Abstract
Purpose
To determine whether cancer risks for carriers and noncarriers from families with a mismatch repair (MMR) gene mutation are increased above the risks of the general population.Patients and methods
We prospectively followed a cohort of 446 unaffected carriers of an MMR gene mutation (MLH1, n = 161; MSH2, n = 222; MSH6, n = 47; and PMS2, n = 16) and 1,029 their unaffected relatives who did not carry a mutation every 5 years at recruitment centers of the Colon Cancer Family Registry. For comparison of cancer risk with the general population, we estimated country-, age-, and sex-specific standardized incidence ratios (SIRs) of cancer for carriers and noncarriers.Results
Over a median follow-up of 5 years, mutation carriers had an increased risk of colorectal cancer (CRC; SIR, 20.48; 95% CI, 11.71 to 33.27; P < .001), endometrial cancer (SIR, 30.62; 95% CI, 11.24 to 66.64; P < .001), ovarian cancer (SIR, 18.81; 95% CI, 3.88 to 54.95; P < .001), renal cancer (SIR, 11.22; 95% CI, 2.31 to 32.79; P < .001), pancreatic cancer (SIR, 10.68; 95% CI, 2.68 to 47.70; P = .001), gastric cancer (SIR, 9.78; 95% CI, 1.18 to 35.30; P = .009), urinary bladder cancer (SIR, 9.51; 95% CI, 1.15 to 34.37; P = .009), and female breast cancer (SIR, 3.95; 95% CI, 1.59 to 8.13; P = .001). We found no evidence of their noncarrier relatives having an increased risk of any cancer, including CRC (SIR, 1.02; 95% CI, 0.33 to 2.39; P = .97).Conclusion
We confirmed that carriers of an MMR gene mutation were at increased risk of a wide variety of cancers, including some cancers not previously recognized as being a result of MMR mutations, and found no evidence of an increased risk of cancer for their noncarrier relatives.Free full text

Colorectal and Other Cancer Risks for Carriers and Noncarriers From Families With a DNA Mismatch Repair Gene Mutation: A Prospective Cohort Study
Abstract
Purpose
To determine whether cancer risks for carriers and noncarriers from families with a mismatch repair (MMR) gene mutation are increased above the risks of the general population.
Patients and Methods
We prospectively followed a cohort of 446 unaffected carriers of an MMR gene mutation (MLH1, n = 161; MSH2, n = 222; MSH6, n = 47; and PMS2, n = 16) and 1,029 their unaffected relatives who did not carry a mutation every 5 years at recruitment centers of the Colon Cancer Family Registry. For comparison of cancer risk with the general population, we estimated country-, age-, and sex-specific standardized incidence ratios (SIRs) of cancer for carriers and noncarriers.
Results
Over a median follow-up of 5 years, mutation carriers had an increased risk of colorectal cancer (CRC; SIR, 20.48; 95% CI, 11.71 to 33.27; P < .001), endometrial cancer (SIR, 30.62; 95% CI, 11.24 to 66.64; P < .001), ovarian cancer (SIR, 18.81; 95% CI, 3.88 to 54.95; P < .001), renal cancer (SIR, 11.22; 95% CI, 2.31 to 32.79; P < .001), pancreatic cancer (SIR, 10.68; 95% CI, 2.68 to 47.70; P = .001), gastric cancer (SIR, 9.78; 95% CI, 1.18 to 35.30; P = .009), urinary bladder cancer (SIR, 9.51; 95% CI, 1.15 to 34.37; P = .009), and female breast cancer (SIR, 3.95; 95% CI, 1.59 to 8.13; P = .001). We found no evidence of their noncarrier relatives having an increased risk of any cancer, including CRC (SIR, 1.02; 95% CI, 0.33 to 2.39; P = .97).
Conclusion
We confirmed that carriers of an MMR gene mutation were at increased risk of a wide variety of cancers, including some cancers not previously recognized as being a result of MMR mutations, and found no evidence of an increased risk of cancer for their noncarrier relatives.
INTRODUCTION
Lynch syndrome is an autosomal dominant inherited disorder of cancer susceptibility caused by germline mutations in the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2. Estimates of carrier frequency of germline mutations of these genes in the population range widely depending on various assumptions, from approximately one in 3,010 individuals (for MLH1 and MSH2 combined1) to one in 360 individuals (for all four MMR genes combined2). Mutation carriers are at substantially increased risk of cancers of the colon, rectum, endometrium, stomach, ovary, ureter, renal pelvis, brain, small bowel, and hepatobiliary tract, and the diagnoses of these cancers generally occur at younger ages than for the general population.3 Estimates of site-specific cancer risks for MMR gene mutation carriers inform optimal clinical management. Screening colonoscopy,4,5 prophylactic hysterectomy, and bilateral salpingo-oophorectomy6 have the potential to decrease the risk of colorectal cancer (CRC), endometrial cancer (EC), and ovarian cancer, respectively, for MMR gene mutation carriers. All studies estimating the risk of cancer for MMR gene mutation carriers have been retrospective and, therefore, may be biased as a result of differential recall of family history of cancer and failure to adjust for ascertainment of subjects recruited because of a family history of cancer.7 Risk estimates using prospective data of mutation carriers with no prior diagnosis of cancer will not be biased but are challenging because they require long-term follow-up to provide sufficient time for cancers to be diagnosed.
Genetic testing of family members of a mutation carrier will identify relatives who have and who have not inherited the family-specific MMR gene mutation. It is not known whether the cancer risk for noncarriers from families with MMR gene mutations is greater than that of the general population. A family history of CRC increases an individual's risk 1.5- to eight-fold, varying with the number and ages of affected relatives and degree of relationship to the proband.8 In Lynch syndrome families, it is possible that modifier genes participate in the milieu in which cancer manifests.9–16 Noncarriers of MMR gene mutations may share predisposing genetic risk with their mutation-carrying relatives other than that caused by the MMR gene mutation and therefore may be at increased risk compared with the general population. If, however, the MMR mutation accounts for all the excess cancer risks in these families, then noncarriers should be at population risk. In this study, we estimated cancer risks for mutation carriers and noncarriers, who had no prior diagnosis of cancer, from families with MMR gene mutations from the Colon Cancer Family Registry, using a prospective cohort.
PATIENTS AND METHODS
Colon Cancer Family Registry
This study, using the Colon Cancer Family Registry, included carriers of pathogenic MMR gene mutations and their noncarrier relatives. Details of the Colon Cancer Family Registry have been published previously17 and can be found at the National Cancer Institute Web site (http://epi.grants.cancer.gov/CFR/). Families were recruited between 1997 and 2010 and were ascertained via CRC cases identified from population cancer registries in the United States (Washington, California, Arizona, Minnesota, Colorado, New Hampshire, North Carolina, and Hawaii), Australia (Victoria), and Canada (Ontario) or from family cancer clinics in the United States (Mayo Clinic, Rochester, MN, and Cleveland Clinic, Cleveland, OH), Australia (Melbourne, Adelaide, Perth, Brisbane, and Sydney), and New Zealand (Auckland). Written informed consent was obtained from all study participants, and the study protocol was approved at each center.
Data Collection
At recruitment, baseline information on demographics, personal characteristics, personal and family history of cancer, cancer screening history, history of polyps, polypectomy, hysterectomy, and other surgeries was obtained from all participants. This participant information was updated approximately 5 and 10 years after baseline. Reported cancer diagnoses and ages at which these occurred were confirmed, where possible, using pathology reports, medical records, cancer registry reports, and/or death certificates. The location, histology, and behavior of cancer diagnoses were coded using International Classification of Diseases for Oncology (ICD-O).18 Blood and tumor tissue samples were collected for genetic testing.
MMR Gene Mutation Testing
MMR gene mutation testing was performed by Sanger sequencing or denaturing high-performance liquid chromatography, followed by confirmatory DNA sequencing. Large duplications and deletions were detected by multiplex ligation-dependent probe amplification.17,19–21 Mutation classification and nomenclature were determined using the InSiGHT Colon Cancer Gene Variant Database (http://www.insight-group.org/mutations), the MMR genes variant database of the Memorial University of Newfoundland (http://www.med.mun.ca/MMRvariants/),22 and the MMR Gene Unclassified Variants Database (http://www.mmruv.info).23 Pathogenic mutations were defined as variants resulting in a stop codon, frameshift mutation, large duplication or deletion, or missense mutation previously reported within scientific literature to be pathogenic.
Eligibility Criteria
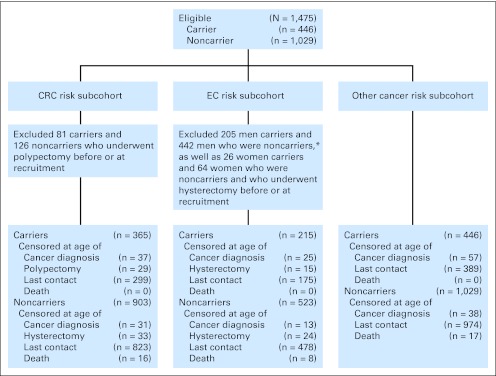
For this study, we assembled three subcohorts to estimate risk of CRC (CRC risk subcohort), risk of EC (EC risk subcohort), and risk of any other cancer (other cancer risk subcohort; Fig 1). Participants were eligible for all subcohorts if they had undergone genetic testing for their specific family germline MMR gene mutation and were confirmed to be either a carrier or noncarrier of this pathogenic mutation, had been followed up at least once since recruitment, and had no cancer diagnosis before or at the time of recruitment. A total of 81 carriers and 126 noncarriers were excluded from the CRC risk subcohort because they had a polypectomy before or at the time of recruitment. A total of 26 carriers and 46 noncarriers were excluded from the EC risk subcohort because they had a hysterectomy before or at the time of recruitment (Fig 1). For this analysis we did not have information on which participants were aware of their mutation status or when they became aware of their status in part because testing may have been conducted clinically, outside the scope of the study.
Statistical Analysis
Observation time began at completion of the baseline questionnaire and ended at the cancer diagnosis, death, or last follow-up, whichever occurred first. For CRC risk, we censored individuals at the time of polypectomy (except when it occurred within a year before the CRC diagnosis, in which case we assumed polypectomy was for the initial CRC diagnosis), and for EC risk, we censored each woman at the time of hysterectomy.
Observed numbers of cancer diagnoses were divided by the expected numbers of cancer diagnoses to calculate standardized incidence ratios (SIRs). Expected numbers of cancer diagnoses were calculated by multiplying the age-, sex-, and country-specific incidence for the general population by the corresponding observation time in the study cohort. Country-, age-, and sex-specific cancer incidences for the general population were obtained for the period from 1998 to 2002 from Cancer Incidence in Five Continents.24 This 5-year period was selected as the closest available data set with respect to the mean calendar year of cancer diagnoses of the sample, and given that it averaged incidence over the 5-year period, it provides more stable estimates of incidence, especially for less common cancers. For cancer cases from the same family, the jackknife method was used to calculate 95% CIs by allowing for any correlation of risk between relatives from the same family25; otherwise, we used exact methods assuming that observed cancer cases followed a Poisson distribution.
We estimated SIRs for the following cancers observed more than once in carriers and noncarriers: colon and/or rectum (ICD-O C18 to C20), pancreas (ICD-O C25), stomach (ICD-O C16), kidney and renal pelvis (ICD-O C64 and C65), urinary bladder (ICD-O C67), and lung (ICD-O C34) for both sexes; endometrium (ICD-O C54 and C55), ovary (ICD-O C56), and breast (ICD-O C50) for females; and prostate (ICD-O C61) for males.
Kaplan-Meier statistics were used to estimate age-dependent cumulative risk (penetrance) at 5 and 10 years. All reported statistical tests were two-sided and P < .05 was considered statistically significant. STATA version 11.0 (STATA, College Station, TX)26 was used for all statistical analyses.
RESULTS
Selection of eligible carriers and noncarriers of an MMR gene mutation from the Colon Cancer Family Registry for each cancer-specific analysis to estimate risk of CRC, EC, and other cancers is depicted in Figure 1.
CRC Risk Subcohort
This subcohort included 365 carriers and 903 noncarriers from 284 families with MMR gene mutations (Table 1). Of the 365 carriers, 310 (85%) were first-degree relatives, 50 (14%) were second-degree relatives, and five (1%) were third-degree relatives of patients with CRC. Of the 903 noncarriers, 583 (64%) were first-degree relatives, 169 (19%) were second-degree relatives, and 151 (17%) were third-degree relatives of patients with CRC.
Table 1.
Baseline Demographics and Clinical Characteristics of Carriers and Noncarriers of a Mismatch Repair Gene Mutation
| Demographic or Clinical Characteristic | CRC Risk Subcohort | EC Risk Subcohort | Other Cancer Risk Subcohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carriers(n = 365) | Noncarriers(n = 903) | Carriers(n = 215) | Noncarriers(n = 523) | Carriers(n = 446) | Noncarriers(n = 1,029) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| MMR gene mutated | ||||||||||||
    MLH1 MLH1 | 134 | 37 | 87 | 41 | 161 | 36 | ||||||
    MSH2 MSH2 | 178 | 49 | 105 | 49 | 222 | 50 | ||||||
    MSH6 MSH6 | 40 | 11 | 18 | 8 | 47 | 11 | ||||||
    PMS2 PMS2 | 13 | 3 | 5 | 2 | 16 | 3 | ||||||
| Sex | ||||||||||||
    Female Female | 201 | 55 | 523 | 58 | 215 | 100 | 523 | 100 | 241 | 54 | 587 | 57 |
    Male Male | 164 | 45 | 380 | 42 | — | — | 205 | 46 | 442 | 43 | ||
| Race | ||||||||||||
    White White | 346 | 96.4 | 847 | 96.3 | 205 | 97.2 | 488 | 96 | 423 | 96.4 | 965 | 96 |
    Latino/Hispanic Latino/Hispanic | 1 | 0.3 | 13 | 1.5 | 1 | 0.5 | 7 | 1 | 4 | 0.9 | 17 | 2 |
    Asian Asian | 9 | 2.5 | 8 | 0.9 | 3 | 1.4 | 5 | 1 | 9 | 2.0 | 9 | 1 |
    Middle Eastern Middle Eastern | 2 | 0.6 | 5 | 0.6 | 2 | 0.9 | 4 | 1 | 2 | 0.5 | 6 | 1 |
    Other Other | 1 | 0.3 | 7 | 0.8 | 0 | 0 | 5 | 1 | 1 | 0.2 | 7 | 1 |
    Unknown Unknown | 6 | 23 | 4 | 14 | 7 | 25 | ||||||
| Country of recruitment | ||||||||||||
    Canada Canada | 24 | 6 | 27 | 3 | 9 | 4 | 23 | 4 | 24 | 5 | 30 | 3 |
    United States United States | 50 | 14 | 110 | 12 | 31 | 14 | 69 | 13 | 75 | 17 | 135 | 13 |
    Australasia Australasia | 291 | 80 | 766 | 85 | 175 | 81 | 431 | 82 | 347 | 78 | 864 | 84 |
| Source of ascertainment | ||||||||||||
    Population based Population based | 59 | 16 | 135 | 15 | 35 | 16 | 77 | 15 | 71 | 16 | 154 | 15 |
    Clinic based Clinic based | 306 | 84 | 768 | 85 | 180 | 84 | 446 | 85 | 375 | 84 | 875 | 85 |
| Age at recruitment, years | ||||||||||||
    Mean Mean | 39.9 | 48.3 | 39.8 | 47.3 | 41.3 | 48.9 | ||||||
    SD SD | 13.8 | 15.6 | 13.1 | 15.9 | 13.9 | 15.4 | ||||||
| Follow-up | ||||||||||||
    Total person-years Total person-years | 1,809 | 4,930 | 1,061 | 2,848 | 2,365 | 5,880 | ||||||
    Mean, years Mean, years | 5.0 | 5.5 | 4.9 | 5.5 | 5.3 | 5.7 | ||||||
    SD, years SD, years | 2.5 | 2.5 | 2.5 | 2.5 | 2.4 | 2.5 | ||||||
    Median, years Median, years | 4.9 | 5.4 | 4.8 | 5.4 | 5.2 | 5.6 | ||||||
    Range, years Range, years | 0.3–11.2 | 0.1–11.9 | 0.2–11.2 | 0.2–11.9 | 0.4–11.2 | 0.1–11.9 | ||||||
Abbreviations: CRC, colorectal cancer; EC, endometrial cancer; SD, standard deviation.
Over a median follow-up of 5 years, 16 mutation carriers (MLH1, n = 9; MSH2, n = 4; MSH6, n = 2; PMS2, n = 1) were diagnosed with CRC at a median age of 49 years (incidence rate, 8.84; 95% CI, 5.42 to 14.43 per 1,000 person-years; SIR, 20.48; 95% CI, 11.71 to 33.27; P < .001; Table 2). The CRC SIRs for carriers of specific MMR gene mutations were 39.40 (95% CI, 18.02 to 74.80) for MLH1, 10.76 (95% CI, 2.93 to 27.55) for MSH2, 17.19 (95% CI, 2.08 to 62.10) for MSH6, and 15.47 (95% CI, 0.39 to 86.21) for PMS2.
Table 2.
Age-, Sex-, and Country-Specific SIRs and Corresponding 95% CIs by Cancer Site for Carriers and Noncarriers of a Mismatch Repair Gene Mutation Compared With the General Population
| Cancer | Observed No. | Expected No. | Age at Diagnosis (years) | SIR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Median | Range | ||||||
| Carriers | |||||||
    Colorectal cancer Colorectal cancer | 16 | 0.78 | 49 | 26–75 | 20.48 | 11.71 to 33.27 | <.001 |
    Endometrial cancer Endometrial cancer | 6 | 0.20 | 53 | 42–66 | 30.62 | 11.24 to 66.64 | <.001 |
    Ovary cancer Ovary cancer | 3 | 0.16 | 52 | 45–56 | 18.81 | 3.88 to 54.95 | <.001 |
    Renal cancer* Renal cancer* | 3 | 0.27 | 71 | 70–77 | 11.22 | 2.31 to 32.79 | <.001 |
    Pancreas cancer Pancreas cancer | 2 | 0.19 | 64 | 63–65 | 10.68 | 2.68 to 47.70 | .001 |
    Gastric cancer Gastric cancer | 2 | 0.20 | 59 | 31–88 | 9.78 | 1.18 to 35.30 | .009 |
    Urinary bladder cancer Urinary bladder cancer | 2 | 0.21 | 62 | 55–68 | 9.51† | 1.15 to 34.37 | .009 |
    Breast cancer‡ Breast cancer‡ | 7 | 1.77 | 56 | 42–62 | 3.95 | 1.59 to 8.13 | .001 |
    Prostate cancer Prostate cancer | 3 | 1.21 | 54 | 50–62 | 2.49 | 0.51 to 7.27 | .18 |
| Noncarriers | |||||||
    Colorectal cancer Colorectal cancer | 5 | 4.88 | 60 | 55–73 | 1.02 | 0.33 to 2.39 | .97 |
    Lung cancer Lung cancer | 3 | 4.68 | 69 | 46–75 | 0.64 | 0.13 to 1.87 | .51 |
    Breast cancer‡ Breast cancer‡ | 5 | 6.95 | 59 | 52–75 | 0.72 | 0.23 to 1.68 | .52 |
    Prostate cancer Prostate cancer | 9 | 5.53 | 67 | 57–82 | 1.63 | 0.74 to 3.09 | .18 |
Abbreviation: SIR, standardized incidence ratio.
Five noncarriers were diagnosed with CRC at a median age of 60 years (incidence rate, 1.01; 95% CI, 0.42 to 2.44 per 1,000 person-years). The overall SIR for noncarriers was 1.02 (95% CI, 0.33 to 2.39; P = .97). All of these CRCs were observed in first-degree relatives of patients with CRC. The SIR was 1.43 (95% CI, 0.46 to 3.34) when analysis was restricted to noncarriers who were first-degree relatives of patients with CRC.
Cumulative risks of CRC were 4.14% (95% CI, 2.35% to 7.24%) at 5 years and 8.05% (95% CI, 4.46% to 14.29%) at 10 years of prospective follow-up for carriers and 0.39% (95% CI, 0.12% to 1.20%) at 5 years and 2.04% (95% CI, 0.49% to 8.30%) at 10 years for noncarriers (Fig 2 and Table 3).
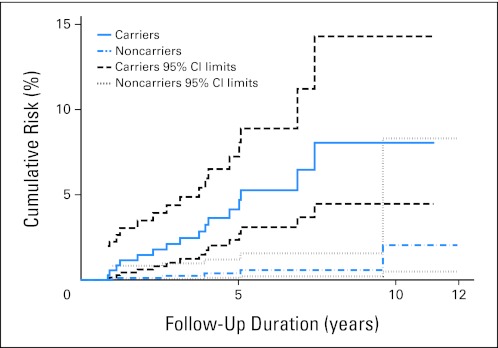
Kaplan-Meier curves for cumulative risks and their corresponding 95% CIs of colorectal cancer at 5 and 10 years.
Table 3.
Cumulative Risk of Cancer Over 5 and 10 Years and Corresponding 95% CIs for Carriers and Noncarriers of a Mismatch Repair Gene Mutation Who Had No Previous Diagnosis of Cancer at Baseline
| Cancer | 5 Years | 10 Years | ||
|---|---|---|---|---|
| Cumulative Risk (%) | 95% CI (%) | Cumulative Risk (%) | 95% CI (%) | |
| Carriers | ||||
    Colorectal cancer Colorectal cancer | 4.14 | 2.35 to 7.24 | 8.05 | 4.46 to 14.29 |
    Endometrial cancer Endometrial cancer | 2.84 | 1.06 to 7.46 | 9.84 | 3.45 to 26.33 |
    Ovary cancer Ovary cancer | 0.29 | 0.04 to 20.5 | 3.11 | 0.82 to 11.43 |
    Renal cancer* Renal cancer* | 0.80 | 0.26 to 2.45 | — | |
    Pancreas cancer Pancreas cancer | 0.33 | 0.05 to 2.34 | 0.95 | 0.22 to 3.97 |
    Gastric cancer Gastric cancer | 0.53 | 0.13 to 2.10 | — | |
    Urinary bladder cancer Urinary bladder cancer | 0.28 | 0.04 to 1.97 | 2.71 | 0.47 to 14.93 |
    Breast cancer† Breast cancer† | 0.85 | 0.27 to 2.65 | 4.49 | 1.78 to 11.08 |
| Noncarriers | ||||
    Colorectal cancer Colorectal cancer | 0.39 | 0.12 to 1.20 | 2.04 | 0.49 to 8.30 |
The frequency of colonoscopy screening was not different between individuals without CRC (unaffected) and those with CRC (affected). On average, unaffected carriers were screened once every 2.7 years (95% CI, 2.5 to 3.0 years), whereas affected carriers were screened every 2.5 years (95% CI, 1.4 to 3.5 years; P = .63). Unaffected noncarriers were screened every 3.9 years (95% CI, 3.7 to 4.1 years), whereas affected noncarriers were screened every 3.9 years (95% CI, 0 to 8.6 years; P = .98). Colonoscopy during the follow-up period for carriers and noncarriers is shown in Appendix Table A1 (online only).
EC Risk Subcohort
This female subcohort included 215 carriers and 523 noncarriers from 229 families with MMR gene mutations (Table 1). Over a median follow-up of 5 years, six carriers were diagnosed with EC at a median age of 53 years (incidence rate, 5.66; 95% CI, 2.54 to 12.59 per 1,000 person-years). The corresponding SIR was 30.62 (95% CI, 11.24 to 66.64; P < .001) for carriers of any MMR gene mutation (Table 2). EC was diagnosed in two MLH1 mutation carriers (SIR, 27.18; 95% CI, 6.80 to 108.66) and four MSH2 mutation carriers (SIR, 44.92; 95% CI, 16.86 to 119.68). Cumulative risks were estimated to be 2.84 (95% CI, 1.06 to 7.46) at 5 years and 9.84 (95% CI, 3.45 to 26.33) at 10 years of prospective follow-up (Table 3). No EC was observed in noncarriers.
Other Cancer Risk Subcohort
This subcohort included 446 carriers and 1,029 noncarriers from 300 families with MMR gene mutations (Table 1). Over a median follow-up of 5 years, for carriers, we observed three ovarian cancers (SIR, 18.81; 95% CI, 3.88 to 54.95; P < .001), three renal cancers (two kidney cancers and one renal pelvis cancer; SIR, 11.22; 95% CI, 2.31 to 32.79; P < .001), two pancreatic cancers (SIR, 10.68; 95% CI, 2.68 to 47.70; P = .001), two gastric cancers (SIR, 9.78; 95% CI, 1.18 to 35.30; P = .009), two urinary bladder cancers (SIR, 9.51; 95% CI, 1.15 to 34.37; P = .009), seven female breast cancers (SIR, 3.95; 95% CI, 1.59 to 8.13; P = .001), and three prostate cancers (Table 2). Cancers observed in only one carrier each included cancer of the esophagus (ICD-O C15.9), biliary tract (ICD-O C24.0), liver (ICD-O C22.1), adrenal gland (ICD-O C74.9), small intestine (ICD-O C17.9), and ureter (ICD-O C66.9).
For noncarriers, there was no evidence of an increased risk of lung, breast, and prostate cancers. In noncarriers, we also observed one case each of pancreatic (ICD-O C25.9), urinary bladder (ICD-O C67.9), thyroid (ICD-O C73.9), and esophageal (ICD-O C15.9) cancer (Table 2).
DISCUSSION
We confirmed that MMR gene mutation carriers were at increased risk of a wide variety of cancers and found that there was no evidence of an increased risk of cancer for their noncarrier relatives. A major strength of our study is the prospective nature of the design, because observation time for carriers and noncarriers commenced before cancer diagnosis. This design, therefore, avoided ascertainment bias commonly present in retrospective analyses, particularly when estimates of cancer risk are made using relatives who are enrolled in cancer clinics because they have been diagnosed with cancer. A further strength of the prospective analysis is that it provides an estimate, based on empirical data, of future cancer risk at the time they come under clinical care. Our estimated 10-year risk of CRC for carriers with a median age of 49 years (8.05%; 95% CI, 4.46% to 14.29%) is comparable to our previous estimate (using an ascertainment-corrected retrospective analysis) of the 10-year risk for MSH6 mutation carriers at age 50 years (6% [95% CI, 3% to 9%] for men and 3% [95% CI, 1% to 5%] for women).27 It seems to be lower than a previous retrospective estimate of the 10-year estimates for MLH1 and MSH2 carriers (26% for men and 13% for women).28
An unanticipated finding of our study is the confirmation of an increased risk for cancers of the breast and pancreas for MMR gene mutation carriers. Although risk of pancreatic cancer has been investigated in Lynch syndrome,29–33 the evidence was inconsistent. The study showing evidence for an association was that of Kastrinos et al,34 which recently showed an increased risk of pancreatic cancer in Lynch syndrome (hazard ratio, 8.6; 95% CI, 4.5 to 15.7). This result is consistent with our finding (test for difference, P = .79). However, given the limited evidence for efficacy of screening for pancreatic cancers,35 expert opinion has not yet advocated pancreatic cancer screening in the context of Lynch syndrome.
A more controversial association with Lynch syndrome is that of breast cancer. Initially raised by Lynch et al36 several decades ago, the issue of breast cancer risk in Lynch syndrome has been debated with evidence for and against this association. Multiple case reports describing MMR-deficient breast cancers, including cases of male breast cancer, have been published.37–41 Walsh et al40 showed that about half of breast cancers observed in MMR gene mutation carries have loss of expression in the tumor of the gene that is mutated in the germline. However, this and other studies were not able to address whether the MMR deficiency caused breast cancer or was a phenotype of a breast cancer caused by another factor. In this report, we observed an increased risk of breast cancer for MMR gene mutation carriers followed prospectively. Although the numbers are small, the risk was estimated to be increased four-fold above that of the general population, suggesting that MMR mutation carriers may benefit from enhanced screening. Further clarification of age-specific magnitude of risk is needed to determine whether ages at screening or methods such as use of magnetic resonance imaging should be modified in Lynch syndrome.
In addition to defining spectrum and cancer risks for MMR gene mutation carriers, it is important to clarify the risk of CRC and other cancers for noncarrier relatives. Identification of mutation noncarriers is considered to be one of the major benefits of MMR gene testing, providing reassurance that these people are at substantially lower risk than their mutation-carrying relatives and, therefore, not subject to the same costly, frequent, and invasive screening and risk-reductionstrategies. Because no cancer arises, to our knowledge, as a result of a single gene mutation, even highly penetrant disorders such as Lynch syndrome might arise on the background of multiple other genetic variants that modify cancer risks. If that is the case, then relatives who do not carry the family-specific pathogenic MMR mutation may still have a cancer risk that is elevated compared with the general population. In this study, we found no evidence of an increased risk of cancers for the noncarrier relatives, whereas carriers of an MMR gene mutation from the same families were at increased risk of a wide variety of cancers. In a similar scenario, it has been observed that there is no evidence of increased risk of breast cancer42,43 or ovarian cancer44 for noncarriers from families carrying mutations of BRCA1 or BRCA2.
Estimating penetrance for CRC is particularly challenging in the high-risk family setting because surveillance colonoscopy is likely to be offered to those with a strong family history of CRC or if Lynch syndrome has been diagnosed in a relative. The colonoscopy likely changes the natural history of the disease by removing the precursor lesion at least some of the time. We observed that those who were mutation carriers (although they may not have known about their mutation status at the time) were having frequent colonoscopies (approximately once every 3 years) yet still manifested an appreciable high risk for development of CRC. In contrast, those who were not mutation carriers (although they also may not have known of their mutation status) were also having colonoscopies almost as frequently as the carriers (approximately once every 4 years). Therefore, we cannot dismiss the possibility that noncarriers who are relatives of mutation carriers are at increased risk of CRC, but their frequent colonoscopies may have reduced their risk.
We also found no evidence of increased risk of extracolonic cancers for the noncarriers. It is unlikely that this finding can be attributed to increased screening for these cancers because, unlike CRC, screening does not alter the risk for extracolonic cancers. For example, screening for urothelial, endometrial, and ovarian cancers does not reduce the risk of these diseases, because it is done for early detection only and not for removal of precursor lesions, and therefore, screening would not influence the estimates of these cancer risks.
There are some limitations to our study, most notably the small number of events and a short follow-up duration. We do not have sufficient power to exclude a two-fold increased risk of CRC for the noncarriers. Another possible limitation is that censoring each individual at the age of polypectomy could have resulted in underestimating the true cancer risk.
In conclusion, our prospective analysis provides unbiased estimates of cancer risks for MMR gene mutation carriers, including significantly increased risks of recognized Lynch syndrome–associated cancers (colorectal, endometrial, ovarian, renal, gastric, and urinary bladder) and of breast cancer and pancreatic cancer. For the noncarrier relatives of family-specific MMR gene mutations, we found no evidence that their risks of CRC or other cancers exceed the population risks.
Acknowledgment
Supported by the National Cancer Institute, National Institutes of Health under Grant No. RFA-CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and Principal Investigators. A.K.W. is supported by a grant from the Cancer Council Victoria and the Picchi Brothers Foundation, Australia. J.L.H. is a National Health and Medical Research Council (NHMRC) Australia Fellow, and M.C.S. is an NHMRC Senior Research Fellow.
Collaborating centers include the Australasian Colorectal Cancer Family Registry (Grant No. U01 CA097735), Familial Colorectal Neoplasia Collaborative Group (Grant No. U01 CA074799) [University of Southern California], Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (Grant No. U01 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (Grant No. U01 CA074783), Seattle Colorectal Cancer Family Registry (Grant No. U01 CA074794), and University of Hawaii Colorectal Cancer Family Registry (Grant No. U01 CA074806). We thank all study participants of the Colon Cancer Family Registry and staff for their contributions to this project.
Appendix
Table A1.
Colonoscopy During the Follow-Up Period for Carriers and Noncarriers of a Mismatch Repair Gene Mutation Involved in This Study
| Colonoscopy During Follow-Up | Carriers | Noncarriers | ||||||
|---|---|---|---|---|---|---|---|---|
| CRC Present | CRC Absent | CRC Present | CRC Absent | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Colonoscopy | ||||||||
    Yes Yes | 9 | 90 | 149 | 76 | 3 | 75 | 224 | 43 |
    No No | 1 | 10 | 48 | 24 | 1 | 25 | 293 | 57 |
    Unknown Unknown | 6 | 152 | 1 | 381 | ||||
| Total | 16 | 349 | 5 | 898 | ||||
Abbreviation: CRC, colorectal cancer.
Footnotes
The content of this article does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Cancer Family Registries (CFRs); in addition, mention of trade names, commercial products, or organizations does not imply endorsement by the US Government or the CFR. Authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Aung Ko Win, Mark A. Jenkins
Provision of study materials or patients: Joanne P. Young, Noralane M. Lindor, Katherine M. Tucker, Dennis J. Ahnen, Graeme P. Young, Daniel D. Buchanan, Graham G. Giles, Ingrid Winship, Finlay A. Macrae, Jack Goldblatt, Melissa C. Southey, Julie Arnold, Stephen N. Thibodeau, Bharati Bapat, John A. Baron, Graham Casey, Steven Gallinger, Loïc Le Marchand, Robert W. Haile, John L. Hopper,Mark A. Jenkins
Collection and assembly of data: Aung Ko Win, Noralane M. Lindor, Katherine M. Tucker, Daniel D. Buchanan, Melissa C. Southey, Shanaka R. Gunawardena, Mark A. Jenkins
Data analysis and interpretation: Aung Ko Win, Joanne P. Young, Noralane M. Lindor, Katherine M. Tucker, Dennis J. Ahnen, Graeme P. Young, Daniel D. Buchanan, Mark Clendenning, Graham G. Giles, Ingrid Winship, Finlay A. Macrae, Jack Goldblatt, Melissa C. Southey, Julie Arnold, Stephen N. Thibodeau, Bharati Bapat, John A. Baron, Graham Casey, Steven Gallinger, Loïc Le Marchand, Polly A. Newcomb, Robert W. Haile, John L. Hopper, Mark A. Jenkins
Manuscript writing: All authors
Final approval of manuscript: All authors
Affiliations:
Aung Ko Win, Graham G. Giles, Ingrid Winship, Melissa C. Southey, John L. Hopper, and Mark A. Jenkins, The University of Melbourne; Ingrid Winship and Finlay A. Macrae, The Royal Melbourne Hospital, Parkville; Graham G. Giles, Cancer Epidemiology Centre, Cancer Council Victoria, Carlton, Victoria; Joanne P. Young, Daniel D. Buchanan, and Mark Clendenning, Queensland Institute of Medical Research; Joanne P. Young, School of Medicine, University of Queensland, Herston, Queensland; Katherine M. Tucker, Hereditary Cancer Clinic, Prince of Wales Hospital, Randwick, New South Wales; Graeme P. Young, Flinders Centre for Cancer Prevention and Control, Flinders University, Adelaide, South Australia; Jack Goldblatt, Genetic Services of Western Australia and School of Paediatrics and Child Health, University of Western Australia, Perth, Australia; Julie Arnold, New Zealand Familial Gastrointestinal Cancer Registry, Auckland City Hospital, Auckland, New Zealand; Bharati Bapat and Steven Gallinger, Samuel Lunenfeld Research Institute, Mount Sinai Hospital; Bharati Bapat, University of Toronto; Steven Gallinger, Cancer Care Ontario, Toronto, Ontario, Canada; Noralane M. Lindor, Stephen N. Thibodeau, and Shanaka R. Gunawardena, Mayo Clinic, Rochester, MN; Dennis J. Ahnen, Denver Veterans Affairs Medical Center, School of Medicine, University of Colorado, Denver, CO; John A. Baron, University of North Carolina, Chapel Hill, NC; Graham Casey and Robert W. Haile, University of Southern California, Los Angeles, CA; Loïc Le Marchand, University of Hawaii Cancer Center, Honolulu, HI; and Polly A. Newcomb, Fred Hutchinson Cancer Research Center, Seattle, WA.
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2011.39.5590
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3341109?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1200/jco.2011.39.5590
Article citations
The intersection of homologous recombination (HR) and mismatch repair (MMR) pathways in DNA repair-defective tumors.
NPJ Precis Oncol, 8(1):190, 05 Sep 2024
Cited by: 0 articles | PMID: 39237751 | PMCID: PMC11377838
Review Free full text in Europe PMC
Bile Acids in Pancreatic Carcinogenesis.
Metabolites, 14(7):348, 21 Jun 2024
Cited by: 0 articles | PMID: 39057671 | PMCID: PMC11278541
Review Free full text in Europe PMC
Modifiable risk factors for cancer among people with lynch syndrome: an international, cross-sectional survey.
Hered Cancer Clin Pract, 22(1):10, 14 Jun 2024
Cited by: 0 articles | PMID: 38877502 | PMCID: PMC11177364
Understanding the Genetic Landscape of Pancreatic Ductal Adenocarcinoma to Support Personalized Medicine: A Systematic Review.
Cancers (Basel), 16(1):56, 21 Dec 2023
Cited by: 2 articles | PMID: 38201484 | PMCID: PMC10778202
Review Free full text in Europe PMC
Neoplasia risk in patients with Lynch syndrome treated with immune checkpoint blockade.
Nat Med, 29(10):2458-2463, 16 Oct 2023
Cited by: 3 articles | PMID: 37845474 | PMCID: PMC10870255
Go to all (181) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome.
J Natl Cancer Inst, 104(18):1363-1372, 28 Aug 2012
Cited by: 116 articles | PMID: 22933731 | PMCID: PMC3529597
Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome.
J Natl Cancer Inst, 105(4):274-279, 05 Feb 2013
Cited by: 53 articles | PMID: 23385444 | PMCID: PMC3576323
Body mass index in early adulthood and colorectal cancer risk for carriers and non-carriers of germline mutations in DNA mismatch repair genes.
Br J Cancer, 105(1):162-169, 10 May 2011
Cited by: 37 articles | PMID: 21559014 | PMCID: PMC3137400
Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.
Eur J Cancer Prev, 23(4):246-257, 01 Jul 2014
Cited by: 44 articles | PMID: 24614649
Review
Funding
Funders who supported this work.
NCI NIH HHS (8)
Grant ID: U01 CA074794
Grant ID: U01 CA097735
Grant ID: U01 CA074783
Grant ID: P30 CA071789
Grant ID: U01 CA074799
Grant ID: P30 CA015704
Grant ID: U01 CA074800
Grant ID: U01 CA074806