Abstract
Free full text

Age and Sex Specific Prevalence and Incidence of Mild Cognitive Impairment, Dementia and Alzheimer’s dementia in Blacks and Whites: A Report From The Einstein Aging Study
Introduction
In 2005, it was estimated that over 24 million people worldwide had dementia. Dementia rates were higher in developed countries (North America and Western Europe) and lower in developing regions (Latin America, China, and the Western Pacific).1 The U.S. Census Bureau predicts that the population of adults over age 65 will double to constitute nearly 20% of the U.S. population in the next 25 years.2 Age-associated illnesses, particularly mild cognitive impairment (MCI) and dementia, are projected to have profound consequences for older adults, caregivers, the health care delivery system and society. Detailed knowledge of age, gender, and race specific rates of onset of MCI and dementia, as well as their subtypes, are required to target interventions and develop preventive strategies.
Prior work has demonstrated that rates of dementia increase exponentially with age.3, 4 The influence of race is less well established. Some studies report that the incidence and prevalence of dementia are higher in blacks than whites,5–7 while others suggest that differences in dementia rates by race may be attributable to differences in education, socio-economic status, health or cultural factors.8, 9 Additional studies are necessary to characterize rates of onset for dementia and cognitive impairment in ethnically diverse community-based studies to address these issues.
There has been increased interest in defining and diagnosing the stages of cognitive impairment that precede of dementia, such as amnestic mild cognitive impairment (aMCI) and non-amnestic mild cognitive impairment (naMCI).10 However, few population based longitudinal studies have reported incidence and prevalence of these conditions. In addition, there is a paucity of information regarding age, sex, and race specific rates of aMCI and naMCI. Studies that seek to enrich our understanding of these pre-clinical states will support health planning and facilitate the development of innovative preventative and treatment efforts that take age, gender, and race into account.
The Einstein Aging Study (EAS) is a longitudinal study of cognitive aging and dementia; the sample includes systematically recruited older adults drawn from an urban, multi-ethnic, community-dwelling population in Bronx County, NY. Participants receive comprehensive annual medical and neuropsychological evaluations. Using data from this cohort, we report total and age-specific prevalence and incidence rates for dementia, Alzheimer’s dementia (AD), aMCI and naMCI overall and categorized by sex and race.
Methods
Study Population/Eligibility
The EAS cohort has employed systematic recruiting methods to reduce the selection biases that arise from clinic-based samples and to capture the racial diversity within the Bronx community. Since 1993, a total of 1944 participants have been enrolled. Between 1993 and 2004, Health Care Financing Administration/ Centers for Medicaid and Medicare Services (HCFA/CMS) rosters of Medicare eligible persons aged 70 and above were used to develop sampling frames of community residing participants in Bronx County. Since 2004, New York City Board of Elections registered voter lists for the Bronx have been used due to changes in policies for release of HCFA/CMS rosters. Individuals were mailed introductory letters regarding the study and were then telephoned to complete a brief screening interview.11 Potential participants who met preliminary eligibility criteria on the telephone were invited for further screening at the EAS clinical research center to determine final eligibility.
Eligible participants were at least 70 years of age, Bronx residents, non-institutionalized, and English speaking. Exclusion criteria included visual or auditory impairments that preclude neuropsychological testing, active psychiatric symptomatology that interfered with the ability to complete assessments, and non-ambulatory status. Written informed consent was obtained at the initial clinic visit. The local institutional review board approved the study protocol.
EAS Assessment Battery
In-person evaluations were completed at baseline and at subsequent 12-month intervals.
Demographic and health status
Demographic information included self-reported race/ethnicity as defined by the U.S. Census Bureau in 1994, education, previous occupations, income, marital status, and religion. Medical history, medications, family history and health behaviors were obtained. Functional status was assessed by the self-administered CERAD C1-ALT,12 a cognitive/functional impairment instrument, and the Instrumental Activities of Daily Living scale(IADL), a subscale on the Lawton Brody Activities of Daily Living Scale.13 The score on the IADL was based on 5 domains of function that were common to both elderly men and women. Scores for each domain were dichotomized as impaired vs. not impaired and then the domain scores were summed. If the participant agreed, an informant completed the CERAD C2-ALT12, a cognitive/functional impairment instrument, and the Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE)14 forms.
Psychosocial Status
The 15-item Geriatric Depression Scale (GDS) was used to screen for depression.15 GDS ranged from 0 to 15 with scores of six or above indicating clinical depression. Anxiety was assessed using The Beck Anxiety Inventory.16 These instruments have high reliability and validity in community-based samples.17
Neurological examination
The standard neurological physical examination was adapted from the Unified Parkinson’s Disease Rating Scale.18 The evaluation assessed the participant’s memory for significant recent events in the news and personal events. The coherence and focus of responses, repetitiveness, and language were determined. When possible, informants were interviewed to ascertain whether they noted any cognitive changes in the participant, and to assess accuracy of the participant’s responses. The neurologist also assessed each participant for abnormal behaviors, fluctuation in cognition, and history of sleep disturbance and visual/auditory hallucinations. The neurologist assigned an Hachinski Ischemic Score (HIS),19 the Clinical Dementia Rating (CDR),20 and provided a clinical impression of presence or absence of dementia.
Neuropsychological testing
Global cognitive status was ascertained by the Blessed Information–Memory-Concentration test (BIMC).21 Memory was measured using the Free and Cued Selective Reminding Test (FCSRT22 and the Logical Memory I23 subtest from the WMS-R. Attention was measured using the Trail Making Test part A,25 and the Digit Span subtest of the WAIS-III.24 Executive function was measured using the Trail Making Test part B25) and the Letter Fluency “FAS” task.26 Visuospatial construction was measured using the Block Design subtest from the WAIS-III and the Digit Symbol subtest from the WAIS-III.24 Language was measured using the Category Fluency task (animals, vegetables, fruits)27 and the Boston Naming Test.28
Physical measures
Anthropometrics, blood pressure, grip strength, and peak flow were measured according to standard protocols and a fasting blood sample was obtained.
EAS Outcomes
Dementia and AD
A diagnosis of dementia was based on standardized clinical criteria from the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV)29 and required impairment in memory plus at least one additional cognitive domain, accompanied by evidence of functional decline. Diagnoses were assigned at consensus case conferences, which included comprehensive review of cognitive test results, relevant neurological signs and symptoms, and functional status. Memory impairment was defined as scores in the impaired range on any of the memory tests in the neuropsychological battery. (FCSRT ≤ 2430 or 1.5 standard deviations below the age-adjusted mean on Logical Memory) Functional decline was determined at case conference based on information from self or informant report, impairment score on the IADL Lawton Brody Scale,13 clinical evaluation, and informant questionnaires.
AD was diagnosed in participants with dementia meeting clinical criteria for probable or possible disease established by the National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA).31 Herein, AD refers to AD alone or in combination with other dementia disorders. Incident dementia and AD were diagnosed in persons free of dementia at baseline who met criteria at follow-up.
A subset of individuals who participated in the clinical studies of the EAS came to autopsy, providing an important quality control for diagnostic accuracy. A clinical diagnosis of dementia had a positive predictive value (PPV) of 96% for significant pathology upon autopsy. A clinical diagnosis of possible or probable AD had a PPV of 79% for the presence of NIA-Reagan intermediate or high likelihood Alzheimer type pathology based on an autopsy sample of 175.
Mild Cognitive Impairment (MCI)
Participants were classified as having aMCI if the memory domain was impaired or naMCI if there was impairment in one or more domains other than memory as defined below. For incidence analyses, participants were classified according to the subtype of MCI that occurred first. Persons eligible for incident MCI were therefore free of dementia and MCI at baseline.
aMCI was diagnosed according to updated criteria10 and required objective memory impairment as stated above, subjective memory impairment indicated by responses to self or informant reports(self: CERAD,12 informant: CERAD12 or IQ CODE14), absence of functional decline (based on the self or informant report, or absence of impairment on any of the domains measured in the IADL Lawton Brody scale13), and they were not classified as clinically demented. The aMCI group included both multiple and single domain aMCI as anyone who met the aMCI memory impairment criterion was included in the aMCI group regardless of whether they had cognitive impairments in other domains.
naMCI was diagnosed in non-demented participants without functional impairment who did not meet memory criteria for aMCI, but had impairment (1.5 standard deviations below the age-adjusted mean) in at least one non-memory cognitive domain of attention, executive function, visuospatial ability, or language.
Data Analysis
Prevalence rates for dementia, AD, aMCI, and naMCI were calculated according to status at baseline assessment. Incidence rates were calculated as estimates of cases per 100 person years of follow-up in persons free of the outcomes at baseline overall, by age and by age within sex, and race. Race specific analyses were presented for those who identified themselves as non-Hispanic white (NHW) or non-Hispanic black (NHB). As these two groups constituted 94.8% of the EAS population, analyses within other racial/ethnic subgroups was not possible due to low sample size and inadequate numbers of events. Person-years of follow-up were calculated as the time between the baseline clinic visit and final follow-up examination, incident event, or death, whichever occurred earliest. For age-specific incidence, both cases and person time were allocated to the appropriate age range. Confidence intervals were calculated using over-dispersed Poisson regression models. Trend tests were performed for each of the gender and race specific subgroups using quasi-likelihood regression models which included interaction terms. Cox proportional hazards models with chronological age as the time scale were used to estimate the association of incident dementia, AD, aMCI, and naMCI as a function of education, sex, and race. The proportional hazards assumption was tested for these models using sums of weighted residuals.
Results
Baseline demographics
The total EAS cohort included 1944 individuals. History of myocardial infarcts was reported by 10%, 9% had prior stroke, 57% had hypertension, and 17% had prevalent diabetes. The GDS identified 10.8% with clinically meaningful depression. Prevalence of these co-morbidities were similar to rates for persons over age 65 in the U.S. population.32 At baseline, 7.5% of the participants were current smokers and 46% were prior smokers.
At baseline, 126 of the 1944 participants (6.8%) were classified as having dementia (prevalent dementia) and 95 (4.9%) were subtyped as AD. Of the 1818 remaining individuals, 211(11.6%) had prevalent aMCI, and 179 (9.9%) had prevalent naMCI. Sixty four per cent (1168 participants) had at least one follow-up evaluation. Incidence rates were estimated for the 1168 participants who were free of dementia at baseline and had at minimum of 2 clinic evaluations (See Table 1). The mean age of this cohort at baseline was 78.8 years; 39.3% were male, and 70.0% were non-Hispanic white. Based on an average of 3.9 years of follow-up (range 1 to 16 years), we identified 130 incident dementia cases, 127 incident aMCI cases, and 132 incident naMCI cases. Those who developed incident dementia or incident MCI were slightly older, less educated, performed less well on a test of global cognitive status, and were more likely to be black or female. Of the 130 incident dementia cases, 58 were prevalent MCI at baseline and 39 developed incident MCI and progressed to dementia (Table 1).
Table 1
Baseline Characteristics by status at enrollment and follow-up
| Cohort at baseline* | Never developed MCI+ or dementia | Normal at baseline, incident MCI, no dementia | Normal at baseline, MCI not observed, incident dementia | Normal at baseline, incident MCI, incident dementia | MCI at baseline, no dementia | MCI at baseline, incident dementia | |
|---|---|---|---|---|---|---|---|
| Sample size | 1168 | 660 | 220 | 33 | 39 | 158 | 58 |
| Age at baseline–Mean (S.D.) | 78.8 (5.42) | 78.2 (4.98) | 79.0 (6.00) | 81.1 (4.96) | 80.3(5.80) | 79.4 (5.82) | 81.7 (5.31) |
| Males (%) | 39.3 | 39.2 | 39.0 | 27.3 | 38.5 | 43.0 | 37.9 |
| Education in years – Mean (S.D.) | 13.5 (3.50) | 13.9 (3.49) | 13.1 (3.27) | 12.0 (3.24) | 13.4(3.39) | 12.5 (3.43) | 13.2(4.00) |
| Non-Hispanic White (%) | 70.0 | 75.0 | 64.5 | 63.6 | 71.8 | 59.5 | 65.5 |
| B.I.M.C. Test – Mean (S.D.) | 2.45(2.34) | 1.76 (1.88) | 2.64 (2.05) | 4.06 (2.89) | 2.90(2.35) | 3.54 (2.58) | 5.14(2.94) |
Incidence of dementia and AD
Table 2 shows the number of cases and the sex, age and race specific incidence rates for all-cause dementia and specifically for AD. The overall incidence rate of dementia for this population was 2.89 (2.33 – 3.58) per 100 person-years. As age increases, the rates of dementia increase overall for both males and females (trend test: males, p=0.003; females, p=0.00003), and for whites and blacks (trend test: whites, p=0.00007; blacks, p=0.0003). The increase for dementia approximately doubled with each five-year age interval, ranging from 0.66 per 100 person-years at ages 70–74 years to 11.30 per 100 person-years for those 90 and over (see Figure 1). Age specific rates for blacks were not significantly higher than for whites based on the interaction term in the quasi-likelihood regression model
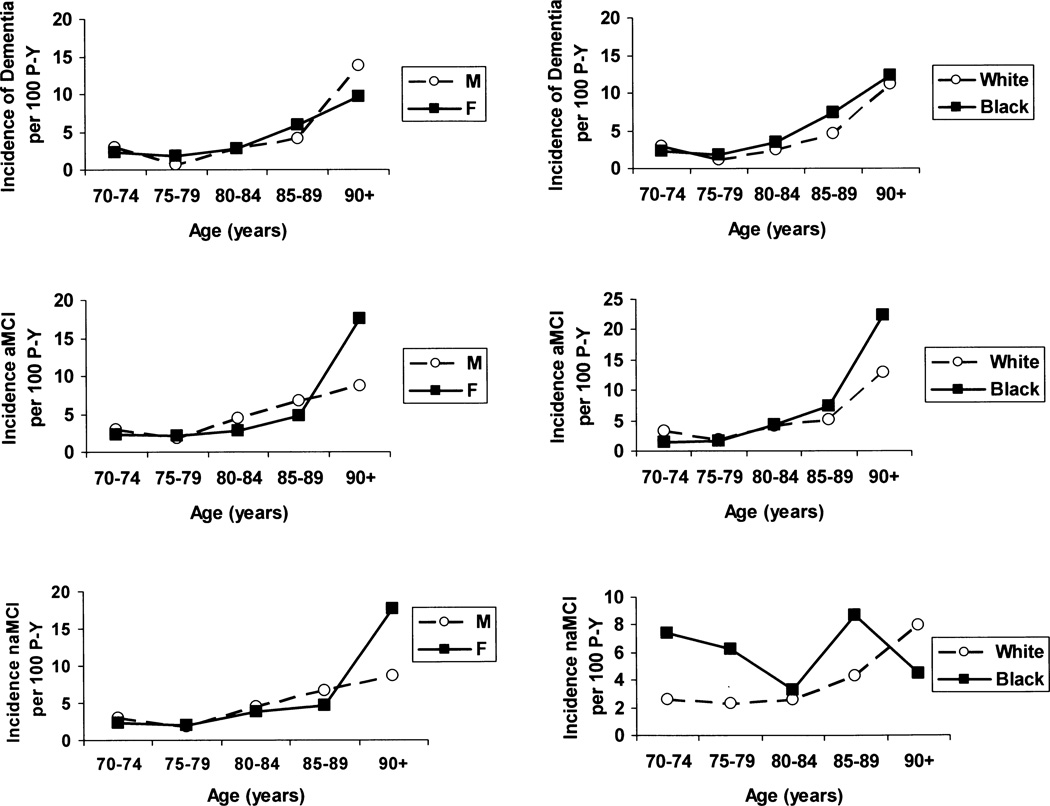
Age specific incidence of dementia, AD, aMCI and naMCI by sex and by race. P-Y=person years of follow-up; AD=Alzheimer’s dementia; aMCI=amnestic mild cognitive impairment; naMCI=non-amnestic cognitive impairment.
Table 2
Incidence of All-Cause Dementia and AD in the EAS Cohort: Person-years at risk, number of cases and rates by sex, age and race (95% CI in parentheses)
| Incident Dementia | Incident Alzheimer’s Dementia | |||||
|---|---|---|---|---|---|---|
| Gender or Race by Age Group | Person- years | No. of cases | Rate per 100 Person-years | Person- years | No. of cases | Rate per 100 Person- years |
| Total Cohort | ||||||
| 70–74 | 628.21 | 4 | 0.64(0.17–2.39) | 628.21 | 4 | 0.64(0.17–2.39) |
| 75–79 | 1368.23 | 19 | 1.39(0.78–2.48) | 1368.23 | 14 | 1.02(0.50–2.09) |
| 80–84 | 1444.74 | 40 | 2.79(1.52–5.03) | 1444.74 | 31 | 2.15(1.29–3.56) |
| 85–89 | 859.23 | 44 | 5.12(3.01–8.70) | 859.23 | 33 | 3.84(1.95–7.58) |
| 90+ | 203.57 | 23 | 11.30(5.55–22.99) | 203.57 | 20 | 9.82(4.61–20.92) |
| Total | 4504.00 | 130 | 2.89(2.33–3.58) | 4504.00 | 102 | 2.26(1.76–2.91) |
| Males | ||||||
| 70–74 | 249.00 | 1 | 0.40(0.03.–6.27) | 249.00 | 1 | 0.40(0.03–6.27) |
| 75–79 | 551.27 | 4 | 0.73(0.12–4.51) | 551.27 | 2 | 0.36(0.01–9.44) |
| 80–84 | 570.46 | 16 | 2.80(0.91–8.65) | 570.46 | 12 | 2.10(1.00–4.41) |
| 85–89 | 345.02 | 14 | 4.06(1.38–11.96) | 345.02 | 10 | 2.90(0.67–12.48) |
| 90+ | 79.97 | 11 | 13.76(4.21–44.93) | 79.97 | 9 | 11.25(3.12–40.53) |
| Total | 1795.72 | 46 | 2.56(1.77–3.71) | 1795.72 | 34 | 1.89(1.22–2.94) |
| Females | ||||||
| 70–74 | 379.21 | 3 | 0.79(0.17–3.61) | 379.21 | 3 | 0.79(0.17–3.61) |
| 75–79 | 816.96 | 15 | 1.84(1.06–3.19) | 816.96 | 12 | 1.47 (0.78–2.76) |
| 80–84 | 874.29 | 24 | 2.75(1.43–5.28) | 874.29 | 19 | 2.17(1.10–4.30) |
| 85–89 | 514.22 | 30 | 5.83(3.23–10.55) | 514.22 | 23 | 4.47(2.13–9.40) |
| 90+ | 123.61 | 12 | 9.71(4.18–22.56) | 123.61 | 11 | 8.90(3.60–22.00) |
| Total | 2708.27 | 84 | 3.10(2.38–4.04) | 2708.27 | 68 | 2.50(1.85–3.41) |
| Whites* | ||||||
| 70–74 | 373.70 | 2 | 0.53(0.09–3.19) | 373.70 | 2 | 0.54(0.09–3.19) |
| 75–79 | 900.63 | 11 | 1.22(0.54–2.78) | 900.63 | 10 | 1.11(0.45–2.72) |
| 80–84 | 1052.36 | 26 | 2.47(1.07–5.71) | 1052.36 | 19 | 1.81(0.94–3.48) |
| 85–89 | 658.56 | 30 | 4.55(2.47–8.40) | 658.56 | 21 | 3.19(1.39–7.31) |
| 90+ | 161.12 | 18 | 11.17(4.81–25.93) | 161.12 | 16 | 9.93(4.16–23.73) |
| Total | 3146.37 | 87 | 2.77(2.13–3.60) | 3146.37 | 68 | 2.16(1.58–2.96) |
| Blacks* | ||||||
| 70–74 | 201.65 | 1 | 0.50(0.03–7.42) | 201.65 | 1 | 0.50(0.03–7.42) |
| 75–79 | 399.68 | 7 | 1.75(0.75–4.09) | 399.68 | 4 | 1.00(0.34–2.97) |
| 80–84 | 352.38 | 12 | 3.41(1.56–7.46) | 352.38 | 10 | 2.84(1.14–7.08) |
| 85–89 | 175.36 | 13 | 7.41(2.53–21.74) | 175.36 | 11 | 6.27(1.80–21.88) |
| 90+ | 40.49 | 5 | 12.35(3.41–44.74) | 40.49 | 4 | 9.88(2.12–46.08) |
| Total | 1169.57 | 38 | 3.25(2.17–4.87) | 762.67 | 30 | 3.54(2.26–5.56) |
The overall incidence for AD was 2.26 (1.76–2.91) per 100 person years. As age increased, the rate of AD increased for both males and females (trend test: males, p=0.0016; females, p=0.00017), and for whites and blacks (trend test: whites, p=0.0001; blacks, p=0.001). Men had non-significantly lower rates of AD in the younger age groups, and there were no differences in AD incidence by race.
Incidence of aMCI and naMCI
The overall incidence rate for aMCI was 3.79 (3.03–4.73) per 100 person-years and for naMCI it was 3.94 (3.17–4.89) per 100 person-years. Table 3 shows the sex, age and race specific incidence rates for aMCI and naMCI. The rates of aMCI increased with age in males (trend test, p=0.039) and in blacks (trend test, p=0.002). Rates increased with age in females and in whites, but the trend was not significant. The rate of aMCI increased for individuals aged 90 and above in all subgroups of gender and race (Wald test: males p=.059, females p=.0085, whites p=.018, blacks p=.0048). In contrast, the naMCI rates did not increase sharply with age for any subgroup(Figure 1).
Table 3
Incidence of aMCI and naMCI in the EAS Cohort: Person-years at risk, number of cases and rates by sex, age and race (95% CI in parenthesis)
| Incident aMCI | Incident naMCI | ||||||
|---|---|---|---|---|---|---|---|
| Race, Gender, Age Groups | Person- years | No. of cases | Rate per 100 Person- years | Person- years | No. of cases | Rate per 100 Person-years | |
| Total Cohort | |||||||
| 70–74 | 499.08 | 13 | 2.60(1.37–4.94) | 499.08 | 23 | 4.61(2.91–7.30) | |
| 75–79 | 1057.96 | 21 | 1.98(0.32–12.50) | 1057.96 | 40 | 3.78(2.09–6.85) | |
| 80–84 | 1084.41 | 44 | 4.06(2.42–6.80) | 1084.41 | 31 | 2.86(1.45–5.62) | |
| 85–89 | 563.34 | 31 | 5.50(1.84–16.44) | 563.34 | 28 | 4.97(2.67–9.24) | |
| 90+ | 125.42 | 18 | 14.35(3.14–65.58) | 125.42 | 10 | 7.97(4.00–15.88) | |
| Total | 3354.33 | 127 | 3.79(3.03–4.73) | 3354.33 | 132 | 3.94(3.17–4.89) | |
| Males | |||||||
| 70–74 | 198.40 | 6 | 3.02(1.16–7.90) | 198.40 | 9 | 4.54(2.35–8.76) | |
| 75–79 | 424.56 | 8 | 1.88(0.70–5.07) | 424.56 | 18 | 4.24(2.37–7.59) | |
| 80–84 | 405.69 | 18 | 4.44(2.02–9.72) | 405.69 | 13 | 3.20(1.20–8.53) | |
| 85–89 | 225.24 | 15 | 6.66(2.56–17.33) | 225.24 | 8 | 3.55(1.37–9.22) | |
| 90+ | 45.89 | 4 | 8.72(4.17–18.23) | 45.89 | 2 | 4.36(1.22–15.62) | |
| Total | 1308.93 | 51 | 3.90(2.70–5.61) | 1308.93 | 50 | 3.82(2.69–5.43) | |
| Females | |||||||
| 70–74 | 300.67 | 7 | 2.33(0.99–5.46) | 300.67 | 14 | 4.66(2.48–8.74) | |
| 75–79 | 633.40 | 13 | 2.05(0.11–37.45) | 633.40 | 22 | 3.47(1.32–9.15) | |
| 80–84 | 678.73 | 26 | 3.83(1.93–7.59) | 678.73 | 18 | 2.65(1.05–6.70) | |
| 85–89 | 338.10 | 16 | 4.73(0.70–31.90) | 338.10 | 20 | 5.92(2.73–12.82) | |
| 90+ | 79.52 | 14 | 17.61(2.67–116.26) | 79.52 | 8 | 10.06(4.61–21.95) | |
| Total | 2045.40 | 76 | 3.72(2.81–4.91) | 2045.41 | 82 | 4.01(3.05–5.28) | |
| Whites* | |||||||
| 70–74 | 312.16 | 10 | 3.20(1.53–6.73) | 312.16 | 8 | 2.56(1.31–5.02) | |
| 75–79 | 730.40 | 14 | 1.92(0.13–29.35) | 730.40 | 17 | 2.33(0.68–7.99) | |
| 80–84 | 845.40 | 34 | 4.02(2.29–7.07) | 845.40 | 22 | 2.60(1.36–5.00) | |
| 85–89 | 463.02 | 24 | 5.18(1.27–21.09) | 463.02 | 20 | 4.32(2.05–9.09) | |
| 90+ | 101.12 | 13 | 12.86(1.63–101.42) | 101.12 | 8 | 7.91(3.69–16.94) | |
| Total | 2462.32 | 95 | 3.86(2.96–5.03) | 2462.32 | 75 | 3.05(2.31–4.01) | |
| Blacks* | |||||||
| 70–74 | 148.28 | 2 | 1.35(0.29–6.37) | 148.28 | 11 | 7.42(3.67–15.01) | |
| 75–79 | 286.81 | 5 | 1.74(0.73–4.16) | 286.81 | 18 | 6.28(3.61–10.92) | |
| 80–84 | 213.18 | 9 | 4.22(1.12–15.87) | 213.18 | 7 | 3.28(0.50–21.71) | |
| 85–89 | 80.63 | 6 | 7.44(2.56–21.63) | 80.63 | 7 | 8.68(2.69–27.97) | |
| 90+ | 22.34 | 5 | 22.38(7.71–64.99) | 22.34 | 1 | 4.48(0.27–74.54) | |
| Total | 762.67 | 27 | 3.54(2.26–5.56) | 762.67 | 44 | 5.77(3.90–8.54) | |
aMCI: Amnestic Mild Cognitive Impairment
naMCI: Non-amnestic Mild Cogniive Impairment
Demographic risk factors
Table 4 shows the results of the Cox proportional hazard models analyzing the effect of demographic variables on the risk of dementia, aMCI, and naMCI. A similar model was used for AD (not shown). Race, education, and sex were not significant risks factor for dementia, AD, or aMCI. Blacks were twice as likely as whites (HR=2.04, CI: 1.39–3.01) to develop naMCI; education (HR=0.95, CI=0.90–0.997) was protective for naMCI.
Table 4
Hazard Ratios for Dementia, aMCI and naMCI in the EAS Cohort:*
| Variable | Hazard Ratio | 95% CI | P value |
|---|---|---|---|
| Dementia | |||
| Sex(Male) | 0.97 | 0.92–1.02 | 0.18 |
| Education (per year) | 0.89 | 0.61–1.29 | 0.53 |
| Race (Black) | 1.31 | 0.88–1.94 | 0.18 |
| aMCI | |||
| Sex (Male) | 1.11 | 0.77–1.62 | 0.57 |
| Education (per year) | 1.01 | 0.96–1.06 | 0.84 |
| Race(Black) | 0.93 | 0.60–1.45 | 0.74 |
| naMCI | |||
| Sex (Male) | 1.07 | 0.73–1.56 | 0.73 |
| Education (per year) | 0.95 | 0.90–0.997 | 0.04 |
| Race (Black) | 2.04 | 1.39–3.01 | 0.0003 |
aMCI: Amnestic Mild Cognitive Impairment
naMCI: Non-amnestic Mild Cogniive Impairment
Discussion
We documented overall prevalence of dementia, AD, aMCI, and naMCI in a community sample of older adults in the Bronx. In addition, we assessed the age, sex, and race specific incidence rates in this community. The EAS sample was diverse; it included 25% non-Hispanic blacks, and individuals with a broad range of educational achievement (0 to 25 years). Age ranged from 70 to 101 years. Our results highlight the importance of preclinical cognitive disease in the population. Over 20% of the EAS sample was classified as having either prevalent aMCI or naMCI. Elderly black individuals appear to be at increased risk for naMCI.
A number of factors may explain the variability in rates across studies. Differences may be due in part to varying definitions of dementia, AD, aMCI, and naMCI or to the application of these definitions. Geographical differences in rates may be attributable to variations in survival rates and in the prevalence of putative and protective factors. These factors may be environmental, genetic, or both.
Prevalence/ Incidence of Dementia and AD
In the EAS, the prevalence of dementia was 6.5% and prevalence of AD was 4.9%. These rates are consistent with those reported in North American populations of comparable age.33
The overall incidence rate of dementia for the EAS cohort was 2.89 per 100 person-years. When taking the age distributions into account, the EAS rate is generally consistent with those reported by other population-based studies.9,34–36 Launer et al.37 pooled results from four European countries included in the European Studies of Dementia network (EURODEM) to provide overall and age-specific dementia rates. The overall rate of dementia was lower than EAS (1.84 per 100 person-years) most likely due to the younger age distribution in these samples. However, the age-specific rates for the incidence of dementia were very similar. Similarly, Kukull et al.34 reported an overall dementia rate of 2.03 per 100 person-years for members of the Group Health Cooperative of Puget Sound. Again, the lower dementia rate is likely due to the younger age of the cohort; however when age specific rates were compared, they were similar. Incidence in the 90+ age group was reported in a cohort study of residents of Laguna Woods, California.36 The rate of dementia in 90–94 year olds was 12.7% year, comparable to our estimate of 11.3% per year in the EAS 90+ group (mean age 92.8 years).
AD alone or in association with other dementia disorders comprised 77% of all dementias within the EAS. The overall AD incidence rate was 2.26 per 100 person-years in EAS. For persons up to age 85, the age specific rates for AD were similar to those reported in other studies.1 Comparisons for the age 90+ group are difficult, as the AD specific above age 90 are not uniformly reported. However, EAS rates are higher than the EURODEM and Puget Sound studies in that age stratum.
Dementia and AD: Age Trends
In the EAS, the rates of dementia increased sharply with age. Similar findings in a meta-analysis presented by Jorm and Jolley35 showed that among 23 studies, age-specific incidence rates rose exponentially up to the age of 90. Corrada et al.36 reported similar results for age distributions beginning with 90–94 years (12.7% per year) and ending with rates for a 100+ age group (40.7% per year). AD rates also increased exponentially with age in the EAS similar to prior reports.1, 34, 37
Dementia and AD: Sex-Specific Rates
Dementia rates were similar in men and women; both groups showed an increase in rates with age. The results from Jorm and Jolley35, Corrada36, and Fitzpatrick9 agree with these results with the exception of those for the oldest-old. Prior reports have shown that in the 90+ age group, women tend to have a higher incidence of dementia, and in particular, AD.9, 34, 35, 37 In contrast, EAS AD rates for men were non-significantly lower than those for women prior to age 90.
Dementia and AD: Race-Specific Rates
Few studies have compared dementia rates by race within the same geographic area, and results have been inconsistent across studies. In the EAS population-based sample, we observed no differences in rates of total dementia among whites and blacks. The Cox models showed that race was not a significant risk factor for dementia after adjustment for sex and education. The model for AD showed similar non-significant results.
Blacks have been reported to have higher incidence of AD compared with non-Hispanic whites in a cohort of community-based residents of Northern Manhattan.6, 7 Gurland et al.6 reported higher prevalence and incidence of all cause dementia for African Americans compared with non-Latino whites in the North Manhattan Aging Project, although the excess incidence among African Americans was observed only below age 85. Tang et al. has reported higher incidence of AD for blacks than for non-Hispanic whites in a community-based sample from northern Manhattan. These differences were not attributable to differences in education, or in frequency of cardiovascular comorbidities. In contrast, other studies have not observed race differences in dementia8 or have found that differences are diminished after adjustment for age and education.9 Fillenbaum et al.8 reported no differences in incidence rates for all cause dementia between blacks and whites in the Piedmont area of North Carolina. Fitzpatrick estimated incidence rates for total dementia in the Cardiovascular Health Study (CHS) and found racial differences, with higher rates for blacks. However, the difference was attenuated when adjusted for age and education and with further adjustment for differences in case ascertainment. Similarly, analyses by dementia subtype demonstrated higher incidence of AD among African Americans, but the difference was only of borderline statistical significance and was not evident after adjustment for differences in case ascertainment.
Prevalence/ Incidence of aMCI and naMCI
The overall prevalence of MCI (aMCI and naMCI combined) in the EAS was 21.5% at the baseline assessment. This rate is similar to rates reported by other community-based cohorts in the United States.38, 39 We found little difference in rates of prevalent MCI between men and women (21.0% in women, 22.2% in men) and lower rates in whites than in blacks(19.1% vs. 27.3%). These results were consistent with Manly’s findings.39 Our observed difference in MCI by race was due entirely to a higher prevalence of naMCI in blacks (16.30% vs. 6.86% in whites). Petersen et al.38 reported prevalence rates of 11.1% for aMCI and 4.9% for naMCI in a cohort from the Mayo Clinic. The EAS prevalent aMCI rates are similar but prevalent naMCI rates were higher in the EAS; similar diagnostic criteria were used in both studies. This difference may be attributable to differences in racial/ethnic or general health characteristics of the study populations. In particular, the Mayo Clinic population is predominately white.
In our study, the incidence of aMCI was 3.8 and for naMCI it was 3.9 per 100 person-years. In the Northern Manhattan study, incidence rates for aMCI was 2.3 and for MCI without memory impairment, 2.8 per 100 person-years.39 The higher rates in the EAS may be due to due to its older age distribution.
aMCI and naMCI: Age, Sex and Race- Specific Incidence Rates
There is a paucity of data regarding age, sex, and gender specific incidence rates of aMCI and naMCI. In the EAS, rates of aMCI increase after the age of 80, for males and females, and for whites and blacks. In the Cox proportional hazard model, sex, education, and race were not significant risk factors for incident aMCI. In contrast, there was no marked increase in naMCI with increasing age or between males and females in the EAS cohort. However, naMCI incidence rates were higher in blacks compared with whites. When controlling for sex and education in the Cox model, we observed a two-fold increased risk of naMCI among blacks compared to whites. This observation is consistent with prior studies showing higher rates of cerebrovascular disease and more prevalent cardiovascular risk factors among African Americans compared with whites,40 and with recent reports suggesting that naMCI often has a vascular etiology.33, 41 Roberts et al.41 have reported that cardiovascular disease is more strongly associated with naMCI than with aMCI. Similarly, Reitz et al33 have reported that the association between hypertension and total MCI is driven by an association with naMCI.
Strengths and Limitations
The operational definition of MCI and its’ subtypes has evolved over time and continues to vary considerably among studies Some definitions are primarily based on clinical impression, while others rely on neuropsychological cut-scores and still others combine approaches. These neuropsychological tests also vary in sensitivity and specificity for identification of impairment. Classification of cognitive status in the EAS was based on a comprehensive neuropsychological test battery supplemented by a complete neurological exam. Diagnosis of dementia was based on standard criteria applied at a consensus case conference. The definitions of aMCI and naMCI reported here follow widely-adopted diagnostic guidelines,10 while dementia and AD were also defined according to standard criteria.20, 29, 31 Given the many sources of unreliability in ascertaining these outcomes, the consistency of EAS results with available data is reassuring.
The prevalence of dementia and AD may be underestimated in the EAS since individuals with severe dementia are less likely to participate in a community based study and those who are institutionalized were not included. Another limitation of this study was that persons lost to follow-up may not develop cognitive impairment at the same rate as do those who remain in the sample, a phenomenon known as informative censoring. When comparing baseline characteristics of the 1168 EAS participants with at least one follow-up clinic visit with those of participants with only baseline evaluations, there were no significant age, gender, or race differences. However, the mean years of education was significantly lower (p<0.0001) and the BIMC was significantly higher (p<0.0001) for the group not completing a follow-up visit. Since those lost to follow-up tended to have reduced global cognitive performance and lower education, the incidence rates we reported may be underestimates. Another limitation is the relatively short follow-up interval. For those with more than one annual visit, the mean follow-time was 3.9 years (range 1–16 years). In any cohort, censoring prior to death or dementia diagnosis results in under ascertainment of cases. Finally, when sub-types of dementia and MCI were examined, demographic comparisons were limited by small sample size.
This study had several notable strengths. First, the cohort was systematically recruited and comparisons with data from the US Census indicate that the EAS cohort was similar to the elderly population of Bronx County, NY, in distributions of age, sex and education. Another strength was the racial and educational diversity of this cohort. Clinic-based studies often include only those with more severe cognitive impairment compared with a community-based sample.
In summary, this study contributes valuable information regarding the prevalence and incidence of cognitive impairment in community dwelling individuals. In particular, few prior studies have reported incidence of aMCI and naMCI in an ethnically diverse community-based sample. As the population ages, both MCI and dementia will present an increasing burden on the health care system as well as families and caretakers. The high prevalence of preclinical dementia documented here underscores the need to develop interventions that will delay or prevent the onset of dementia.
Acknowledgements
The Authors would like to thank Charlotte Magnotta, Diane Sparracio, and April Russo for assistance with participant recruitment, Betty Forro, Alicia Gomez, Wendy Ramratan, and Mary Joan Sebastian for assistance with clinical and neuropsychological assessments, Michael Potenza for assistance with data management, and all of the study participants who generously gave their time in support of this research. The work presented in this paper was supported by National Institute on Aging Grant AG03949.
Sources of Support:
Supported by National Institutes of Health NIA AG03949. Dr. Sanders is supported by the CTSA Grant UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mindy J. Katz, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Richard B. Lipton, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y. Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y. Institute for Aging Research, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Charles B. Hall, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y. Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Molly E. Zimmerman, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Amy E. Sanders, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Joe Verghese, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
Dennis W. Dickson, Department of Neuroscience and Neuropathology, Mayo Clinic, Jacksonville, FL.
Carol A. Derby, Saul B. Korey Department of Neurology, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y. Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/wad.0b013e31823dbcfc
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3334445?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/wad.0b013e31823dbcfc
Article citations
Trajectory of Cognitive Decline Before and After Stroke in 14 Population Cohorts.
JAMA Netw Open, 7(10):e2437133, 01 Oct 2024
Cited by: 0 articles | PMID: 39356504 | PMCID: PMC11447567
Independent and joint associations of cardiometabolic multimorbidity and depression on cognitive function: findings from multi-regional cohorts and generalisation from community to clinic.
Lancet Reg Health West Pac, 51:101198, 12 Sep 2024
Cited by: 0 articles | PMID: 39308753 | PMCID: PMC11416683
Blood Pressure, Antihypertensive Use, and Late-Life Alzheimer and Non-Alzheimer Dementia Risk: An Individual Participant Data Meta-Analysis.
Neurology, 103(5):e209715, 14 Aug 2024
Cited by: 0 articles | PMID: 39141884
Associations between Cognitive Impairment, Weight Status and Comorbid Conditions in Hospitalized Adults of 55 Years and Older in Guadeloupe.
Healthcare (Basel), 12(17):1712, 27 Aug 2024
Cited by: 1 article | PMID: 39273736 | PMCID: PMC11395463
Lifestyle and incident dementia: A COSMIC individual participant data meta‐analysis.
Alzheimers Dement, 20(6):3972-3986, 27 Apr 2024
Cited by: 1 article | PMID: 38676366 | PMCID: PMC11180928
Go to all (226) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A Birth Cohort Analysis of Amnestic Mild Cognitive Impairment Incidence in the Einstein Aging Study (EAS) Cohort.
J Alzheimers Dis, 70(s1):S271-S281, 01 Jan 2019
Cited by: 5 articles | PMID: 31256119 | PMCID: PMC6700647
Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia.
JAMA Neurol, 73(1):93-101, 01 Jan 2016
Cited by: 196 articles | PMID: 26569387 | PMCID: PMC4710557
Association of diabetes with amnestic and nonamnestic mild cognitive impairment.
Alzheimers Dement, 10(1):18-26, 03 Apr 2013
Cited by: 88 articles | PMID: 23562428 | PMCID: PMC3830601
Differential and subtype-specific neuroimaging abnormalities in amnestic and nonamnestic mild cognitive impairment: A systematic review and meta-analysis.
Ageing Res Rev, 80:101675, 17 Jun 2022
Cited by: 8 articles | PMID: 35724862
Review
Funding
Funders who supported this work.
NCRR NIH HHS (3)
Grant ID: UL1 RR025750
Grant ID: KL2 RR025749
Grant ID: TL1 RR025748
NIA NIH HHS (3)
Grant ID: P01 AG003949-24
Grant ID: P01 AG003949
Grant ID: AG03949





