Abstract
Objective
Studies examining the link between objective measures of total daily physical activity and incident Alzheimer disease (AD) are lacking. We tested the hypothesis that an objective measure of total daily physical activity predicts incident AD and cognitive decline.Methods
Total daily exercise and nonexercise physical activity was measured continuously for up to 10 days with actigraphy (Actical®; Philips Healthcare, Bend, OR) from 716 older individuals without dementia participating in the Rush Memory and Aging Project, a prospective, observational cohort study. All participants underwent structured annual clinical examination including a battery of 19 cognitive tests.Results
During an average follow-up of about 4 years, 71 subjects developed clinical AD. In a Cox proportional hazards model adjusting for age, sex, and education, total daily physical activity was associated with incident AD (hazard ratio = 0.477; 95% confidence interval 0.273-0.832). The association remained after adjusting for self-report physical, social, and cognitive activities, as well as current level of motor function, depressive symptoms, chronic health conditions, and APOE allele status. In a linear mixed-effect model, the level of total daily physical activity was associated with the rate of global cognitive decline (estimate 0.033, SE 0.012, p = 0.007).Conclusions
A higher level of total daily physical activity is associated with a reduced risk of AD.Free full text

Total daily physical activity and the risk of AD and cognitive decline in older adults
Abstract
Objective:
Studies examining the link between objective measures of total daily physical activity and incident Alzheimer disease (AD) are lacking. We tested the hypothesis that an objective measure of total daily physical activity predicts incident AD and cognitive decline.
Methods:
Total daily exercise and nonexercise physical activity was measured continuously for up to 10 days with actigraphy (Actical®; Philips Healthcare, Bend, OR) from 716 older individuals without dementia participating in the Rush Memory and Aging Project, a prospective, observational cohort study. All participants underwent structured annual clinical examination including a battery of 19 cognitive tests.
Results:
During an average follow-up of about 4 years, 71 subjects developed clinical AD. In a Cox proportional hazards model adjusting for age, sex, and education, total daily physical activity was associated with incident AD (hazard ratio = 0.477; 95% confidence interval 0.273–0.832). The association remained after adjusting for self-report physical, social, and cognitive activities, as well as current level of motor function, depressive symptoms, chronic health conditions, and APOE allele status. In a linear mixed-effect model, the level of total daily physical activity was associated with the rate of global cognitive decline (estimate 0.033, SE 0.012, p = 0.007).
Conclusions:
A higher level of total daily physical activity is associated with a reduced risk of AD.
Physical activity is a modifiable behavior that is associated with Alzheimer disease (AD),1–3 but several recent reports have concluded that there is insufficient evidence linking physical activity and AD.4,5 Most prior studies have relied on self-report measures of physical activity without objective validation. Studies of older persons are especially challenging since self-reported physical activity can be affected by recall bias, chronic health conditions, low fitness levels, and various functional limitations. Furthermore, prior studies have not measured nonexercise physical activity which, through its contribution to total daily energy expenditure, may play an important role in the benefits which accrue from physical activity.6–9
Although 2 recent studies have examined the association of daytime actigraphy or active energy expenditure and cognition in older individuals, studies which quantify total daily physical activity are needed.10,11 The current study used data from 716 participants of the Memory and Aging Project to test the hypothesis that total daily physical activity is associated with incident AD and cognitive decline.12 Actigraphs worn on the wrist recorded movements 24 hours/day for up to 10 days, providing an objective measure of total daily physical activity which circumvents recall bias.13
METHODS
Participants.
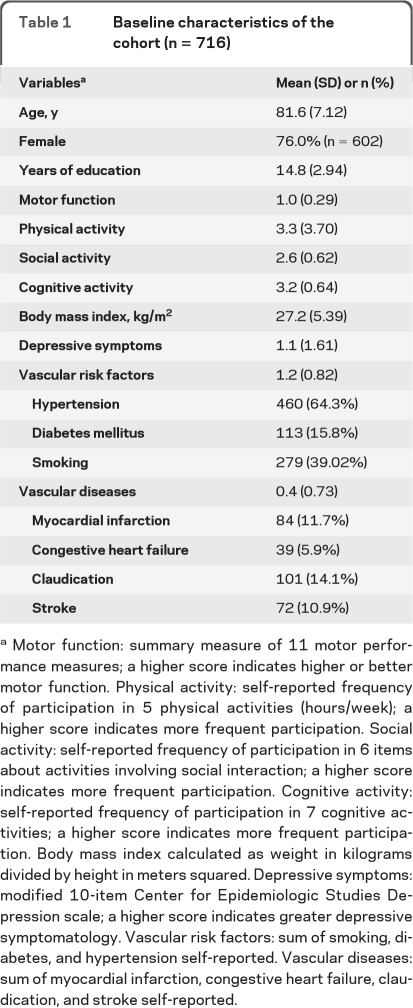
Participants were individuals from the Memory and Aging Project, an ongoing longitudinal, community study of common chronic conditions of old age. Although the Memory and Aging Project began in 1997, actigraphy data collection was not added until 2005. Of 1,258 living participants, 154 refused actigraphy testing and 190 await testing. Of 914 who had completed actigraphy testing there were 9 missing cognitive testing during the Actical cycle and 12 had missing actical data due to device failure. Of 893 with valid actigraphy and cognitive testing, 106 were excluded due to the presence of clinical AD and non-AD dementia (see below) at the time of actigraphy testing and 71 did not have a second follow-up cognitive test (21 died before follow-up testing, 46 have not been in the study long enough, and 4 had missing data). There were 716 participants without dementia with 2 or more annual cognitive assessments and valid baseline actigraphy included in these analyses with baseline characteristics shown in table 1.
Table 1
Baseline characteristics of the cohort (n = 716)
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all study participants.
Assessment of cognitive function and diagnosis of AD.
Cognitive function was assessed using a battery of 19 tests which were used to create a composite measure of global cognition as described elsewhere.12 Cognitive testing was scored by computer and reviewed by a neuropsychologist to diagnose cognitive impairment. Participants were then evaluated by a clinician who used all cognitive and clinical data to diagnose AD using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria.
Assessment of total daily physical activity.
Total daily exercise and nonexercise physical activity was measured 24 hours/day for up to 10 days with actigraphs worn on the nondominant wrist. Physical activity was measured with actigraphy (Actical®; Mini Mitter, Bend, OR). Figure 1 shows a time series for a single participant and illustrates the distribution of activity during 10 days. Total daily physical activity was the average sum of all daily activity counts recorded.13 On average, there were more than 3 × 105 activity counts/day; raw counts were divided by 1 × 105 (about 1 SD) to facilitate presentation and interpretation of the results.
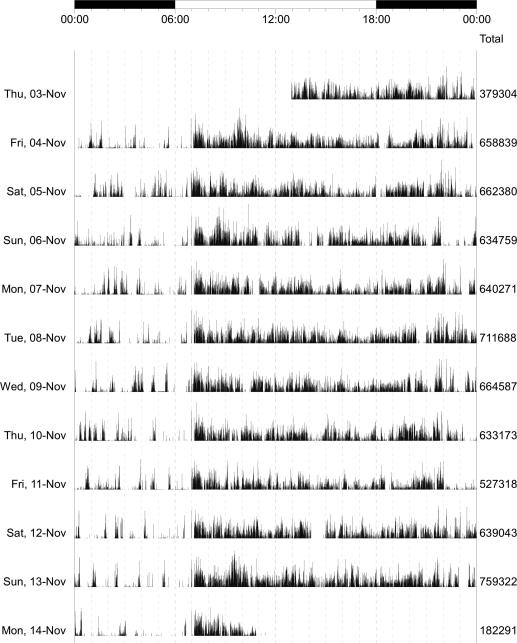
A graphical summary of total daily physical activity from a single Memory and Aging participant. In this recording, the device recorded and averaged physical activity every 15 seconds for 10 complete days from 7 pm on November 3 through 7 pm on November 13. The time of day is displayed on the top of the figure (0.00 to 0.00). Activity can be seen to vary during the day, with low or absent activity usually noted between 23:00 and 7:00, when the participant was likely sleeping. The column on the right of the figure shows the total daily physical activity counts which were averaged to obtain mean total daily physical activity.
Assessment of late-life physical, social, and cognitive activities.
Self-report assessment of late-life physical activity was based on questions adapted from the 1985 National Health Interview Survey. Five activities included walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming or water exercise. Minutes spent engaged in each of these activities were summed and expressed as hours of activity/week.14 Frequency of participation in social activity was based on 6 items about activities involving social interaction over the past year.14 Frequency of participation in cognitively stimulating activities was quantified for 7 cognitive activities over the past year.14
Other covariates.
Age was computed from self-reported date of birth and date of actigraphy. Sex and years of education were recorded at the study entry.
Motor function was based on 11 motor performance tests which were scaled and averaged to obtain a summary measure. Grip and pinch strength were measured using the Jamar® hydraulic dynamometer (Lafayette Instruments). Motor performances included finger tapping, Purdue pegboard testing, and 7 tests of lower extremity function as previously described.15
Body mass index was based on measured weight and height. Depressive symptoms over the prior week were assessed with a 10-item version of the Center for Epidemiologic Studies Depression (CES-D) scale.16 The number of 3 self-reported vascular risk factors and the number of 4 self-reported vascular diseases were used in these analyses.14 APOE genotyping was done and individuals were dichotomized into those with at least 1 copy of the ϵ4 allele vs those without a copy.17
Statistical analysis.
We first examined the crude associations of actigraphy measures with age, sex, and education. The distribution of total daily physical activity was slightly positively skewed so we used a square root transformation before the following analyses. Next, we examined the association of total daily physical activity with incident AD using a discrete-time Cox proportional hazards model with terms that adjusted for age, sex, and education.18 Next we added terms to this core model to examine potential confounders and modifiers. Linear mixed-effect models adjusted for age, sex, and education were used to examine the relationship between total daily physical activity, the rate of change in global cognition, and 5 cognitive abilities. Similar analyses were used to examine the association of intensity of total daily physical activity, incident AD, and cognitive decline. Model validation was performed graphically and analytically and there was no evidence of lack of fit. Programming was done in SAS.19
RESULTS
Descriptive properties of total daily physical activity.
There were 716 participants and their baseline characteristics are included in table 1. Participants wore the Actical for an average of 9.3 days (SD = 1.1 day). Total daily physical activity levels ranged from 0.16 × 105 counts/day to 13.56 × 105 counts/day (mean: 3.06 × 105 counts/day; SD = 1.55 × 105 counts/day). Total daily physical activity was related to age (r −0.23, p < 0.001) and there was a trend for education (r −0.07, p = 0.052). Women had marginally higher levels of total daily physical activity (women 3.12 [SD = 1.53] vs men 2.85 [SD = 1.62]; t[714] = 1.94, p = 0.053).
Total daily physical activity and AD.
Over a mean of 3.5 years (SD = 1.54) of follow-up, 71 (9.9% of 716) persons developed AD. Using a Cox proportional hazards model adjusted for age, sex, and education, level of total daily physical activity was associated with the risk of developing AD (table 2, model A). Figure 2 shows that a person with low total daily physical activity (10th percentile) had a more than 2-fold (2.3×) higher risk of developing AD as compared to a participant with high total daily physical activity (90th percentile).
Table 2
Total daily physical activity and incident ADa
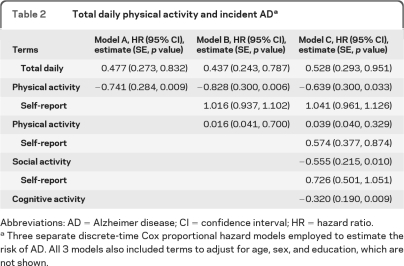
Abbreviations: AD = Alzheimer disease; CI = confidence interval; HR = hazard ratio.
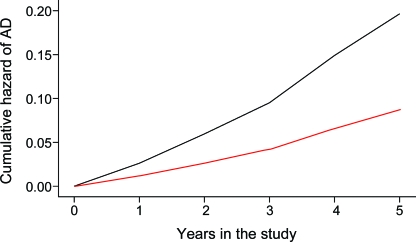
Expected risk of Alzheimer disease (AD) based on the entire cohort is illustrated for 2 hypothetical average participants with high (dotted line: 90th percentile, 4.90 × 105 activity counts per day) and low levels (solid line: 10th percentile, 1.24 × 105 activity counts per day) of total daily physical activity.
We hypothesized that the association of total daily physical activity and AD could be due to aspects of physical activity not captured by traditional activity measures. In further analyses total daily physical activity remained associated with risk of AD even after adjusting for common self-report physical activity (table 2, model B). Total daily physical activity remained associated with AD even after adjusting for self-report physical activity as well as the frequency of social and cognitive activities in a single model (table 2, model C).
We repeated the core model (table 2, model A) and added terms for the interaction of demographic variable with the level of total daily physical activity. The association between total daily physical activity and incident AD did not vary with age, sex, or education (results not shown).
In sensitivity analyses, the association between total daily physical activity and risk of AD remained significant when we excluded individuals with a history of stroke or PD which might cause cognitive impairment (hazard ratio [HR] 0.478, 95% confidence interval [CI] 0.268–0.851). Further, the association persisted after excluding cases with the lowest 10% of global cognition (HR = 0.433, 95% CI 0.201–0.932) or excluding those who developed AD in the first 2 years after actigraphy (HR = 0.467, 95% CI 0.220–0.992).
Total daily physical activity and AD: Examining other potential confounders.
We repeated the core model (table 2, model A) and added terms to examine whether several potential confounders attenuated the association of total daily physical activity and AD. Prior studies in this cohort suggest that current motor function could affect either total daily physical activity or risk of AD or both.20 The association of total daily physical activity with AD remained significant when adjusting for a summary measure of motor function (HR 0.490, 95% CI 0.264–0.910).
Chronic health conditions might also affect either total daily activity or risk of AD or both. Adding terms for body mass index (BMI), depressive symptoms, vascular risk factors, and vascular diseases did not affect the association of total daily physical activity and the risk of AD (HR 0.435, 95% CI 0.244–0.778).
Genetic factors such as APOE4 have been reported to affect both cognitive and motor function.17 Adding a term for the presence of the APOE4 allele did not affect the association of total daily physical activity and risk of AD (HR 0.511, 95% CI 0.287–0.909).
Total daily physical activity and cognitive decline.
AD develops slowly over many years and its clinical hallmark is change in cognitive function. Thus, to some extent a diagnosis of AD consists of placing a cutpoint along a continuum of cognition. To ensure that our results were not influenced by diagnostic misclassification or individuals with lower cognition, we examined the association of total daily physical activity with cognitive decline using a composite measure of global cognition.12
We used a series of linear mixed-effects models to test the association of total daily physical activity and the rate of cognitive decline. Global cognition declined by 0.125 unit/year. Baseline total daily physical activity was associated with both the level and annual rate of change of global cognition (table 3). Global cognition is comprised of measures of 5 related but relatively dissociable cognitive systems. In further analyses, total daily physical activity was associated with the rate of decline of episodic and working memory and perceptual speed and there was a trend for an association with declining visuospatial abilities (table 3).
Table 3
Total and intensity of daily physical activity and the rate of cognitive declinea

Cognitive decline prior to actigraphy, and baseline total daily physical activity.
Lower cognition might lead to decreased activity, so we examined whether the rate of cognitive decline prior to actigraphy was associated with baseline total daily physical activity. Using a mixed-effect model, we determined the person-specific rate of cognitive decline in 438 cases with 2 or more cognitive tests prior to actigraphy. In a regression model controlled for age, sex, and education, the rate of cognitive decline before actigraphy was not associated with total daily physical activity (estimate 0.161, SE 0.124, p = 0.195).
Baseline cognition and subsequent decline in total daily physical activity.
Given that baseline total daily physical activity predicted subsequent cognitive decline (table 3), we next examined whether baseline global cognition predicted the subsequent decline of total daily physical activity. A total of 595 cases had 2 or more actigraphy tests. We used a generalized estimating equation which controlled for age, sex, education, and baseline global cognition and their interaction with time. On average, total daily physical activity declined by 0.064 × 105 counts/day/year (estimate −0.064, SE 0.006, p < 0.001). Baseline global cognition was not associated with the rate of decline of total daily physical activity (global cognition × time, estimate 0.002, SE 0.012, p = 0.862).
Intensity of daily physical activity, incident AD, and cognitive decline.
Recent work has suggested that benefits of physical activity may accrue from aerobic physical activity. As a surrogate for aerobic activity, we calculated the intensity of daily activity by dividing the total daily physical activity counts by the total hours/day of all nonzero epochs to yield the intensity of daily physical activity (activity counts/hour/day). Intensity of daily physical activity ranged from 0.04 × 105 counts/hour/day to 0.71 × 105 counts/hour/day (mean 0.29 × 105 [SD = 0.10 × 105 counts/hour/day]). Intensity of total daily physical activity also predicted incident AD (HR 0.016, 95% CI 0.001–0.253) and was associated with the rate of cognitive decline in global cognition and all cognitive abilities except semantic memory (table 3).
DISCUSSION
In a group of more than 700 older persons without dementia, an objective measure of higher level of total daily physical activity was associated with a lower risk of the subsequent development of AD. Total daily physical activity remained associated with risk of AD after adjusting for a wide range of late-life activities, including physical, cognitive, and social activities, suggesting that it captures aspects of physical activity not assessed by traditional physical activity questionnaires. This association was robust and not attenuated by the current level of motor function, BMI, vascular risk factors or diseases, or APOE4 allele status. In separate analyses, total daily physical activity was related to the level of cognition and also the subsequent annual rate of cognitive decline. These findings lend support for the link between physical activity, cognition, and risk of AD in old persons.
The number of Americans older than 65 years will double to about 80 million by 2030, with the most rapid growth in those 80 years or older.21 Thus, whether physical activity, a modifiable risk factor, is associated with cognitive decline and AD has important public health consequences. Some but not all prior observational studies have reported an association between physical activity and cognition in old age.2,3 The reliance in prior studies on self-report questionnaires to assess physical activity without objective validation has restricted the type of activities that are sampled and may be affected by recall bias and may have contributed to the small effect sizes observed. A recent study reported an association between daytime actigraphy and level of cognition in older women adjusting for self-report walking and physical function.11 However, this study did not assess total daily nonexercise physical activity, an important component of total daily energy expenditure, and a likely determinant of the health benefits which accrue from physical activity.7–9 The current study extends prior work by using actigraphy in the community setting to circumvent recall bias and to provide an objective measure of both total daily exercise and nonexercise physical activity and determine its association with cognitive decline and incident AD.
The finding that total daily physical activity is associated with incident AD as well as with the level and rate of change in cognitive function provides support for efforts to encourage physical activity even in very old individuals. In further analyses, this association remained significant after adjusting for a wide range of late-life activities including physical, cognitive, and social activities. Thus, not only exercise but also higher levels of nonexercise activity are associated with cognition in old age. This finding has important implications not only for observational studies but also for the design of physical activity intervention studies and cognition in old age. Older individuals, for whom participation in formal exercise may be constrained because of underlying health problems, may nonetheless benefit from a more active lifestyle through increases in the full spectrum of routine activities which are included under the rubric of nonexercise physical activity. Further, studies are needed to delineate the determinants of exercise and nonexercise physical activity in older individuals as well as their relative contributions to cognition in old age.
Rapid advances in technology have increased the availability of minimally intrusive devices which can provide objective measures of physical activity for days to weeks in the community setting, and do not rely on participant recall, allowing investigators to study the full spectrum of health and disabilities in old age.11,22–25 Recent studies suggest that laboratory testing may not reflect physical activity measured in the community setting.26 Furthermore, older individuals may decrease their nonexercise activities when they increase their exercise activity.27 Thus, it may be particularly important to measure exercise and nonexercise activities in older persons. Recording continuously 24 hours/day provides objective measures of the total daily physical activity as well as the distribution of activity and rest periods. For example, both periods of physical activity at night which may represent disrupted sleep patterns (sleep fragmentation) as well as prolonged rest periods during the day which may represent daytime naps (activity fragmentation) can be determined. Further work is needed to examine the temporal dynamics of activity and rest periods and their health consequences.28,29 It is likely that with falling cost and technologic advances, the use of these devices will become more commonplace, increasing the precision and specificity of physical activity measures and facilitating efforts to explicate the link between physical activity and cognition in old age.
There are limitations to this study. Inferences regarding causality must be drawn with caution from observational studies. However, it is important to note that if the benefits of physical activity are small and cumulative over many years, they may be beyond resolution by a randomized clinical trial. Thus, the field may be forced to draw inferences regarding this association from well-designed epidemiologic studies. The complementary analyses in the current study provide important data suggesting that physical activity may be protective of and forestall the development of AD. The percentage of female participants was high and this was a volunteer cohort, and thus may not be representative of the general population of older adults. Further, the actigraphs used in this study do not differentiate the types of activities that were performed, and removal of the device cannot always be distinguished from periods of no activity.
The main strength of this study is that we obtained objective measures of total daily physical activity from a relatively large number of well-characterized older persons who may be more representative of the cognitive and physical function spectrum observed in the community setting. In addition, our analyses adjusted for a wide range of late-life activities and potential confounders including robust measurements of both cognition and motor function, evaluated as part of a uniform clinical evaluation.
ACKNOWLEDGMENT
The authors thank the participants and the staff of the Rush Memory and Aging Project and the Rush AD Center for this work, and Liping Gu, MS, for help with statistical programming.
GLOSSARY
| AD | Alzheimer disease |
| BMI | body mass index |
| CES-D | Center for Epidemiologic Studies Depression |
| CI | confidence interval |
| HR | hazard ratio |
AUTHOR CONTRIBUTIONS
Study concept or design: Dr. Buchman, Dr. Wilson, Dr. Bennett. Analysis or interpretation of the data: Dr. Buchman, Dr. Boyle, Dr. Yu, Dr. Shah, Dr. Wilson, Dr. Bennett. Drafting of the manuscript: Dr. Buchman, Dr. Boyle, Dr. Yu, Dr. Shah, Dr. Wilson, Dr. Bennett. Statistical analysis: Dr. Buchman, Dr. Yu. Obtaining funding: Dr. Buchman, Dr. Bennett.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0b013e3182535d35
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3335448?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1212/wnl.0b013e3182535d35
Article citations
The Effect of Two Somatic-Based Practices Dance and Martial Arts on Irisin, BDNF Levels and Cognitive and Physical Fitness in Older Adults: A Randomized Control Trial.
Clin Interv Aging, 19:1829-1842, 06 Nov 2024
Cited by: 0 articles | PMID: 39525874
Performance Under Fire: Older Adult Cognitive Risks and Protections Under Heat Strain.
Gerontologist, 64(11):gnae116, 01 Nov 2024
Cited by: 0 articles | PMID: 39166357 | PMCID: PMC11467403
Cardiorespiratory Fitness and Sleep, but not Physical Activity, are Associated with Functional Connectivity in Older Adults.
Sports Med Open, 10(1):113, 19 Oct 2024
Cited by: 0 articles | PMID: 39425826 | PMCID: PMC11490599
Relationship between objectively measured conversation time and social behavior in community-dwelling older adults.
Front Neurol, 15:1479296, 07 Oct 2024
Cited by: 0 articles | PMID: 39434836 | PMCID: PMC11491351
What contributes to a decline in cognitive performance among home care clients? Analysis of interRAI data from across Canada.
BMC Geriatr, 24(1):822, 12 Oct 2024
Cited by: 0 articles | PMID: 39395942 | PMCID: PMC11470726
Go to all (362) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons.
Arch Gen Psychiatry, 67(3):304-310, 01 Mar 2010
Cited by: 214 articles | PMID: 20194831 | PMCID: PMC2897172
Hemoglobin level in older persons and incident Alzheimer disease: prospective cohort analysis.
Neurology, 77(3):219-226, 13 Jul 2011
Cited by: 66 articles | PMID: 21753176 | PMCID: PMC3136057
Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age.
Am J Geriatr Psychiatry, 19(11):961-969, 01 Nov 2011
Cited by: 84 articles | PMID: 21430509 | PMCID: PMC3123692
Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons.
Arch Neurol, 66(11):1339-1344, 01 Nov 2009
Cited by: 213 articles | PMID: 19901164 | PMCID: PMC2838435
Funding
Funders who supported this work.
NIA NIH HHS (8)
Grant ID: R01 AG024480
Grant ID: R01AG24480
Grant ID: R01 AG034374-03
Grant ID: R01AG34374
Grant ID: R01AG17917
Grant ID: R01 AG017917-09
Grant ID: R01 AG017917
Grant ID: R01 AG034374






