different versions in three stages of pilot testing. The aim of this approach was to add quality with every step in terms of conceptual equivalence between the source and target version as well as in terms of comprehensibility for the patients. While the CRQ-IA was easy comprehensible for patients, we noticed through pilot testing that patients had difficulties completing the individualised dyspnoea items of the initial German translation of the CRQ-SA independently. However by modifying and pilot testing the instructions of this individualised dyspnoea domain we were able to develop an improved version.
The standardised dyspnoea domains produced higher cross-sectional correlations than the individualised dyspnoea domains. This finding is important because it indicates that the standardised CRQ dyspnoea domain allows for better discrimination between different degrees of COPD severity. These results are consistent with those of a recent study in which discriminative properties of the standardised dyspnoea questions of the English CRQ also proved superior[18]
We found for item 9 ("How often during the last two weeks have you felt embarrassed by your coughing or heavy breathing?") a very low item-total correlation of -0.03. Because there is no apparent explanation for this finding, we used the data set of patient group 3 (test-retest) and analysed the item-total correlations of the emotional function domain. We found item-total correlations of 0.51 for the CRQ-IA and 0.50 for the CRQ-SA for item 9. For all items of the emotional function domain the item-total correlation was between 0.44 and 0.85. Thus we assume that the low item-total correlation in patient group 2 was due to chance.
Conclusions
The careful development of the German CRQ has led to reliable and valid self- and interviewer administered CRQ formats and individualised and standardised dyspnoea questions. The need of an interviewer and the time-consuming selection process of the individualised dyspnoea questions are no longer a hindrance for the use of the CRQ: Investigators can choose between self-and interviewer administered formats and individualised and standardised dyspnoea questions based on efficiency considerations. The brevity of the standardised CRQ-SA with good validity, reliability and responsiveness makes the CRQ-SA an attractive choice for trials as well as for clinical practice.
Abbreviations
COPD = Chronic Obstructive Pulmonary Disease
CRQ = Chronic Respiratory Questionnaire
CRQ-IA = Chronic Respiratory Questionnaire Interviewer-Administered
CRQ-SA = Chronic Respiratory Questionnaire Self-Administered
FT = Feeling Thermometer
HRQL = Health Related Quality of Life
SD = Standard Deviation
Funding
GlaxoSmithKline Switzerland and the Swiss Lung League funded this study with grants to the Horten Centre (MP). The sponsors were not involved in the study design, conduction of the trial, analysis of data and manuscript writing.
Authors contributions
MP, MB, MF, OB, HS and GG designed and organised the study; MB, MF, TG, OB and AL collected the data and supervised the study at their study sites, MP and HS analysed the data and wrote the first draft of the manuscript, MB, MF, TG, OB, AL and GG critically reviewed the manuscript and MP and HS prepared the final version of the manuscript.
Acknowledgment
The CRQ-IA and CRQ-SA are copyrighted by McMaster University; Principal Authors Dr. Gordon Guyatt and Dr. Holger Schünemann. Use of the instrument requires licensing.
We would like to thank Cornelia Flamann (Zuercher Hoehenklinik Wald) and Dr. Marco Laschke (Klinik Barmelweid) for data collection in their centres.
References
- Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. [Abstract] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. [Abstract] [Google Scholar]
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. 10.1016/S0140-6736(03)12459-2. [Abstract] [CrossRef] [Google Scholar]
- Behnke M, Taube C, Kirsten D, Lehnigk B, Jorres RA, Magnussen H. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med. 2000;94:1184–1191. 10.1053/rmed.2000.0949. [Abstract] [CrossRef] [Google Scholar]
- Puhan MA, Koller M, Brandli O, Steurer J. [Pulmonary rehabilitation of COPD in Switzerland--an assessment of current status] Schweiz Rundsch Med Prax. 2003;92:111–116. [Abstract] [Google Scholar]
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. [Europe PMC free article] [Abstract] [Google Scholar]
- Harper R, Brazier JE, Waterhouse JC, Walters SJ, Jones NM, Howard P. Comparison of outcome measures for patients with chronic obstructive pulmonary disease (COPD) in an outpatient setting. Thorax. 1997;52:879–887. [Europe PMC free article] [Abstract] [Google Scholar]
- Wijkstra PJ, TenVergert EM, van Altena R, Otten V, Postma DS, Kraan J, Koeter GH. Reliability and validity of the chronic respiratory questionnaire (CRQ) Thorax. 1994;49:465–467. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldstein RS, Todd TR, Guyatt G, Keshavjee S, Dolmage TE, van Rooy S, Krip B, Maltais F, LeBlanc P, Pakhale S, Waddell TK. Influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 2003;58:405–410. 10.1136/thorax.58.5.405. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tsukino M, Nishimura K, McKenna SP, Ikeda A, Hajiro T, Zhang M, Izumi T. Change in generic and disease-specific health-related quality of life during a one-year period in patients with newly detected chronic obstructive pulmonary disease. Respiration. 2002;69:513–520. 10.1159/000066456. [Abstract] [CrossRef] [Google Scholar]
- Lacasse Y, Brosseau L, Milne S, Martin S, Wong E, Guyatt GH, Goldstein RS. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD003793. [Abstract] [Google Scholar]
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. 10.1016/S0895-4356(96)00206-5. [Abstract] [CrossRef] [Google Scholar]
- Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. 10.1016/S0895-4356(99)00071-2. [Abstract] [CrossRef] [Google Scholar]
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. 10.1016/0197-2456(89)90005-6. [Abstract] [CrossRef] [Google Scholar]
- Wyrwich KW, Fihn SD, Tierney WM, Kroenke K, Babu AN, Wolinsky FD. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease: an expert consensus panel report. J Gen Intern Med. 2003;18:196–202. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones PW. Issues concerning health-related quality of life in COPD. Chest. 1995;107:187S–193S. [Abstract] [Google Scholar]
- Williams JE, Singh SJ, Sewell L, Guyatt GH, Morgan MD. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR) Thorax. 2001;56:954–959. 10.1136/thorax.56.12.954. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schunemann Holger J., Griffith Lauren, Jaeschke Roman, Goldstein Roger, Stubbing David, Austin Peggy, Guyatt Gordon H. A Comparison of the Original Chronic Respiratory Questionnaire With a Standardized Version. Chest. 2003;124:1421–1429. 10.1378/chest.124.4.1421. [Abstract] [CrossRef] [Google Scholar]
- M.A. Puhan, M. Behnke, M. Laschke, A. Lichtenschopf, O. Brandli, G.H. Guyatt, Schunemann HJ. Self-administration and standardisation of the chronic respiratory questionnaire: A randomised trial in three German-speaking countries. Respiratory Medicine. 2004. [Abstract]
- Bullinger M. Creating and Evaluation Cross-Cultural Instruments. In: SpilkerB, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Second. Vol. 69. Philadelphia, Lippincott; 1996. pp. 659–668. [Google Scholar]
- Kirsten DK, Taube C, Lehnigk B, Jorres RA, Magnussen H. Exercise training improves recovery in patients with COPD after an acute exacerbation 1. Respir Med. 1998;92:1191–1198. [Abstract] [Google Scholar]
- Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41:1359–1366. 10.1016/0277-9536(95)00115-N. [Abstract] [CrossRef] [Google Scholar]
- Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, Turner-Lawlor PJ, Payne N, Newcombe RG, Ionescu AA, Thomas J, Tunbridge J, Lonescu AA. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362–368. 10.1016/S0140-6736(99)07042-7. [Abstract] [CrossRef] [Google Scholar]
- Fries JF, Ramey DR. "Arthritis specific" global health analog scales assess "generic" health related quality-of-life in patients with rheumatoid arthritis. J Rheumatol. 1997;24:1697–1702. [Abstract] [Google Scholar]
- Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22:128–139. 10.1016/S0149-2918(00)87984-9. [Abstract] [CrossRef] [Google Scholar]
- Schunemann HJ, Griffith L, Stubbing D, Goldstein R, Guyatt GH. A clinical trial to evaluate the measurement properties of 2 direct preference instruments administered with and without hypothetical marker states. Med Decis Making. 2003;23:140–149. 10.1177/0272989X03251243. [Abstract] [CrossRef] [Google Scholar]
- Streiner D, Norman G. Health measurement scales. Second. Oxford, Oxford University Press; 1998. pp. 60–65. (Oxford Medical Publications). [Google Scholar]
Articles from Health and Quality of Life Outcomes are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1477-7525-2-1
Read article for free, from open access legal sources, via Unpaywall:
https://hqlo.biomedcentral.com/track/pdf/10.1186/1477-7525-2-1
Citations & impact
Impact metrics
Article citations
Anxiety Inventory for Respiratory Disease: Cross-Cultural Adaptation and Semantic Validity of the Brazilian Version for Individuals with Chronic Obstructive Pulmonary Disease.
J Multidiscip Healthc, 17:3283-3293, 09 Jul 2024
Cited by: 0 articles | PMID: 39010932 | PMCID: PMC11247127
Development of Urdu version of Chronic Respiratory Disease Questionnaire Self-Administered Standardized (CRQ-SAS); validity and reliability analysis in COPD patients.
PLoS One, 18(12):e0293981, 28 Dec 2023
Cited by: 0 articles | PMID: 38153959 | PMCID: PMC10754428
The German version of the Pulmonary Embolism Quality of Life (PEmb-QoL) questionnaire: reliability, responsiveness and structural validity.
Qual Life Res, 31(7):2235-2245, 14 Mar 2022
Cited by: 0 articles | PMID: 35286537 | PMCID: PMC8919155
Effectiveness of Pulmonary Rehabilitation in Severe and Critically Ill COVID-19 Patients: A Controlled Study.
Int J Environ Res Public Health, 18(17):8956, 25 Aug 2021
Cited by: 15 articles | PMID: 34501549 | PMCID: PMC8430691
Doctor-Diagnosed Arthritis and Self-Reported Physical Health Function Among Middle-Aged and Older Adults With Serious Mental Illness.
J Nerv Ment Dis, 207(11):908-912, 01 Nov 2019
Cited by: 3 articles | PMID: 31517715 | PMCID: PMC7053215
Go to all (146) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A randomised trial to evaluate the self-administered standardised chronic respiratory questionnaire.
Eur Respir J, 25(1):31-40, 01 Jan 2005
Cited by: 71 articles | PMID: 15640320
[Self assessment quality of life and lung diseases (SAQOL in patients with pulmonary carcinoma: influence to survival and impact of chronic obstructive pulmonary disease].
Pneumologie, 59(7):446-455, 01 Jul 2005
Cited by: 0 articles | PMID: 16047278
Validation of the Clinical COPD questionnaire in Italian language.
Health Qual Life Outcomes, 3:9, 07 Feb 2005
Cited by: 21 articles | PMID: 15698477 | PMCID: PMC549036
Combining scores from different patient reported outcome measures in meta-analyses: when is it justified?
Health Qual Life Outcomes, 4:94, 07 Dec 2006
Cited by: 54 articles | PMID: 17156420 | PMCID: PMC1712224
Review Free full text in Europe PMC

 1
1