Abstract
Free full text

Statins, inflammation and deep vein thrombosis: a systematic review
Abstract
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism. The 2009 JUPITER trial showed a significant decrease in DVT in non-hyperlipidemic patients, with elevated C-reactive protein (CRP) levels, treated with rosuvastatin. The effects of statins on thrombosis are unclear, prompting this literature review. A literature search was performed (1950 to February 2011) with MEDLINE, EMBASE, and PUBMED databases including the following keywords: “statins”, “hydroxymethylglutaryl-CoA reductase inhibitors”, “VTE”, “PE”, “DVT”, and either “anti-coagulation” or “inflammation”. Editorials, reviews, case reports, meta-analysis and duplicates were excluded. Inflammatory biomarkers of DVT, include interleukin (IL)-6, CRP, IL-8, and monocyte chemotactic protein 1 (MCP-1). Statin therapy reduces IL-6 expression of CRP and MCP-1, usually elevated in VTE. Reduction of IL-6 induced MCP-1 has been linked to vein wall fibrosis, promoting post thrombotic syndrome (PTS) and recurrent DVT in patients. Also, our review suggests that the anti-thrombotic effects are likely exhibited through the anti-inflammatory properties of statins. This work supports that statin therapy has the ability to decrease the incidence and recurrence of VTE and the potential to decrease PTS. This is mainly due to the anti-inflammatory effects of statins and may explain why normolipidemic patients, with elevated CRP, appear to have the greatest reduction in VTE. Given their low risk of bleeding, statins have the potential to serve as a safe adjunctive pharmacological therapy to current treatments in select patients with VTE, however further investigations into this concept are needed and essential.
Introduction
Venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). In a recent study, the estimated total annual cases of VTE occurring in the United States exceeded 900,000, of which more than 250,000 were fatal [1]. An international study that involved six countries within the European Union, reported 465,715 estimated annual cases of DVT; 295,982 PE events and 370,012 VTE-related deaths [2]. Clinically, DVT is divided into two distinct phases: acute and chronic. It is during the acute phase of DVT that PE, a serious and potentially fatal condition may occur as a result of a dislodged thrombus. In addition, post-thrombotic syndrome (PTS), which is a long-term sequelae of DVT [3–5], affects 23–60% of patients and carries with it a high level of morbidity [6]. Despite the progress in medicine and vascular biology, there has been no decrease in the incidence of VTE in the last 25 years [7]. The standard protocol for a patient presenting with a DVT is initial treatment with heparin, followed by long-term anticoagulation with warfarin in order to reduce the chance of PE, complications of PTS and recurrence of DVT. However, all of these medications are associated with a high risk of bleeding [8]. An overview of bleeding complications demonstrated that the rate of intracranial bleeding was 1.15% per year and the case fatality rate of major bleeding was 13% [8]. Thus, investigation into new treatment options for DVT is warranted.
The effect of statins on DVT was brought to light in 2009 through a clinical randomized controlled trial (RCT) called the JUPITER Trial. In this study, relatively healthy people with high levels of C-reactive protein (CRP) and normal low density lipoprotein cholesterol (LDL-C) levels, were given either a placebo or rosuvastatin [9]. It showed that the rate of DVT was significantly decreased in patients treated with rosuvastatin versus controls, 34 versus 60 patients respectively, with a median follow-up point of 1.9 years [9]. Rosuvastatin, along with other statins, inhibits hydroxymethylglutaryl (HMG)-CoA reductase, and are used as a treatment for hypercholesterolemia.
Several mechanisms have been proposed for the reduction in thrombosis in patients treated with statins, including decreased tissue factor [10, 11], plasminogen activator inhibitor-1 (PAI-1) [11, 12], increased tissue plasminogen activator (tPA) expression, decreased platelet aggregation, and increased thrombomodulin expression [11, 13]. The effect of rosuvastatin on DVT is not clear, and its investigation will provide an important contribution to the field. In addition to their anti-thrombotic/anti-inflammatory potential, statins have years of data that show no bleeding side effects, making them a potential adjunctive therapy for DVT. We have thus conducted a systematic review of the literature regarding the non-lipid effects of statins and the potential link between DVT, inflammation, and statins. We hypothesize that DVT, inflammation and statins are interrelated due to the probable anti-thrombotic and anti-inflammatory properties of statins.
Materials and methods
Eligibility criteria
The literature search was limited to the English language. All articles primarily related to DVT, statins, anti-coagulation, and inflammation were evaluated to determine if they met inclusion criteria. Editorials, reviews, case series, and duplicates were then excluded. Reviews of similar topics were read by the authors, but not included as a primary study. A review of references from the final list of manuscripts was done in order to include all pertinent studies not obtained in our search.
Search strategy
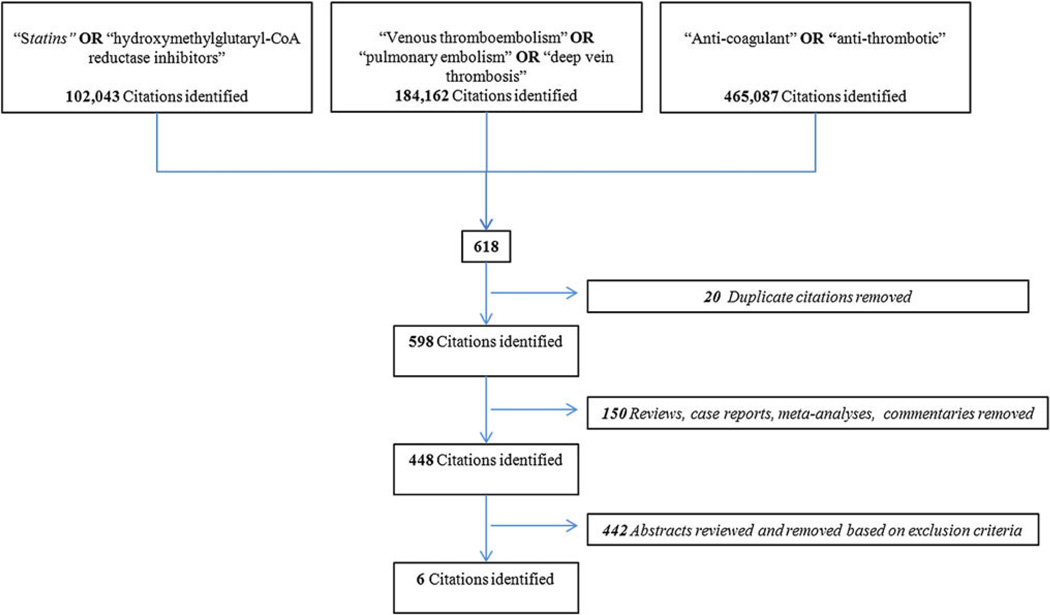
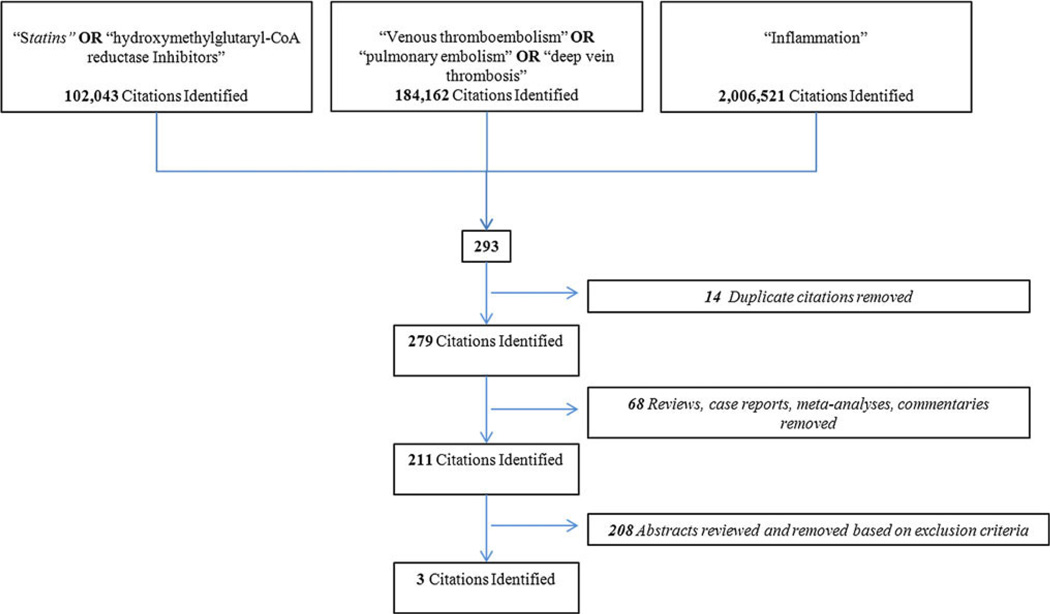
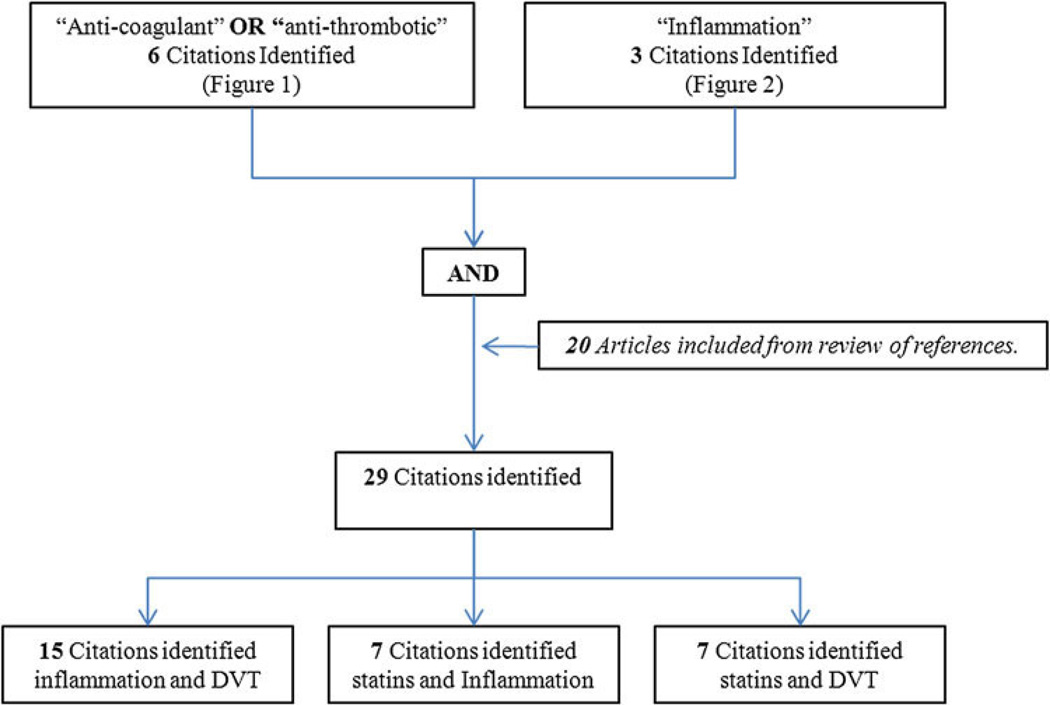
An English language literature search from 1950 to February 2011 was performed with MEDLINE, EMBASE, and PUBMED databases. The search included the following keywords: “statins”, “HMG-CoA reductase inhibitors”, “venous thromboembolism”, “pulmonary embolism”, “deep vein thrombosis”, and either or “inflammation”. The inclusion and exclusion criteria as well as the results of the search are outlined in Figs. 1 and and2.2. A total of 911 manuscripts were initially identified, after removal of duplicates the remaining 877 abstracts [598 anti-coagulation, Fig. 1 + 279 inflammation, Fig. 2] were then reviewed. Two independent reviewers evaluated all abstracts (A.L.R. and J.A.D.) based on the inclusion and exclusion criteria. All relevant review articles generated by the search were analyzed. In addition, articles were added to the final analysis based on evaluation of the references of the articles previously included, and using the same inclusion/exclusion criteria applied for the systematic review (Fig. 3). Disputes between authors on inclusion were reexamined together and only those that were agreed upon by both authors were included in the final analysis.
Data review and analysis
A standardized data retrieval form was created. Two independent reviewers (A.L.R. and J.A.D.) evaluated data extracted from articles including: study design, patient population, sample size, interventions, primary end point, and primary and secondary outcomes. Articles were then sub-classified into subject areas in order to allow for a more efficient analysis.
Results
A total of 29 articles were identified that fit the eligibility criteria, shown in Figs. 1 and and2.2. Common investigative topics were identified and articles categorized by subject (Fig. 3). The inflammatory biomarkers and inflammatory events that were reviewed in this work are shown in Table 1.
Table 1
Inflammatory biomarkers and inflammatory events included in this manuscript that fit the eligibility criteria
| Inflammatory biomarkers | Ref # |
|---|---|
| hs-CRP | [9] |
| MCP-1, monocyte recruitment | [14] |
| sP-selectin | [15] |
| MCP-1, IL-8, neutrophil recruitment, VEGF. | [16] |
| Microparticle tissue factor activity (MPTFA), sP-selectin | [17] |
| D-dimer, sPselectin, CRP, MP | [18] |
| sP-selectin - vein wall inflammation, coagulation parameters | [19] |
| IL-6, IL-8, CRP | [20] |
| IL-6, CRP | [21] |
| CRP | [22] |
| IL-6, IL-8, MCP-1 | [23] |
| IL-8 | [24] |
| IL-6 | [25] |
| hs-CRP | [26] |
| TNFα, IL-6, MCP-1, IL-8 | [27] |
| P-selectin, TNFα | [28] |
| IL-6, CCL2, monocyte recruitment | [29] |
| CRP | [30] |
| CRP | [31] |
| MCP-1, IL-6, IL-6R | [33] |
| TNFα R2, IL-6, hs-CRP, TNFα, TLR2, TLR4. | [34] |
| thrombomodulin, eNOS, IL-8, MCP-1, PAI-1, endothelin | [35] |
| IL-6, IL-8, MCP-1 | [36] |
Inflammation and DVT
The link between DVT and inflammation facilitating thrombosis and the development of post-thrombotic syndrome (PTS) was discussed in 9 clinical studies (2 cohort studies, 3 prospective non randomized, 4 case–control studies) and 6 basic science animal studies[14–28] (Table 2, Section 1).
Table 2
Articles included in this manuscript that fit the eligibility criteria
| Ref nos. | ±* | Increases** | Decreases** | No change** | Sample size | Comments | Type of study |
|---|---|---|---|---|---|---|---|
| Section 1: Inflammation and DVT | |||||||
| [14] | (+) | MCP-1 | 192 Mice | Found that increased levels of MCP-1 in mice and rats increased thrombus resolution, independent of monocyte recruitment. | Basic science in vivo | ||
| [15] | (+) | sP-selectin | 116 Patients 129 Controls | Case–control study with 245 patients and controls found increased sP-selectin was associated with VTE. | Case–control study | ||
| [16] | (+) | Thrombus dissolution, neovascularization, thrombus blood flow, PMN’s, fibrosis, absolute femoral venous pressure | Vascular endothelial growth factor | MCP-1, macrophage inflammatory protein-1alpha | 42 Rats | Basic science study utilizing a rodent model of DVT and treating with IV IL-8 daily. Overall found that administration of IL-8, which induced a pro-inflammatory state, enhanced thrombus resolution. | Basic science in vivo |
| [17] | (+) | Percent vein reopening | Vein wall inflammation, microparticle tissue factor activity (MPTFA), sP-selectin | 9 Primates | Basic science study utilizing a primate model of DVT compared outcomes in animals treated with enoxaparin, P-selectin inhibitor (PSI-421), and controls. Found that PSI-421 treated animals’ demonstrated reduced MPTFA and vein wall inflammation in addition to greater vein reopening. | Basic science in vivo | |
| [18] | (+) | D-dimer, sPselectin CRP, Wells score, platelet derived MP | Leukocyte derived MP | 318 Participants | Study included 318 participants, 208 were analyzed. Thirty healthy controls, 62 were positive for DVT via compression ultrasound, 116 presented with leg pain but were DVT negative via duplex scan. Overall found that the combination of Wells score and sP-selectin levels could both confirm and exclude DVT effectively. | Prospective non randomized | |
| [19] | (+) | IL-6, IL-8, CRP | 99 Patients | Elevated inflammatory biomarkers were observed to be the highest on day of admission for DVT and decreased thereafter. | Prospective non randomized | ||
| [20] | (+) | IL-6, CRP | 110 Patients | Elevated baseline IL-6 levels were found to be associated with increased VOR at 3 months, post DVT. | Prospective non randomized | ||
| [21] | (+) | F VIII, vWF, FVII CRP | 19,237 Patients | Prospective study with 19,237 patients over a 7.8 year follow-up. | Cohort study | ||
| [22] | (+) | IL-6, IL-8, MCP-1 | 532 Patients | Biomarkers were found to be elevated in patients with a history of recurrent DVT. | Case–control study | ||
| [23] | (+) | IL-8 | 948 Patients | Case–control study found elevated levels of IL-8 were a risk factor for VTE and levels remained elevated in chronic DVT. | Case–control study | ||
| [24] | (−) | IL-6 | 355 Participants | No association was observed between IL-6 and VTE. Interleukin-6 was measured in 128 patients with DVT, 105 with PE, and 122 healthy controls. | Case–control study | ||
| [25] | (−) | hs-CRP | 318 Participants | Association was initially seen between hs-CRP levels and VTE, but after adjustment for BMI this association was no longer observed. Levels were measured in 117 patients with DVT, 97 patients with PE, and 104 healthy controls. | Case–control study | ||
| [26] | (+) | TNFa, IL-6, MCP-1, IL-8 | 6 Baboons | Elevated levels of inflammatory markers were observed in a baboon venous thrombosis model. | Basic science in vivo | ||
| [27] | (+) | P-selectin, TNFa | 28 Rats | Inhibition of P-selectin and TNF in a rat venous thrombosis model suggested that the neutralization of these biomarkers could play a role in attenuation of inflammation in venous thrombosis. | Basic science in vivo | ||
| [28] | (+) | CCL2, monocyte recruitment, vein wall intimal thickness, fibrosis | 136 Mice | Basic science study utilizing a mouse model of DVT and treatment with IL-6 Ab, to neutralize IL-6, led to outcomes listed. This suggested IL-6 could serve as a target in order to prevent complications generally seen in PTS. | Basic science in vivo | ||
| Section 2: Statins and Inflammation | |||||||
| [30] | (+) | CRP | 2,884 Participants | Prospective randomized double blinded study and open label study using 40 mg pravastatin, which found decreased CRP at 12 and 24 weeks with pravastatin use. | RCT | ||
| [31] | (+) | CRP | Cell cultures | Basic science study that found statins to reduce CRP production directly in hepatocytes. | Basic science in vitro | ||
| [32] | (+) | HDL, Apo A1. | LDL-C, TC, TG, Apo B, C3c, LDL-Ab, F VII, F I, F II, Ag-tPA bo, PAI-1 ao, thrombin, antithrombin | 67 Participants | Treatment with statins was found to exert a dose-dependent effect in lowering risk of thrombotic events in patients who have suffered a myocardial infarction (ao: after occlusion, bo: before occlusion). | Cross-sectional study | |
| [33] | (+) | MCP-1, IL-6, IL-6R | Cell cultures | Basic science study that found decreased expression of various biomarkers in human aortic endothelial cells stimulated with IL-6, when in the presence of statins. | Basic science in vitro | ||
| [34] | (−) | LDL | ApoB, TNFa R2, IL-6, hsCRP, TNFa, TLR2, TLR4, monocyte stimulation | 20 Participants | RCT that found no effect of statins on plasma inflammatory biomarkers. | RCT | |
| [35] | (+) | Thrombomodulin, eNOS | IL-8, MCP-1, PAI-1, endothelin | Cell cultures | Basic science study that concluded that statins directly change gene expression in endothelial cells. | Basic science in vitro | |
| [29] | (+) | Inflammatory biomarkers: Serum IL-6, IL-8, MCP-1. In vitro study: mRNA expression IL-6, IL-8, MCP-1 | 107 Participants | Inflammatory biomarkers: Decreased serum cytokines were observed in hypercholesterolemic patients treated with simvastatin for 6 months. In vitro study: involving HUVECs and monocytes from healthy volunteers. | Basic science in vitro | ||
| Section 3: Statins and DVT | |||||||
| [9] | (+) | DVT rate, hs-CRP | 17,802 Participants | RCT found decreased incidence of DVT in patients with elevated CRP and normal LDL levels who were treated with statins. | RCT | ||
| [36] | (+) | DVT rate | 2,427 Participants | Case control, which found a lower risk of venous thrombosis in current statins users. | Case–control study | ||
| [37] | (+) | VTE rate | 740 Participants | Case control that found reduction in occurrence of VTE in patients with a history of solid organ tumor with the use of statins for at least 2 months. | Case–control study | ||
| [38] | (+) | VTE rate | 754 Participants | Case control that found association between VTE and statin use. | Case–control study | ||
| [39] | (+) | Mice (n = 245) and cell cultures | Basic science study in rodents found that NCX6560, an atorvastatin derivative that releases nitric oxide, possessed anti-inflammatory, vasorelaxant and anti-thrombotic properties while maintaining inhibitory effects on cholesterol biosynthesis in vitro and superior in vivo lipid lowering properties. This study compared the effects of this new agent, versus atorvastatin and found that NCX6560 had improved anti-inflammatory, anti-thrombotic, and lipid-lowering properties. | Basic science in vivo | |||
| [40] | (+) | DVT rate | 4,538 Participants | Case control study that found that use of statins were associated with a reduced risk of DVT. When stratified by statin type, pravastatin was found to have a greater benefit than simvastatin. | Case–control study | ||
| [41] | (+) | DVT rate | 125,862 Participants | Retrospective cohort study found a reduction in rate of DVT in statin users. | Cohort study | ||
While multiple biomarkers were shown to increase the risk of thrombosis, interleukin (IL)-6, IL-8, P-selectin, monocyte chemotactic protein 1 (MCP-1), tumor necrosis factor alpha (TNFα), and CRP each demonstrated a prothrombotic effect in two or more studies. In particular, IL-6, IL-8, and CRP were elevated on the first day of admission for patients with DVT [19]. In terms of recurrent DVT, IL-6, IL-8, and MCP-1 were increased in patients after their first thrombotic event [22]. Regarding potential mechanisms, inhibition of IL-6 was shown to reduce the effects of PTS following DVT in a mouse model of DVT, likely by down-regulating synthesis of MCP-1 [28]. TNFα was elevated in a primate model of DVT [26]. Additionally, TNFα inhibition, in a rat model of DVT, led to a reduction in thrombosis [27]. Soluble P-selectin (sP-selectin) was elevated in patients with recurrent VTE during at least one unprovoked event of DVT or PE [15]. Additionally, inhibition of P-selectin was found to reduce venous thrombosis and vein wall inflammation in rats and primates [17, 27].
While the majority of the studies report a correlation between an increased thrombotic effect with the inflammatory biomarker of interest, other studies found that certain inflammatory markers were associated with thrombus resolution. A rodent model of DVT in which animals were injected with IL-8 showed increased vascular endothelial growth factor and thrombus resolution compared to controls [16]. Additionally, IL-6, MCP-1 and high sensitivity C-reactive protein (hs-CRP) were each shown in single studies to have no correlation with thrombosis [24, 25].
Statins and inflammation
The relationship between statins and inflammation was investigated in 2 RCTs, 1 cross-sectional study and 4 basic science (one including serum inflammatory biomarkers [29]) studies [29–35]. There is increasing evidence in support of statins having an anti-inflammatory effect and this is substantiated by 6 of the 7 articles (Table 2, Section 2).
Statins were associated with reductions in CRP, IL-6, IL-8, MCP-1, and PAI-1 [29–33, 35]. In addition, statins were also associated with increased levels of endothelial nitric oxide synthase (eNOS), thrombomodulin, and apolipoprotein A1 [33, 35]. The patient populations across the studies, showing an anti-inflammatory effect, included men and women with and without a history of cardiovascular disease or currently suffering from myocardial infarction along with matched controls. Basic science studies showed decreased inflammatory biomarkers with statin use across multiple cell lines including, hepatocytes, human aortic endothelial cells, and human umbilical vein endothelial cells (HUVEC) [29, 31, 33, 35].
One RCT did not support the possible anti-inflammatory effects of statins. The patient population in this study included normolipidemic patients with normal CRP levels who received short-term high dose therapy with atorvastatin. The authors investigated several plasma inflammatory biomarkers and found no association with statin use [34].
Statins and DVT
The potential anti-thrombotic nature of statins was elucidated in 1 RCTs, 4 case–controlled studies, 1 retrospective cohort study and 1 basic science study [9, 36–41]. All 7 studies, including the JUPITER trial, concluded that statin use was associated with a decreased rate of DVT, PE, or overall VTE (Table 2, Section 3).
The JUPITER trial was a prospective clinical RCT involving 17,802 patients, in 26 countries, and included a 5-year follow up [9]. This study showed a decreased incidence of DVT in patients, with normal LDL-C levels and elevated CRP, treated with rosuvastatin, 20 mg daily [9]. Several prospective and retrospective studies have demonstrated a similar effect on other patient populations including post-menopausal woman, patients with solid organ tumors for 2 months, and patients with recurrent DVT [36–38, 40, 41]. One case–control study concluded that pravastatin had a greater effect on reducing DVT rate than simvastatin, suggesting a difference in efficacy by statin type [40]. A potential mechanism is elucidated in a rodent study in which an atorvastatin derivative caused a release of nitric oxide, leading to a vasorelaxant effect [39].
Discussion
The recent JUPITER trial showed that statins might be beneficial for patients suffering from deep vein thrombosis. The JUPITER trial involved 17,802 non-hyperlipidemic patients with elevated CRP levels where rosuvastatin treatment caused a significant decrease in the rate of DVT [9]. This prompted our review of the literature in order to determine what evidence currently exists in support of or in opposition to this claim.
Multiple inflammatory biomarkers were found to be associated with VTE including IL-6, IL-8, sP-selectin, MCP-1, TNFα, and CRP. However, it is difficult to conclude whether the elevations in these biomarkers are from systemic inflammation or play an integral part in the formation of thrombosis. Direct inhibition of IL-6, TNFα, and P-selectin was found to reduce VTE and its sequelae, PTS [17, 27, 28]. This evidence supports pharmacological therapy targeted at inflammatory mediators to decrease the incidence and recurrence of VTE and may explain the underlying anti-thrombotic effect of statins.
In fact, several studies found that statin use led to decreased levels of inflammatory biomarkers including IL-6, IL-8, MCP-1 and CRP. These inflammatory biomarkers were specifically found to be elevated in patients with VTE and affected the physiology of endothelial cells. Most of the current research involving statins and inflammation focuses on the arterial side of the circulation and while this review aims to discuss mainly the venous side, it is important to look at biomarkers that both have in common. IL-6 was the most common inflammatory biomarker analyzed and is primarily studied because it is a known regulator in the activation of an acute phase response. It is usually analyzed both on an individual basis and also in conjunction with IL-8, MCP-1, and CRP [31, 32, 34]. The study by Arnaud et al. [31] looked at levels of IL-6 induced CRP in which they found them to be decreased by statins. In addition, the study by Jougaski also examined levels of MCP-1, a cytokine that is induced by IL-6. This study found that statins suppressed IL-6 induced MCP-1 expression [33]. One RCT that analyzed plasma inflammatory biomarkers by Millar et al. [34], found no changes in the expression of IL-6, hs-CRP and other inflammatory biomarkers with either the in vivo or ex vivo use of statins. However, the patient population consisted of normolipidemic adults with normal CRP levels, versus the elevated CRP levels in the participants of the JUPITER trial, suggesting baseline CRP levels are an important marker in predicting the efficacy of reducing VTE with statins [9, 34].
Statin therapy was found to reduce VTE in all studies included in this review. While many patient populations were studied, the strong support for effective statin use exists for normolipidemic patients with elevated levels of CRP, indicating increased inflammation. The studies were comprised of various statins including: pravastatin, simvastatin, atorvastatin, rosuvastatin, lovastatin, fluvastatin and cerivastatin. The degree of risk reduction was found to differ between statins. Khemasuwan et al. [37], Ramcharan et al. [40], and Doggen et al. [36] were all in accordance with the beneficial effect of simvastatin in decreasing the rate of DVT, but a discrepancy was seen in regards to the level of benefit conferred by this statin in comparison to others. Khemasuwan et al. [37] found simvastatin led to fewer occurrences of DVT, when compared to atorvastatin. Ramcharan discovered a beneficial preventative effect of DVT with pravastatin, which was higher in comparison to simvastatin. Doggen found that pravastatin showed no benefit in reducing the rate of DVT and the initial protective effect on DVT observed was seen only in those treated with simvastatin [36, 37]. While this observation made by Doggen is in opposition to the conclusion made by Ramcharan, the number of patients in Doggen’s study using simvastatin was approximately three times higher than those treated with pravastatin (simvastatin 16:5 pravastatin). This discrepancy brings into question the reliability of this conclusion. Furthermore, this study was conducted only in postmenopausal women, which decreases the ability to generalize results and accurately compare them to those from the other studies. The remainder of the studies reviewed consisted of both male and female participants in approximately equal ratios [9, 36–41].
In summary, there is overwhelming support that statin therapy has the ability to decrease the incidence and recurrence of VTE. Further work must be done to uncover the mechanism leading to this anti-thrombotic affect and to more clearly define appropriate patient populations. However, our review suggests that the anti-thrombotic effects are likely exhibited through the anti-inflammatory properties of statins. Cytokines that most likely are involved include IL-6, IL-8, CRP, and MCP-1. Specifically, statin therapy reduces the IL-6 induced expression of both CRP and MCP-1, which are elevated in patients with VTE [31–33]. Reduction of IL-6 induced MCP-1 has also been linked to vein wall fibrosis, which would predispose a patient to PTS and recurrent DVT [28]. Collectively, this may explain why anti-inflammatory effects of statins in normolipidemic patients, with elevated CRP levels, appear to lead to the greatest reduction in the incidence and recurrence of VTE and potentially could decrease the incidence of PTS. Given their low risk of bleeding, statins have the potential to serve as a safe adjunctive pharmacological therapy to current treatments in select patients with VTE, however further investigations into this concept are needed and essential.
Limitations
It may be possible that positive findings tend to be published much more often than negative or non-conclusive findings. This was observed in the articles that matched our criteria as most of them tended to have findings that supported one viewpoint. This may leads to a bias in terms of the availability of data that is either in support of or in opposition to the link between statins, inflammation and DVT. Another limitation is the fact that the manuscripts included within this review cover very heterogeneous topics with regards to DVT (inflammatory response to acute DVT, chronic inflammation following DVT, post thrombotic syndrome). In addition, the vast majority of the literature focuses more on inflammatory cytokines rather than on cell-based mechanisms. This then leaves another area to be explored with regards to the impact of statins on DVT and inflammation.
Future directions
There is a necessity for more RCTs in order to adequately identify the relationship between DVT and statins in a structured setting and to differentiate between specific statins and doses. There is also a need to investigate the use of statins for both prophylaxis and treatment, in addition to doing comparison studies of statins with and without current therapy or standard care.
Acknowledgments
This work supported in part by NIH 1PO1HL089407. The authors also would like to acknowledge and extend their gratitude to the Michigan Institute for Clinical & Health Research (MCRiT) program, all the members of the Jobst Vascular Surgery Laboratory at the University of Michigan for their time, training, encouragement and continued support.
Abbreviations
| VTE | Venous thromboembolism |
| DVT | Deep vein thrombosis |
| PE | Pulmonary embolism |
| PTS | Post-thrombotic syndrome |
| RCT | Randomized controlled trial |
| CRP | C-reactive protein |
| LDL-C | Low density lipoprotein cholesterol |
| HMG-CoA | reductase Hydroxymethylglutaryl-CoA reductase |
| PAI-1 | Plasminogen activator inhibitor-1 |
| tPA | Tissue plasminogen activator |
| IL | Interleukin |
| MCP-1 | Monocyte chemotactic protein 1 |
| TNFα | Tumor necrosis factor alpha |
| sP-selectin | Soluble P-selectin |
| hs-CRP | High sensitivity C-reactive protein |
| eNOS | Endothelial nitric oxide synthase |
| HUVEC | Human umbilical vein endothelial cells |
Contributor Information
April L. Rodriguez, Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA.
Brandon M. Wojcik, Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA.
Shirley K. Wrobleski, Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA.
Daniel D. Myers, Jr., Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA. Unit for Laboratory Animal Medicine, University of Michigan, Ann Arbor, MI, USA.
Thomas W. Wakefield, Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA.
Jose A. Diaz, Department of Surgery, Section of Vascular Surgery, Conrad Jobst Vascular Research Laboratories, School of Medicine, University of Michigan, A570A MSRB II, 1150 W. Medical Center Dr., Dock #6, Ann Arbor, MI 48109, USA.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s11239-012-0687-9
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3338886?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s11239-012-0687-9
Article citations
Statins for the primary prevention of venous thromboembolism.
Cochrane Database Syst Rev, 11:CD014769, 05 Nov 2024
Cited by: 0 articles | PMID: 39498835
Review
Risk of Venous Thromboembolism in Statin Users Compared to Fibrate Users in the United Kingdom Clinical Practice Research Datalink (UK CPRD) GOLD.
Clin Epidemiol, 16:683-697, 05 Oct 2024
Cited by: 0 articles | PMID: 39386131 | PMCID: PMC11463176
Dispensed prescription medications and short-term risk of pulmonary embolism in Norway and Sweden.
Sci Rep, 14(1):20054, 29 Aug 2024
Cited by: 0 articles | PMID: 39209867 | PMCID: PMC11362151
Predictive factors of postoperative complications related to free flap reconstruction in head and neck cancer patients admitted to intensive care unit: a retrospective cohort study.
BMC Anesthesiol, 24(1):258, 29 Jul 2024
Cited by: 1 article | PMID: 39075344 | PMCID: PMC11285200
Association between the use of statins and in-hospital mortality risk in patients with sepsis-induced coagulopathy during ICU stays: a study based on medical information mart for intensive care database.
BMC Infect Dis, 24(1):738, 25 Jul 2024
Cited by: 0 articles | PMID: 39061029 | PMCID: PMC11282707
Go to all (87) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Statin therapy associated with improved thrombus resolution in patients with deep vein thrombosis.
J Vasc Surg Venous Lymphat Disord, 7(2):169-175.e4, 16 Jan 2019
Cited by: 2 articles | PMID: 30660579
Statin use and the prevention of venous thromboembolism: a meta-analysis.
Int J Clin Pract, 64(10):1375-1383, 01 Sep 2010
Cited by: 42 articles | PMID: 20716146
Review
Statins during Anticoagulation for Emergency Life-Threatening Venous Thromboembolism: A Review.
Medicina (Kaunas), 60(8):1240, 30 Jul 2024
Cited by: 0 articles | PMID: 39202521 | PMCID: PMC11356097
Review Free full text in Europe PMC
Statins as a preventative therapy for venous thromboembolism.
Cardiovasc Diagn Ther, 7(suppl 3):S207-S218, 01 Dec 2017
Cited by: 16 articles | PMID: 29399524 | PMCID: PMC5778529
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (3)
Grant ID: 1P01HL089407
Grant ID: P01 HL089407
Grant ID: P01 HL089407-01A1