Abstract
Free full text

Improving Outcomes for Pulmonary Vascular Disease
Associated Data
Abstract
Recognizing the importance of improving lung health through lung disease research, the National Heart, Lung, and Blood Institute (NHLBI) convened a workshop of multidisciplinary experts for the following purpose: (1) to review the current scientific knowledge underlying the basis for treatment of adults and children with pulmonary vascular diseases (PVDs); (2) to identify gaps, barriers, and emerging scientific opportunities in translational PVD research and the means to capitalize on these opportunities; (3) to prioritize new research directions that would be expected to affect the clinical course of PVDs; and (4) to make recommendations to the NHLBI on how to fill identified gaps in adult and pediatric PVD clinical research. Workshop participants reviewed experiences from previous PVD clinical trials and ongoing clinical research networks with other lung disorders, including acute respiratory distress syndrome, chronic obstructive lung disease, and idiopathic pulmonary fibrosis, as well. Bioinformatics experts discussed strategies for applying cutting-edge health information technology to clinical studies. Participants in the workshop considered approaches in the following broad concept areas: (1) improved phenotyping to identify potential subjects for appropriate PVD clinical studies; (2) identification of potential new end points for assessing key outcomes and developing better-designed PVD clinical trials; and (3) the establishment of priorities for specific clinical research needed to advance care of patients with various subsets of PVDs from childhood through adulthood. This report provides a summary of the objectives and recommendations to the NHLBI concentrating on clinical research efforts that are needed to better diagnose and treat PVDs.
Background
Participants agreed that PVD encompasses a wide variety of disorders with different diagnostic criteria, treatment, and prognosis. The underlying PVD may differ substantially for the various types of clinical pulmonary hypertension (PH). Precise phenotyping of PVD is essential for understanding disease pathogenesis, natural history, and response to therapy, and is essential for designing high-quality studies. The most recent World Health Organization (WHO) clinical classification of PH (Table 1) categorizes a spectrum of conditions all defined clinically by a pulmonary hypertensive state (1). The Dana Point classification provides a helpful format for understanding diverse causes of PH, but this classification does not adequately reflect differences in the causes of PH in newborns, infants, and children, and it does not acknowledge the potential importance of developmental mechanisms in pediatric disease. In addition, PVDs may occur in the absence of PH, which is not addressed in the current classification. Recently, a new classification system for understanding pediatric pulmonary vascular disorders has been proposed as a complement to the Dana Point scheme (Table 2) (2). Although still in its early stages and likely to undergo further revision with time, the recent pediatric classification system provides an initial framework to highlight unique aspects of pediatric PVDs. A detailed review of the diagnosis and treatment strategies currently in place for all categories and classifications of adult and pediatric PVDs is beyond the scope of this workshop report.
TABLE 1.
DANA POINT 2008 CLASSIFICATION
| 1. Pulmonary arterial hypertension (PAH) |
| 1.1. Idiopathic (IPAH) |
| 1.2. Familial (FPAH) |
| 1.3. Associated with (APAH): |
| 1.3.1. Connective tissue disorder |
| 1.3.2. Congenital systemic-to-pulmonary shunts |
| 1.3.3. Portal hypertension |
| 1.3.4. HIV infection |
| 1.3.5. Drugs and toxins |
| 1.3.6. Other (thyroid disorders, glycogen storage disease, Gaucher disease, hereditary hemorrhagic telangiectasia, hemoglobinopathies, chronic myeloproliferative disorders, splenectomy) |
| 1.4. Associated with significant venous or capillary involvement |
| 1.4.1. Pulmonary veno-occlusive disease (PVOD) |
| 1.4.2. Pulmonary capillary hemangiomatosis (PCH) |
| 1.5. Persistent pulmonary hypertension of the newborn |
| 2. Pulmonary hypertension with left heart disease |
| 2.1. Left-sided atrial or ventricular heart disease |
| 2.2. Left-sided valvular heart disease |
| 3. Pulmonary hypertension associated with lung diseases and/or hypoxemia |
| 3.1. Chronic obstructive pulmonary disease |
| 3.2. Interstitial lung disease |
| 3.3. Sleep disordered breathing |
| 3.4. Alveolar hypoventilation disorders |
| 3.5. Chronic exposure to high altitude |
| 3.6. Developmental abnormalities |
| 4. Pulmonary hypertension due to chronic thrombotic and/or embolic disease (CTEPH) |
| 4.1. Thromboembolic obstruction of proximal pulmonary arteries |
| 4.2. Thromboembolic obstruction of distal pulmonary arteries |
| 4.3. Nonthrombotic pulmonary embolism (tumor, parasites, foreign material) |
| 5. Miscellaneous (sarcoidosis, histiocytosis X, lymphangiomatosis, compression of pulmonary vessels [lsqb]adenopathy, tumor, fibrosing mediastinitis[rsqb]) |
TABLE 2.
PEDIATRIC PHVD CLASSIFICATION
| 1. Prenatal or developmental PHVD |
| 2. Perinatal pulmonary vascular maladaptation |
| 3. Cardiovascular disease |
| 4. Bronchopulmonary dysplasia |
| 5. Isolated PHVD |
| 6. Multifactorial PHVD in congenital malformation syndromes |
| 7. Lung disease |
| 8. Thromboembolic disease |
| 9. Hypobaric hypoxia exposure |
| 10. PHVD with systemic disorders |
PHVD indicates pulmonary hypertensive vascular disease.
Pulmonary arterial hypertension (PAH) is a particularly insidious and devastating disorder resulting from elevated pulmonary vascular resistance causing restricted flow through the pulmonary arterial circulation and ultimately death due to right heart failure. It is increasingly recognized that PAH not only involves changes in vascular tone and reactivity, but it is also the result of an imbalance in cell proliferation, cell death, and genetic factors, mechanisms felt to play a large role in the unrestrained growth in cancer.
Recent registry data suggest that the prevalence of PAH may be 15 cases per million (3). Idiopathic PAH has been most intensively studied and has an estimated incidence of 1 to 2 cases per million in the United States. Heritable PAH accounts for 6% of PAH patients without an associated condition, and mutations in bone morphogenic protein receptor 2 and other members of the transforming growth factor β signaling family have been identified in 75% of families with PAH (4). Scleroderma is the most commonly occurring PAH-associated condition with recent epidemiological studies reporting that 10% of scleroderma patients will develop some degree of PH, not all of which is PAH (5).
The natural history of adult idiopathic PAH (IPAH) has been well characterized. The National Institutes of Health registry in the 1980s, before the approval of current PAH therapy, estimated median survival of IPAH adults at 2.8 years, with 1-, 3-, and 5-year survival rates of 68%, 48%, and 34%, respectively (6). During this time and extending into the mid-1990s, care of IPAH and other forms of PAH were largely supportive. Over the past 2 decades, there have been significant advances in the treatment of PAH, for which the vast majority of clinical trials in PH have been conducted. Available therapies for PAH in the United States now consist of prostanoids, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors. Continuous intravenous epoprostenol is the only therapy for IPAH shown to prolong survival. Basic research on the cellular and molecular mechanisms underlying pulmonary vascular tone regulation and abnormal vasoconstriction has been instrumental in identifying the therapeutic potential of PAH drugs now in practice. Many of these agents likely modulate vascular growth and structure and vascular tone, as well. With these pharmacological therapies, the prognosis of adult IPAH is improving, with an 85% survival rate at 1 year (7). Predictors of poor clinical prognosis have also become better appreciated, but the problems of diagnosing, sustaining a treatment benefit, and ultimately curing IPAH remain unsolved. A number of critical concept questions also remain poorly understood, including “What are the cells of origin”? “What are the initiating events in the disease process”? ”Why are the small pulmonary arteries primarily affected”? and “What are the genetic influences in the majority of PAH patients”?
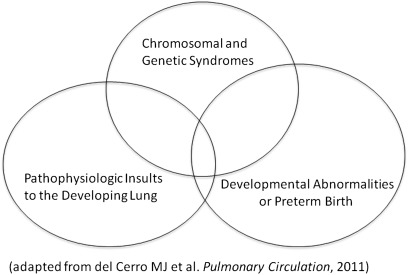
PH and PVDs contribute significantly to the high mortality and morbidity in children and adults, as well. The true incidence and prevalence of PVDs in children is not known, but current estimates suggest a prevalence of 3.7 to 5.0 cases per million children (8, 9). Many disorders associated with PVDs in children are not well reported. Despite similarities with the disorders listed in the Dana Point Classification, many causes of PVD are distinctly different in children versus adults (Table 3). In children, PVD is strongly associated with IPAH, congenital heart disease, and a number of complicated childhood diseases, such as bronchopulmonary dysplasia, congenital diaphragmatic hernia, persistent PH of the newborn, developmental lung diseases, genetic syndromes, and others that are particularly unique to children. Multifactorial conditions likely contribute to many pediatric cases of PVD (Figure 1). With the exception of inhaled nitric oxide therapy for persistent PH of the newborn, specific therapies approved for childhood PVDs are rare, and, as in adults, current strategies are rarely curative. Many pediatric patients are treated with off-label medications based on adult studies, because data from randomized controlled trials in pediatric PVDs are extremely limited. Currently, more studies in children are clearly needed to inform treatment decisions on safety and efficacy. Safety considerations are paramount, given the potential for long-term effects that might impact growing children.
TABLE 3.
UNIQUE FEATURES OF PEDIATRIC PULMONARY HYPERTENSION
| •Developmental biology of the cardiopulmonary system |
| •Timing of vascular injury during susceptible periods of growth and adaptation |
| •Impact of lung vascular disease beyond PH alone (eg, distal lung growth in BPD, congenital diaphragmatic hernia, lung hypoplasia, congenital heart disease) |
| •Differences in genetics, maturational changes in vascular function and growth, and responsiveness to therapeutic strategies |
| •Developmental differences in pharmacokinetics and pharmacodynamics, potential adverse effects |
| •Importance of “preventive” strategies and approaches that target “reverse remodeling,” as well |
| •Maturational changes in right and left ventricular function |
PH indicates pulmonary hypertension; BPD, bronchopulmonary dysplasia.
Summary Of Workshop Discussions
Phenotyping in PVDs
The lack of sufficient phenotyping was identified as a significant gap in conducting clinical research in adult and pediatric patients with PVDs. Even the relatively well-studied population of patients with PAH (WHO group I) remains incompletely phenotyped, because each subgroup likely includes significant diversity with regard to cause and response to therapy. Clinical trials in PAH patients have traditionally included a heterogeneous group of patients, and the results of therapeutic trials likely reflect this. Few data are available to help identify specific subpopulations of patients who are most likely to respond to any specific therapy. Identification of “responder phenotypes” to specific therapies will allow clinicians to use treatments with greater success in subsequently identified patients with similar phenotypes. Because premortem histopathology of the pulmonary vasculature is rarely available in adult patients, accurate clinical and molecular phenotyping is of great importance if treatments in this field are to further improve. In addition, precise phenotyping would lead to more powerful mechanistic studies. Several biomarkers have been proposed as predictors of disease severity such as brain natriuretic peptide (10). However, whether their use translates to clinical benefit for patients is still unclear. There is also increasing evidence that inflammation plays an important role in PAH, but current inflammatory markers have not yet been able to characterize disease severity or risk for clinical progression. In addition, current biomarkers are not specific for the pulmonary vasculature. Although mutations in members of the transforming growth factor β signaling family have been identified in a small percentage of PAH patients, it is clear that additional genetic and/or environmental factors are needed to develop disease. The relevant genetic/environmental interactions are unknown, and few studies have addressed the role of epigenetic mechanisms on PAH. In addition, there is scarce information on the genetic influences leading to the development of PH in patients with scleroderma and other associated conditions.
With regard to the other WHO groups of PH, even more basic phenotypic data are lacking. For example, WHO groups 2 and 3 include patients with “out-of-proportion” PH, but there is no clear definition of what an expected level of pulmonary artery pressures would be in these cases. Why some patients develop greater PH, and how this affects outcome, is unknown, and limited studies have been performed in these groups of PVD patients. Group 4 patients, those with chronic thromboembolic pulmonary hypertension, were previously divided into either “proximal (operable)” or “distal (inoperable)” disease. However, this schema was removed in the Dana Point classification because there is no agreement on the definition of “operable” versus “inoperable” disease, and this definition varies from center to center. Identification of genotypes and biomarkers in patients with proximal disease would be of great benefit in determining a more uniform classification and differentiating “operable” from “inoperable” disease. Finally, group 5 patients, comprising a miscellaneous group of patients with PH, are the least well characterized and require more basic studies to help in phenotype identification.
Unique aspects of pediatric PVD phenotyping.
Workshop participants recognized that phenotyping problems are further exacerbated by the differences between the current knowledge bases of adult- and pediatric-onset disease. Pediatric PVD has been largely understudied, and little is known about the natural history and mechanisms of disease.
Pediatric PVDs and the development of PH are primarily linked to issues of lung vascular growth and development, including diverse prenatal and early postnatal influences. The development of PH in the neonate and young infant is often related to impaired functional and structural adaptation of the pulmonary circulation during transition from fetal to postnatal life, which can be disrupted by adverse stimuli, such as hyperoxia, hypoxia, hemodynamic stress, inflammation, and others (Figure 2).
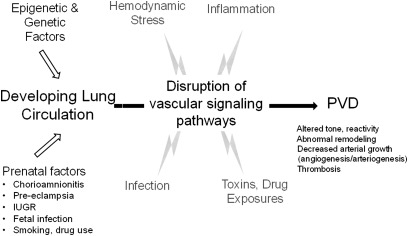
Pulmonary vascular disease may result from epigenetic and genetic factors, prenatal exposures, or postnatal insults that produce sustained alterations of vascular signaling pathways in children and adults. PVD indicates pulmonary vascular disease; IUGR, intrauterine growth retardation.
Recent studies have shown that disruption of lung vascular growth can impair distal airspace structure during development and that decreased angiogenesis contributes to the pathobiology of diverse lung diseases. However, the ability to accurately define a clinical phenotype based on this growing understanding is lacking. Similarly, as observed in airways disease, decreased lung vascular growth during development may persist into adult life and may potentially contribute to PH disease that becomes symptomatic during adulthood (Figure 2). How perinatal events may contribute to adult disease with regard to the pulmonary circulation has been understudied. In addition, current therapeutic strategies for adult pulmonary hypertension have not been sufficiently studied in children with regard to optimal dosing and efficacy and potential short- and long-term toxicities, as well. Such studies will be even more difficult without improving our understanding of pediatric PVD phenotypes.
Phenotyping Research Priorities.
Adult PVD Research
Identify high-fidelity phenotypes of PVDs and the right ventricular (RV) response to PH to enhance mechanistic studies and clinical care. More precise phenotyping would make study data and samples more homogenous, leading to greater ability to detect genetic and other risk factors, especially with the limited numbers of available patients. In particular, this could be accomplished by using physiological, genomic, and proteomic studies to identify specific phenotypes of PVDs, and using “deep phenotyping” (e.g., classification of patients into subsets defined by similar clinical characteristics) and systems biology approaches. For example, identification of patients with an “RV failure phenotype” before initiating therapy would allow for more timely adjustments of therapy, enrollment in clinical trials of salvage therapies, or timely referral for lung transplantation in appropriate patients.
Identify better and more specific biomarkers reflecting PVD as part of improved molecular phenotyping with the use of clinical biosamples and/or scientific biorepositories.
Determine whether patients with exercise-induced pulmonary hypertension constitute a unique group and if this is a precursor of resting PVD.
Determine more accurately “out of proportion” PH in WHO groups 2 and 3.
Predict the development of PH in at-risk populations based on phenotype (e.g., sleep apnea, obesity hypoventilation syndrome).
Apply novel phenotyping approaches to:
Use informatics, including using information from institutional and publically available databases, to help combine various patient databases
Develop personal patient applications to better involve them with clinical research
Use bioengineering/modeling techniques
Incorporate measurements of pharmacokinetic data
Better understand the hemodynamics of PVD, e.g., measurements of impedance of the pulmonary vasculature as a phenotype
Use MRI to better understand RV function and additional techniques aimed at understanding RV function should be investigated.
Pediatric PVD Research
Better establish the longitudinal course and natural history of at-risk pediatric populations (phenotypes), including premature infants, developmental lung diseases, Down syndrome, obstructive sleep apnea, sickle cell disease, complex congenital heart disease, chronic lung diseases, including cystic fibrosis, and others
Develop clinically applicable technologies to assess pulmonary vascular growth and surface area in neonates, infants, and children, through the use of novel imaging strategies in addition to improved echocardiographic approaches
Identify responders and nonresponders, predictors of disease progression or stabilization, and related questions regarding the:
Evaluation and modification of classification systems of pediatric pulmonary hypertension that will enhance clinical research and care;
Utilization of biorepositories and novel genetic, proteomic, and imaging strategies to augment clinical characterization of each patient population.
Evaluation of novel phenotyping, approaches that may include physiological (pulmonary vascular resistance index, exercise, vasoreactivity, lung vascular growth, right heart function), molecular, genetic, and biochemical assessments
Assessments of dosing with pharmacokinetic studies, response to therapy, and develop unique risk/benefit assessments of pulmonary hypertension therapy in infants and young children.
Identifying Novel End Points for Conducting Clinical Trials
Workshop participants agreed that current end points used in PVD clinical trials are not ideal. Surrogate end points have not been validated against clinically important outcomes, and intermediate and composite end points are not established. Mortality is the ultimate end point, but this is not a useful end point for phase II or most phase III studies of investigational therapies. Nearly all studies in PAH have compared changes in exercise capacity (assessed in most studies by measurement of 6-minute walk distance), as the primary end point. However, placebo-controlled studies using “untreated” patient cohorts are no longer possible in PAH therapy trials, and patients being enrolled in studies are often less ill, thereby making improvement in walk distance a more difficult meaningful end point to obtain. In the setting of other therapies, placebo-controlled studies of new treatments may result in smaller effect estimates, requiring larger numbers of subjects. Improved end points are also needed if therapeutic clinical trials are to be expanded beyond WHO group I patients.
There is also a need to develop better end points for assessing disease course and response to therapy in diverse settings that are age appropriate. Such end points should be clinically relevant and include feasible markers that are sufficiently sensitive to changes in clinical course. In young children, there is clearly a lack of applicability of New York Heart Association/WHO functional class, 6-minute walk distance, quality of life, and formal exercise testing, and there is a need to modify the definition of “time to clinical worsening” as applicable to pediatrics. Emerging evidence suggests the use of pulmonary vascular resistance index as a potential end point for children who cannot perform exercise testing or 6-minute walk distance, but more long-term studies to test this concept are needed.
Studies need specific designs to validate surrogate end points (11). True validation of surrogates can only come from randomized clinical trials, so that trials need to incorporate these investigational end points to make advances. Many candidate end points with potential utility were discussed, recognizing that some end points might be appropriate for longitudinal study application to be used in studies with children and adults, whereas others may remain specific for either adult or pediatric PVD clinical research. Approaches that allow for earlier and more sensitive assessments of disease and its progression across all ages, better standardization of clinical assessments, including exercise testing, and understanding how disease comorbidities impact outcomes, were broad priorities. Specific priorities include the following:
Adult PVD Research
Defining surrogate end points (e.g., measurement of end-tidal CO2 changes is easy to perform and may be a surrogate measure of improved vascular function, which could be used in children and adults, as well).
Measurement of circulating protein markers or cells
Identification of circulating biomarkers specific for PVD
Further investigation of autonomic function
Increased and/or innovative use of lung and cardiac imaging (MRI/magnetic resonance angiography/echocardiography)
Serial measurements of pulmonary vascular impedance
Pediatric PVD Research
Novel application of noninvasive studies including:
Establish pediatric-specific developmentally appropriate end points for clinical care and research to determine clinical worsening, need for additional therapy, and response to therapy
Echocardiography to assess vascular impedance, RV function, and estimates of pulmonary vascular resistance index
Radiological imaging approaches via MRI or other strategies
Assessments of distal lung vascular growth and associated changes in alveolarization, such as studies of lung diffusion capacity in infants and young children
Molecular or cell biomarkers that reflect disease activity and provide insights into disease pathobiology, which may have even stronger links with known developmental pathways of the lung vasculature than in adults
Testing of surrogate markers of survival such as ventricular physiology and ventricular-vascular coupling.
“Next-Generation” Clinical Research
End points in past drug trials have largely been limited to short-term effects on 6-minute walk distance and related outcomes. Workshop participants recognized the limitations of such assessments, and generally favored conducting longer studies that extend beyond at least 6 mo to years to establish sustained benefit or possibly the ability to reverse the disease process. In addition, participants agreed strongly that there should be genetic and biomarker components to all clinical studies, especially with multicenter PVD studies. Additional general considerations for pediatric studies include incorporating trial components that assess lung vascular growth, alveolar growth, and lung surface area.
Trials and cohort studies of high priority include the following:
Adult PVD Research
Test whether PAH patients with less severe disease have better long-term outcomes with initial aggressive therapy that includes intravenous prostanoids followed by subsequent transition to less intense therapy;
Evaluate the effect of exercise training on PAH clinical status and disease progression;
Understand the pathophysiology and clinical implications of exercise-induced PH;
Evaluate treatments for use in patients with acute RV failure or chronic RV dysfunction failing currently available pulmonary vascular therapy;
Evaluate the efficacy of warfarin versus platelet inhibitors, e.g., aspirin, in PAH;
Perform phase I and II studies of emerging novel therapeutics, beyond traditional pharmaceutical-initiated studies;
Perform cohort studies of PH and PVD linked with other common conditions: e.g., heart failure with preserved ejection fraction, portal hypertension, and renal failure incorporating a stratification arm of high versus low transpulmonary pressure assessment;
Examine the role of obesity, the metabolic syndrome, and sleep apnea with PVDs and RV dysfunction
Pediatric PVD Research
Examine the pharmacology of pulmonary hypertension therapies in developmentally different pediatric populations such as neonates, infants, and children, in whom risk-benefit profiles may differ
Investigate whether there are unique adverse effects of PH drugs on organ development (e.g., brain, eyes, kidney) or lung structure/function. This will require long-term follow-up studies
Develop prospective collection of placenta and cord blood samples to link basic disease mechanisms with clinical course and outcomes, in “high-risk” population cohorts, especially in preterm infants
Support prospective studies of populations “at risk” to develop PH, such as preterm newborns; infants from pregnancies complicated by preeclampsia, chorioamnionitis, and drug exposures; congenital diaphragmatic hernia; congenital heart disease, Down syndrome; developmental lung diseases and others
Develop longitudinal analyses to determine links between perinatal factors and the development of adult-onset PH and to better assess similarities and differences in PVDs and clinical courses between adult and pediatric patients
Compare relative efficacy between current therapies or combination therapies
Conduct pilot studies of novel interventions based on preclinical studies, e.g., progenitor cell therapies
Use bioinformatics approaches to query existing databases to understand symptoms and studies used to diagnose PVDs and response to therapy
Conclusions
Workshop participants identified the importance of advancing PVD research priority areas through the use of collaborative and consortium-based approaches. Workshop participants suggested that establishing research networks for pediatric and adult PH populations would likely lead to improved phenotyping strategies for clinical research in PVDs, development and incorporation of biorelevant end points in clinical trials, and conducting trials that have high priority for the NHLBI mission. Multicenter PVD programs could better define the natural history and clinical course of at-risk patient populations (Figure 3). The group also strongly supported the goal of enhancing interactions between the adult and pediatric scientific and clinical communities, which would greatly strengthen our understanding of the pathobiology and treatment of PVDs. Encouragement was also given to the larger PH research community to use investigator-initiated applications for clinical trials and leverage ongoing NHLBI support mechanisms.
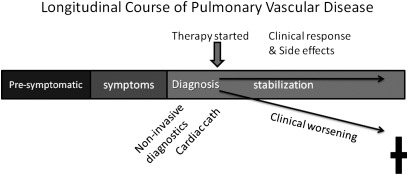
Clinical research designed to understand the longitudinal course of pulmonary vascular disease development will identify disorders and abnormalities contributing to pulmonary vascular dysfunction. Better diagnostic criteria and phenotyping will result, ultimately leading to better therapeutic responses. Understanding the presymptomatic phase of pulmonary vascular disease might also identify interventions to prevent disease onset, especially in the pediatric population.
Acknowledgments
Workshop Participants:Ian Adatia, M.B.Ch.B. (Edmonton, AB, Canada), Eric Austin, M.D., (Nashville, TN), Raouf Amin, M.D. (Cincinnati, OH), Raymond Benza, M.D. (Pittsburgh, PA), Atul Butte, M.D., Ph.D. (Stanford, CA), Wendy Chung, M.D., Ph.D. (New York, NY), James Cimino, M.D. (Bethesda, MD), Gail Deutsch, M.D. (Seattle, WA), Raed Dweik, M.D. (Cleveland, OH), Jonas Ellenberg (Philadelphia, PA), Allen Everett, M.D. (Baltimore, MD), Karen Fagan (Mobile, AL), Jeff Feinstein, M.D., M.P.H. (Stanford, CA), Teri Franks, M.D. (Silver Spring, MD), Victor Gordeuk, M.D. (Washington, DC), Mardi Gomberg-Maitland, M.D. (Chicago, IL), Hakon Hakonarson M.D., Ph.D. (Philadelphia, PA), Dunbar Ivy, M.D. (Aurora, CO), Steve Kawut, M.D. (Philadelphia, PA), Roberto Machado, M.D. (Chicago, IL), Kenneth Mandl, M.D., M.P.H. (Boston, MA), Michael Matthay, M.D. (San Francisco, CA), Fernando Martinez, M.D. (Ann Arbor, MI), Sharon McGrath-Morrow, M.D. (Baltimore, MD), Steve Nathan, M.D. (Falls Church, VA), Lawrence Nogee, MD (Baltimore, MD), Myung Park, M.D. (Baltimore, MD), Robin Shandas, Ph.D. (Aurora, CO), Virend Somers, MD PhD (Rochester, MN), and Duncan Stewart, M.D. (Ottawa, ON, Canada).
NHLBI Staff: Dorothy Gail, Ph.D., Gail Weinmann, M.D., and James Kiley, Ph.D.
Footnotes
This Workshop was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health.
This article is being co-published in the May 1, 2012 issue of Circulation.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
Articles from American Journal of Respiratory and Critical Care Medicine are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1164/rccm.201201-0049ws
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3359939?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1164/rccm.201201-0049ws
Article citations
Prognostic and diagnostic utility of interleukin-6 in pediatric pulmonary arterial hypertension - a case-control study.
Eur J Pediatr, 183(4):1637-1643, 09 Jan 2024
Cited by: 0 articles | PMID: 38193997 | PMCID: PMC11001729
Clinical Characteristics and Transplant-Free Survival Across the Spectrum of Pulmonary Vascular Disease.
J Am Coll Cardiol, 80(7):697-718, 01 Aug 2022
Cited by: 25 articles | PMID: 35953136 | PMCID: PMC9897285
Research priority setting in childhood chronic disease: a systematic review.
Arch Dis Child, 103(10):942-951, 11 Apr 2018
Cited by: 25 articles | PMID: 29643102
Review
CT measurements of central pulmonary vasculature as predictors of severe exacerbation in COPD.
Medicine (Baltimore), 97(3):e9542, 01 Jan 2018
Cited by: 4 articles | PMID: 29504975 | PMCID: PMC5779744
Accelerometry: Improving Objective Assessments of Therapeutic Impact in Pediatric Pulmonary Arterial Hypertension.
Am J Respir Crit Care Med, 196(2):127-129, 01 Jul 2017
Cited by: 0 articles | PMID: 28707968 | PMCID: PMC5519973
Go to all (18) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhancing Insights into Pulmonary Vascular Disease through a Precision Medicine Approach. A Joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop Report.
Am J Respir Crit Care Med, 195(12):1661-1670, 01 Jun 2017
Cited by: 48 articles | PMID: 28430547 | PMCID: PMC5476915
Cell therapy for lung diseases. Report from an NIH-NHLBI workshop, November 13-14, 2012.
Am J Respir Crit Care Med, 188(3):370-375, 01 Aug 2013
Cited by: 22 articles | PMID: 23713908 | PMCID: PMC3778734
Review Free full text in Europe PMC
Accelerating Scientific Advancement for Pediatric Rare Lung Disease Research. Report from a National Institutes of Health-NHLBI Workshop, September 3 and 4, 2015.
Ann Am Thorac Soc, 13(12):385-393, 01 Dec 2016
Cited by: 7 articles | PMID: 27925785 | PMCID: PMC5291498
Strategic plan for pediatric respiratory diseases research: an NHLBI working group report.
Pediatr Pulmonol, 44(1):2-13, 01 Jan 2009
Cited by: 8 articles | PMID: 19086051 | PMCID: PMC2778243
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R56 HL068702
Grant ID: R01 HL068702
 2
2