Abstract
Free full text

Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction
Abstract
Despite mounting pre-clinical and clinical evidence of the beneficial effects of cell-based therapy, optimal cell dosing and delivery approaches have not been identified. Cardiospheres are self-assembling three-dimensional (3D) microtissues formed by cardiac stem cells and supporting cell types. The ability of cardiospheres to augment cardiac function has been demonstrated in animal models of ischemic cardiomyopathy. In this study, we studied the dose dependence of the benefits of human cardiospheres, delivered via intramyocardial injection, upon cardiac function and ventricular remodelling in SCID mice with acute myocardial infarction. Four doses of cardiospheres were used: 1 × 104, 5 × 104, 1 × 105 and 5 × 105 (expressed as number of plated cardiosphere-forming cells). Acute (24 hr) cell retention rates in all groups were similar. Functional assessment and quantitative heart morphometry indicated benefit from higher cell doses (≥5 × 104) in terms of ejection fraction, infarct size and capillary density. Histological analysis indicated that the dose-dependent benefit was primarily because of indirect effects of transplanted cells. The results provide scalable data on cardiosphere dosing for intramyocardial injection.
Introduction
Heart disease remains the number one killer in the western countries. Current treatments at best only slow the progression of disease leading to heart failure and death; once scar has developed, the injury to heart muscle is generally cumulative and irreversible. Stem cell therapy represents a promising strategy to mitigate remodelling and regenerate the damaged myocardium. Cardiospheres (CSps) and cardiosphere-derived cells (CDCs) are natural mixtures of resident cardiac stem cells and supporting cell types [1]. Studies have shown that CSps and CDCs are able to ameliorate cardiac dysfunction in acute and chronic ischaemic cardiomyopathies [1–10]. Despite emerging pre-clinical and clinical evidence of the beneficial effects of cell-based therapy, optimal cell dosing and delivery approaches remain elusive. Previous clinical trials using bone marrow mononuclear cells [11] suggested a cell dose-dependent effect in myocardial function improvement and scar size reduction. However, dose dependence has generally been poorly characterized in animal models prior to clinical application. Most studies in rodents have used astronomically high cell doses when extrapolated to large animal or human doses. For instance, in the SCIPIO trial (ClinicalTrials.gov; NCT00474461) 1 million c-kit+ cardiac stem cells are administered to humans. Interestingly, the same dose (1 million cells) was employed in a pre-clinical rat model by the same group, despite the >200-fold difference in recipient size [12]. Most other cell types have suffered from similar discrepancies in advancing from rodents to large animals and humans [13]. This study was designed to examine the dose-dependent effects of intramyocardial injection of human CSps in a mouse model of acute myocardial infarction (MI). In addition, we sought to determine, on the basis of histological analysis, the mechanisms for this dose-dependent benefit. The hope is to systematically optimize dose in mice and then use these results to guide dosing in large animals and humans.
Materials and methods
Human CSps were derived as described [6, 7]. Briefly, human right ventricle biopsies from male donors were minced into small fragments. After collagenase digestion, the tissue fragments were cultured as ‘explants’ on dishes coated with fibronectin (BD Biosciences, Bedford, MA, USA). Within 1–2 weeks, stromal-like flat cells, and phase-bright round cells, emerged from the tissue fragments and became confluent. These CSp-forming cells were harvested and then plated on poly-d-lysine (BD Biosciences)-coated plates for suspension culture. After 3–5 days, the CSp-forming cells spontaneously aggregated into CSps. Acute MI was created in adult male SCID-beige mice (10–12 weeks old), as described [1, 7], by ligating the left anterior descending artery. As the CSp is a relatively heterogenous product and the numbers may vary from different batches, we define the dose by the number of starting CSp-forming cells [7]. The mice were then subjected to intramyocardial injections with a 30-gauge needle at four points in the infarct border zone, with one of the following five randomly assigned treatments: (i) 20 μL PBS (control group); (ii) 1 × 104 cells formed CSps; (iii) 5 × 104 cells formed CSps; (iv) 1 × 105 cells formed CSps; (v) 5 × 105 cells formed CSps. Cardiac functions were assessed by echocardiography at baseline (4 hrs post-MI) and 3 weeks afterwards [1, 7]. A sub-population of animals from each group was sacrificed at 24 hrs and the hearts were excised for determining the numbers of engrafted cells by quantitative PCR (utilizing the SRY gene located on the Y-chromosome of the injected male human cells). All other animals were killed at 3 weeks and hearts were excised for morphometry and immunohistochemisty. All results are presented as mean ± S.D. Statistical significance between the two groups was determined using the two-tailed unpaired t-test and among groups by anova followed by Bonferroni's post-hoc test. Differences were considered significant when P < 0.05.
Results
When CSp-forming cells were seeded on poly-d-lysine-coated plates, they readily aggregated into 3D clusters, within 3–5 days, to yield a suspension of CSps (Fig. 1A). The size of CSps ranged from 40 to 100 μm. The phenotypes of CSps have been well characterized in previous studies [14, 15]. Generally, CSps consist of distinct layers with the expression of c-kit in the core and the mesenchymal marker CD105 in their periphery (Fig. 1B). Cardiospheres were consistently negative for CD45 (Fig. 1C). qPCR quantitation of human cells in the mouse hearts revealed a ~10% cell retention rate 24 hrs after injection into the injured hearts, regardless of the doses (Fig. 1D). This means that the absolute number of retained cells increased with escalating injected doses.
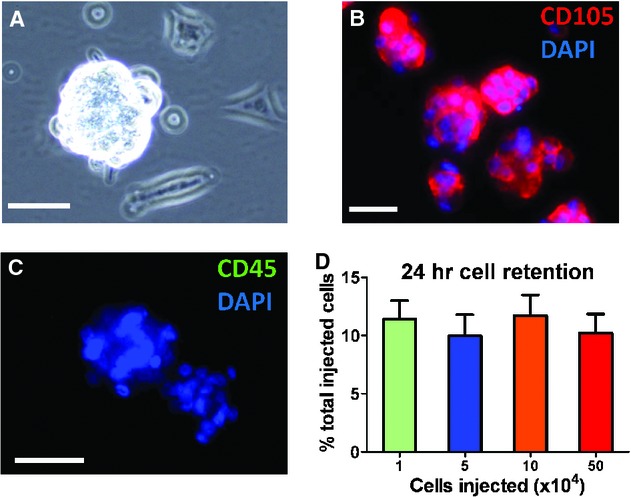
Acute retention of cardiospheres in post-myocardial infarction mouse hearts. (A) Representative phase bright image showing the morphology of a human cardiosphere. The cardiospheres are consistently positive for CD105 (B) and negative for CD45 (C). (D) qPCR quantitation of cell retention rates in hearts injected with male human cardiospheres (n = 5 mice per group).
The most meaningful indicator of therapeutic benefit, in clinical practice, is the ability to produce functional preservation or improvement in the injured heart. Echocardiography revealed that baseline left ventricular ejection fraction (LVEF) after surgery did not differ among groups, indicating comparable degrees of initial injury (Fig. 2A). Three weeks after treatment, LVEFs in mice receiving the three highest doses (5 × 104, 1 × 105 and 5 × 105) outperformed the saline group (Fig. 2B). No significant difference was seen between the saline and the 1 × 104 groups, although a positive trend was evident. To facilitate comparisons of treatment effects among groups, we calculated the change of LVEFs over the 3-week time course. Over the 3 weeks, LVEF declined progressively in the saline group (Fig. 2C), but not in the hearts that had received high doses of CSps. This is in line with previous reports that CSp transplantation preserves LVEFs in post-MI hearts [6, 7]. LVEF slightly declined in the mice which received the 1 × 104 dose, but not as much as in the saline group (not statistically different). Coinciding with the dose-dependent functional benefit is the dose-dependent attenuation of ventricular remodelling. Masson's trichrome staining clearly distinguished scar tissue (blue) from normal myocardium (pink; Fig. 3A). Quantitative morphometry at 3 weeks showed severe LV chamber dilatation and infarct wall thinning in the saline-injected hearts (Fig. 3A). In contrast, high-dose CSp-treated hearts exhibited attenuated LV remodelling and less abnormal heart morphology. Magnification of the infarct region revealed the preservation and/or regeneration of normal myocardium tissue in the infarct. Further quantitation indicated thicker infarcted walls (Fig. 3B) and more viable tissues (Fig. 3C) in the infarct area from the hearts received the three high doses of CSps. The measures from the low-dose group (1 × 104 cells) were somewhat higher than that in the saline group, although not significantly so.
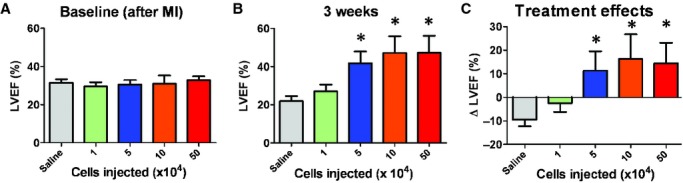
Cardiac function: left ventricular ejection fraction (LVEF) measured by echocardiography at baseline (post-myocardial infarction; A) and 3 weeks afterwards (B) in saline or various dosage groups (n = 5–6 mice per group). (C) Changes of LVEF from baseline to 3 weeks in each group. *P < 0.05 when compared with the saline group or the 1 × 104 group.
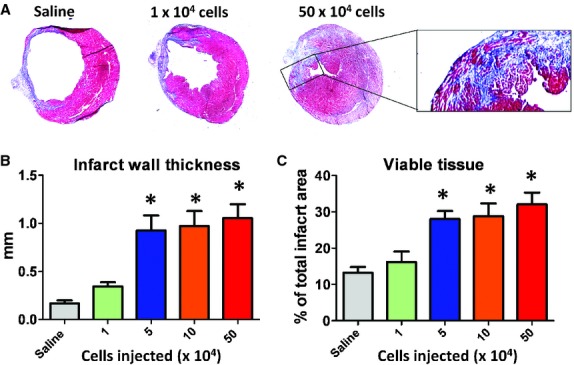
Heart morphometry analysis. (A) Representative Masson's trichrome-stained myocardial sections 3 weeks after treatment. Scar tissue and viable myocardium are identified by blue and red colours, respectively. (B, C) Quantitative analysis and left ventricle morphometric parameters of the Masson's trichrome images (n = 5 mice per group). *P < 0.05 when compared with the saline group or the 1 × 104 group.
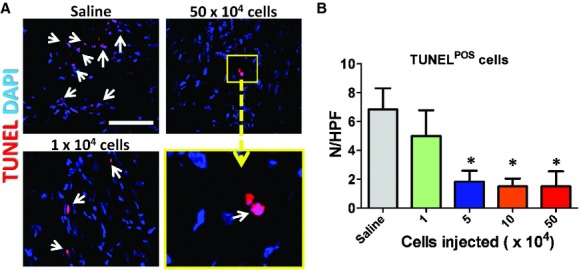
We have found that CSps exert their functional benefits through both direct regeneration and indirect paracrine mechanisms [3], with the latter as the predominant factor. Following this lead, we sought to investigate whether the dose-dependent functional benefit of CSps also involves paracrine contributions, which involve various mechanisms such as promoting angiogenesis and cell cycle re-entry of mature cardiomyocytes and preserving myocardium after ischaemic injury. Myocardial neovascularization assessed by vascular density at 3 weeks was dose dependently enhanced in mice receiving CSp transplantation. Blood vessels were stained with α-smooth muscle actin (Fig. 4A–C). Hearts receiving high doses of CSps exhibited higher vascular densities than those in the low-dose group (1 × 104 cells) or the saline group (Fig. 4D). The vascular densities in the low-dose group were similar to that in the saline group. A similar dose-dependent enhancement was found in promoting cell cycle re-entry of cardiomyocytes. More Ki67+ cardiomyocytes were evident in hearts from the three high-dose groups (Fig. 5), as compared with the low-dose or the saline group. Again, no difference was seen between the low-dose and the saline groups. In addition to tissue regeneration, tissue preservation may be a salutary mechanism of cell therapy for acute MI. To quantify tissue preservation, apoptotic cells in the hearts were Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-stained. Fewer apoptotic cells were detected in the hearts from the three high-dose groups (Fig. 6). No difference was found between the saline and the low-dose groups. It should be noted that, at 3 weeks, the numbers of TUNEL+ cells are a cumulative reflection of maladaptive LV remodelling rather than acute cell death due to ischaemia.
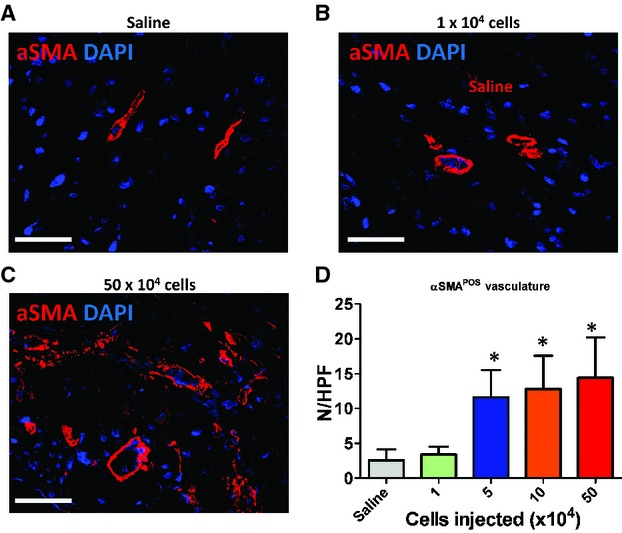
Promotion of angiogenesis by cardiosphere transplantation. (A–C) Representative confocal images showing α-smooth muscle actin-positive vasculature in the heart. (D) Quantitation of α-smooth muscle actin-positive vasculature in various groups (n = 5 mice per group). *P < 0.05 when compared with the saline group or the 1 × 104 group. Bar = 100 μm.
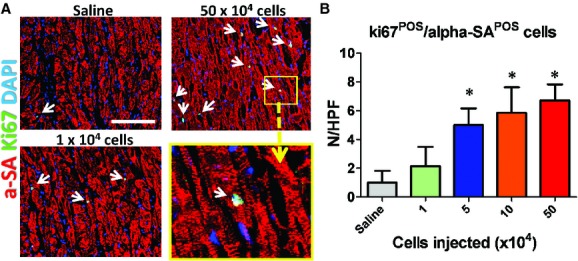
Increase of ki67+ cardiomyocytes induced by cardiosphere injection. (A) Representative confocal images showing ki67+ cardiomyocytes in the heart. (B) Quantitation of ki67+ cardiomyocytes in various groups (n = 5 mice per group). *P < 0.05 when compared with the saline group or the 1 × 104 group. Bar = 200 μm.
Discussion
The last decade witnessed a burst of pre-clinical and clinical data indicating that the transplantation of stem cells may benefit diseased myocardium [13]. Despite mounting pre-clinical and clinical evidence of the positive effects of cell-based therapy, optimal cell dosing and delivery approaches have not been identified. Some salient questions include: (i) Is there a minimally effective cell dose for therapeutic benefit? (ii) When cell dose keeps escalating, does it reach a benefit plateau or even induce detrimental effects? In this study, we explored these questions by using human CSp in a mouse model of acute MI.
The 24-hr cell retention rate was independent of cell dose: ~10% of injected cells engrafted in the myocardium, regardless how many cells were injected (Fig. 1D). This indicates that the myocardial capacity to host transplanted cells is not saturated, at least at the dose of 5 × 105 cells. Previous studies from our laboratory and other groups indicate that cardiac contraction and venous drainage account for a great amount of initial cell loss [2, 9]. A large fraction of cells are simply ‘washed out’ immediately after injection. Therefore, a straightforward way to increase total cell engraftment is to increase the delivered dose.
Improvement of cardiac function was dose dependent (Fig. 2). The three highest doses (5 × 104, 1 × 105 and 5 × 105 cells) of CSps exerted superior functional benefit (i.e. higher values of LVEF) than the saline group or the low-dose (1 × 104) group. There was a trend for the low-dose group to outperform the saline group, but the differences are not statistically significant. Moreover, higher resolution is needed to elucidate whether there are dose-dependent patterns within the three highest doses. A similar dose–benefit relationship was seen in heart morphology (Fig. 3). Better attenuation of ventricular remodelling was evident in the hearts at the high doses but not in the low-dose group. This indicates that the functional benefit from the high-dose groups was at least partially because of prevention of ventricular dilation and wall thinning. We sought to dissect the mechanisms underlying the dose-dependent functional benefit and heart morphology preservation brought about by CSp injections. Our previous studies indicated that cell engraftment at 3 weeks is extremely low (i.e. <1% of total injected cells could be detected at 3 weeks) [2]. Thus, although the high-dose groups could have relatively more engrafted cells at 3 weeks, the absolute numbers of engrafted cells are small and seem insufficient to explain the striking functional benefit. To that end, we focused on examining the indirect paracrine effects of various CSp doses. These effects include promotion of angiogenesis, cardiomyocyte cell cycle re-entry and inhibition of apoptosis. The three high-dose groups exhibited more vessels (Fig. 4) and ki67+ cardiomyocytes (Fig. 5), but fewer TUNEL+ apoptotic cells (Fig. 6). There was no difference between the low-dose and the saline groups. Our previous studies indicate that CSps are rich biological ‘factories’, secreting various cytokines and growth factors (e.g. vascular endothelial growth factor, insulin-like growth factor, hepatocyte growth factor, stromal cell-derived factor-1) [3]. It has been shown that these factors can promote angiogenesis, tissue protection and cardiomyocyte proliferation. Taking these facts together, our data suggest that the dose-dependent functional benefit from CSps may largely come from paracrine effects.
Our study demonstrated dose-dependent functional benefit of CSp transplantation in mice with acute MI. Given that the mouse body weight is ~20–25 g and average human weight is ~70 kg, the corresponding doses in humans, relative to those used here in mice, would be 30 million, 150 million, 300 million and 1.5 billion cells. Based on our results, we would extrapolate 150 M as an efficacious dose in humans and one where the benefit has nearly reached saturation. Taking together these considerations and manufacturing feasibility, we anticipate using ~150 M CSp cell-equivalent dosage in large animals and humans. Nevertheless, future studies with large animals are needed to confirm that extrapolations from rodents are useful for defining a plausible dose range for clinical trials.
Acknowledgments
The authors thank B. Sun and G. Galang for technical assistance. Work in the laboratory was supported by research grants from National Institutes of Health (NIH), California Institute of Regenerative Medicine (CIRM) and the Cedars-Sinai Board of Governors Heart Stem Cell Center. D.S. was supported by Zhengzhou University. E.M. holds the Mark S. Siegel Family Professorship of the Cedars-Sinai Medical Center.
Conflict of interest
E.M. is a founder and equity holder in Capricor Inc. Capricor provided no funding for this study. The remaining authors report no conflicts.
References
Articles from Journal of Cellular and Molecular Medicine are provided here courtesy of Blackwell Publishing
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1582-4934.2011.01512.x
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3337339
Citations & impact
Impact metrics
Citations of article over time
Article citations
Human Stem Cells for Cardiac Disease Modeling and Preclinical and Clinical Applications-Are We on the Road to Success?
Cells, 12(13):1727, 27 Jun 2023
Cited by: 5 articles | PMID: 37443761 | PMCID: PMC10341347
Review Free full text in Europe PMC
Cardiac Cell Therapy for Heart Repair: Should the Cells Be Left Out?
Cells, 10(3):641, 13 Mar 2021
Cited by: 15 articles | PMID: 33805763 | PMCID: PMC7999733
Review Free full text in Europe PMC
The utility of biomedical scaffolds laden with spheroids in various tissue engineering applications.
Theranostics, 11(14):6818-6832, 03 May 2021
Cited by: 16 articles | PMID: 34093855 | PMCID: PMC8171099
Review Free full text in Europe PMC
Challenges and Limitations of Strategies to Promote Therapeutic Potential of Human Mesenchymal Stem Cells for Cell-Based Cardiac Repair.
Korean Circ J, 51(2):97-113, 01 Feb 2021
Cited by: 15 articles | PMID: 33525065 | PMCID: PMC7853896
Review Free full text in Europe PMC
All Roads Lead to Rome (the Heart): Cell Retention and Outcomes From Various Delivery Routes of Cell Therapy Products to the Heart.
J Am Heart Assoc, 10(8):e020402, 06 Apr 2021
Cited by: 35 articles | PMID: 33821664 | PMCID: PMC8174178
Review Free full text in Europe PMC
Go to all (35) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00474461
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Intracoronary delivery of self-assembling heart-derived microtissues (cardiospheres) for prevention of adverse remodeling in a pig model of convalescent myocardial infarction.
Circ Cardiovasc Interv, 8(5):e002391, 01 May 2015
Cited by: 16 articles | PMID: 25953823 | PMCID: PMC4428690
Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction.
J Am Coll Cardiol, 57(4):455-465, 01 Jan 2011
Cited by: 149 articles | PMID: 21251587
Integrin β-3 is required for the attachment, retention and therapeutic benefits of human cardiospheres in myocardial infarction.
J Cell Mol Med, 22(1):382-389, 11 Oct 2017
Cited by: 7 articles | PMID: 29024385 | PMCID: PMC5742734
Heart to heart: cardiospheres for myocardial regeneration.
Heart Rhythm, 9(10):1727-1731, 17 Jul 2012
Cited by: 23 articles | PMID: 22813578
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R01 HL083109
Grant ID: R01 HL083109-07