Abstract
Purpose
The purpose of this study was to determine whether diabetes type 2 causes thinning of retinal layers as a sign of neurodegeneration and to investigate the possible relationship between this thinning and duration of diabetes mellitus, diabetic retinopathy (DR) status, age, sex, and glycemic control (HbA1c).Methods
Mean layer thickness was calculated for retinal layers following automated segmentation of spectral domain optical coherence tomography images of diabetic patients with no or minimal DR and compared with controls. To determine the relationship between layer thickness and diabetes duration, DR status, age, sex, and HbA1c, a multiple linear regression analysis was used.Results
In the pericentral area of the macula, the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL) were thinner in patients with minimal DR compared to controls (respective difference 1.9 μm, 95% confidence interval [CI] 0.3-3.5 μm; 5.2 μm, 95% CI 1.0-9.3 μm; 4.5 μm, 95% CI 2.2-6.7 μm). In the peripheral area of the macula, the RNFL and IPL were thinner in patients with minimal DR compared to controls (respective difference 3.2 μm, 95% CI 0.1-6.4 μm; 3.3 μm, 95% CI 1.2-5.4 μm). Multiple linear regression analysis showed DR status to be the only significant explanatory variable (R = 0.31, P = 0.03) for this retinal thinning.Conclusions
This study demonstrated thinner inner retinal layers in the macula of type 2 diabetic patients with minimal DR than in controls. These results support the concept that early DR includes a neurodegenerative component.Free full text

Early Neurodegeneration in the Retina of Type 2 Diabetic Patients
Abstract
Purpose.
The purpose of this study was to determine whether diabetes type 2 causes thinning of retinal layers as a sign of neurodegeneration and to investigate the possible relationship between this thinning and duration of diabetes mellitus, diabetic retinopathy (DR) status, age, sex, and glycemic control (HbA1c).
Methods.
Mean layer thickness was calculated for retinal layers following automated segmentation of spectral domain optical coherence tomography images of diabetic patients with no or minimal DR and compared with controls. To determine the relationship between layer thickness and diabetes duration, DR status, age, sex, and HbA1c, a multiple linear regression analysis was used.
Results.
In the pericentral area of the macula, the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL) were thinner in patients with minimal DR compared to controls (respective difference 1.9 μm, 95% confidence interval [CI] 0.3–3.5 μm; 5.2 μm, 95% CI 1.0–9.3 μm; 4.5 μm, 95% CI 2.2–6.7 μm). In the peripheral area of the macula, the RNFL and IPL were thinner in patients with minimal DR compared to controls (respective difference 3.2 μm, 95% CI 0.1–6.4 μm; 3.3 μm, 95% CI 1.2–5.4 μm). Multiple linear regression analysis showed DR status to be the only significant explanatory variable (R = 0.31, P = 0.03) for this retinal thinning.
Conclusions.
This study demonstrated thinner inner retinal layers in the macula of type 2 diabetic patients with minimal DR than in controls. These results support the concept that early DR includes a neurodegenerative component.
Introduction
Diabetic retinopathy (DR) is commonly viewed as a microvascular complication of diabetes mellitus. In addition to vascular changes, structural neurodegenerative changes such as neural apoptosis, loss of ganglion cell bodies, glial reactivity, and reduction in thickness of the inner retinal layers have been described in the earliest stages of DR. This loss of neural tissue agrees with previous functional studies showing neuroretinal deficits in patients with diabetes including electroretinogram abnormalities, loss of dark adaptation and contrast sensitivity, color vision disturbances, and abnormal microperimetry.1–7 The introduction of optical coherence tomography (OCT) has allowed imaging and measuring of retinal thickness (RT) with high accuracy, and several groups have been able to show that total RT is decreased in diabetic patients with no or minimal DR compared to normal controls.8–14 The high resolution of spectral domain OCT (SD-OCT) allows measurement of the thickness of all individual retinal layers after automated three-dimensional segmentation.15,16 The layers that can be identified have been interpreted as follows (from the inner to outer surface): retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL) + inner segment photoreceptors (IS), outer segment photoreceptors (OS), and retinal pigment epithelium (RPE). A recent study by this group has shown that the decreased total RT that manifests in type 1 diabetic patients with minimal DR is caused by retinal neuropathy characterized by thinning of the GCL in the pericentral area and RNFL in the peripheral area of the macula.17 The difference in disease mechanism and management of patients with type 1 and type 2 diabetes mellitus (DM) may also result in differential development of neuropathy in these patients.18 The purpose of the present study was to determine whether type 2 diabetes, like type 1, causes thinning of individual retinal layers while correcting for age, sex, duration of DM, DR status, and glycosylated serum hemoglobin (HbA1c).
Materials and Methods
Participants
Patients were recruited from the outpatient clinic of the Department of Ophthalmology at the Academic Medical Center (University Hospital, Amsterdam, The Netherlands). Inclusion criteria were type 2 diabetes and no or minimal DR as evaluated by a retinal specialist through indirect fundoscopy and slit-lamp stereo biomicroscopy. Minimal DR was defined as microaneurysms only, conforming to stage 2 of the International Clinical Diabetic Retinopathy Disease Severity Scale.19 Exclusion criteria were refractive error of more than SE +5 or SE −8 diopters in at least one eye; visual acuity below 20/25; significant media opacity; or a history of glaucoma, uveitis, or retinal disease. Age- and sex-matched subjects free of ocular disease, diabetes, hypertension, or other systemic diseases were recruited as controls from among those who accompanied patients visiting the outpatient clinic. All subjects underwent pupillary dilation and an ophthalmologic examination, including slit-lamp biomicroscopy with a handheld lens (SuperField; Volk Optical, Inc., Mentor, OH) and OCT imaging (3D OCT-1000; Topcon Corporation, Tokyo, Japan). Age, sex, duration of diabetes, and HbA1c were obtained from the patient charts. The mean HbA1c was calculated from all available HbA1c measurements in the year preceding the study visit. The study adhered to the tenets of the Declaration of Helsinki. Investigative Review Board approval was obtained at both the Academic Medical Center and the University of Iowa, and all participants gave written informed consent.
Optical Coherence Tomography Imaging and Layer Segmentation
OCT images of the subjects were obtained with SD-OCT using the 3-D volume scan protocol (6 × 6 × 2.2 mm3). Nine intraretinal surfaces were segmented automatically for all subjects using the authors' algorithm, which entails a fully three-dimensional graph search approach.15,16 The algorithm had previously shown excellent reproducibility of intraretinal layer thickness.20 The identified layers between the intraretinal surfaces were interpreted as follows: A, retinal nerve fiber layer (RNFL); B, ganglion cell layer (GCL); C, inner plexiform layer (IPL); D, inner nuclear layer (INL); E, outer plexiform layer (OPL), F, outer nuclear layer (ONL) + inner segments (photoreceptors) (IS); G, outer segments (photoreceptors) (OS); H, retinal pigment epithelium (RPE). One of the authors (HvD), masked to the demographic characteristics and retinopathy status of each subject, marked two retinal areas centered on the fovea: the pericentral area, with an inner diameter of 1 mm and an outer diameter of 3 mm; and the peripheral area, with an inner diameter of 3 mm and outer diameter of 6 mm. The mean thickness of each of the layers in the pericentral and peripheral areas was calculated automatically using ImageJ 1.41.21
Statistical Analysis
Statistical analyses were performed with SPSS 16.0.2 for Windows (SPSS, Chicago, IL). Analysis of variance (ANOVA) was used to assess differences in mean age between diabetic patients with no and minimal DR and controls. Mean HbA1c and duration of diabetes were compared using the unpaired t-test between patients with no and minimal DR.
Mean layer thicknesses of diabetic patients with minimal DR, diabetic patients without DR, and controls were compared using ANOVA, followed by a Bonferroni post hoc analysis to correct for multiple comparisons. Correlation analysis between individual retinal layers was performed by calculating the Pearson correlation coefficient. A multiple linear regression model was used to determine the relationship between inner retinal layer thickness and the duration of DM, DR status, age, sex, and HbA1c in the diabetic patients.
Results
Sixty-four subjects with type 2 DM were included. Thirty-nine patients had no DR, and 25 patients showed minimal DR. Diabetes duration was longer and HbA1c higher in the patients with minimal DR as compared to the patients without DR. Fifty-seven controls were included. There was no significant difference in age and sex among patient groups and controls (see Table 1).
Table 1.
Demographics of Patients with Type 2 Diabetes and No or Minimal Diabetic Retinopathy (DR) and Controls
Parameters | No DR (N= 39) | Minimal DR (N= 25) | Controls (N= 57) |
| Age (years) | 56 ± 9 | 59 ± 6 | 58 ± 12 |
| Sex (M:F) | 18:21 | 10:15 | 23:34 |
| Duration DM (years) | 8 ± 7 | 16 ± 8 | NA |
| HbA1c (%) | 7.6 ± 1.0 | 7.9 ± 0.8 | - |
Values are the mean ± standard deviation for all subjects in each group. NA, not applicable; -, not performed.
The absolute values and differences in retinal layer thickness (μm) between patients with type 2 DM and no or minimal DR as compared to normal controls in the pericentral and peripheral area of the macula are given in Tables 2 and and3.3. In the pericentral area of the macula, the RNFL, GCL, and IPL were thinner in patients with minimal DR compared to controls (respective difference RNFL: 1.9 μm, 95% CI 0.3–3.5 μm; GCL: 5.2 μm, 95% CI 1.0–9.3 μm; IPL: 4.5 μm, 95% CI 2.2–6.7 μm). In the peripheral area of the macula, the RNFL and IPL were thinner in patients with minimal DR compared to controls (respective difference RNFL: 3.2 μm, 95% CI 0.1–6.4 μm; IPL: 3.3 μm, 95% CI 1.2–5.4 μm). The other retinal layers did not show a significant difference in layer thickness. Patients with diabetes but no DR showed no significant difference in any layer thickness in both areas compared to normal controls.
Table 2.
Mean Layer Thickness Measurements (μm) of the Individual Intraretinal Layers in the Pericentral Area of the Macula in Patients with Type 2 Diabetes with No or Minimal DR Compared to Controls
Parameters | No DR (N= 39) | Mean Difference | 95% CI of the Difference | Minimal DR (N= 25) | Mean Difference | 95% CI of the Difference | Controls (N= 57) |
| RNFL | 22.3 ± 2.3 | 0.9 | −0.5–2.3 | 21.3 ± 3.0 | 1.9 | 0.3–3.5 | 23.2 ± 3.0 |
| GCL | 46.9 ± 6.4 | 2.1 | −1.6–5.7 | 43.8 ± 7.8 | 5.2 | 1.0–9.3 | 49.0 ± 7.5 |
| IPL | 38.3 ± 3.9 | 1.5 | −0.5–3.5 | 35.3 ± 3.3 | 4.5 | 2.2–6.7 | 39.8 ± 4.2 |
| INL | 38.2 ± 3.3 | 1.4 | −3.4–3.2 | 38.6 ± 3.6 | 1.0 | −1.0–3.1 | 39.6 ± 3.8 |
| OPL | 28.3 ± 2.9 | 0.7 | −1.0–2.3 | 28.1 ± 3.0 | 0.9 | −1.0–2.7 | 29.0 ± 3.5 |
| ONL + IS | 90.6 ± 9.3 | 3.1 | −1.3–7.5 | 89.3 ± 9.0 | 4.4 | −0.7–9.4 | 93.7 ± 8.2 |
| OS | 26.3 ± 2.2 | 0.8 | −0.3–1.9 | 26.9 ± 2.4 | 0.2 | −1.0–1.5 | 27.1 ± 2.1 |
| RPE | 39.3 ± 2.2 | −0.1 | −1.2–1.1 | 39.3 ± 2.3 | −0.1 | −1.4–1.3 | 39.2 ± 2.4 |
Values are the mean ± standard deviation for all subjects in each group. The bold values indicate a statistically significant difference between patients and controls.
Table 3.
Mean Layer Thickness Measurements (μm) of the Individual Intraretinal Layers in the Peripheral Area of the Macula in Patients with Type 2 Diabetes with No or Minimal DR Compared to Controls
Parameters | No DR (N= 39) | Mean Difference | 95% CI of the Difference | Minimal DR (N= 25) | Mean Difference | 95% CI of the Difference | Controls (N= 57) |
| RNFL | 34.4 ± 4.2 | 2.2 | −0.6–5.0 | 33.4 ± 5.8 | 3.2 | 0.1–6.4 | 36.6 ± 5.6 |
| GCL | 28.9 ± 2.7 | −0.3 | −2.3–1.7 | 27.0 ± 4.8 | 1.6 | −0.5–3.9 | 28.6 ± 3.7 |
| IPL | 34.4 ± 3.5 | 0.2 | −1.7–2.0 | 31.3 ± 3.2 | 3.3 | 1.2–5.4 | 34.6 ± 3.6 |
| INL | 29.7 ± 1.9 | 0.4 | −1.0–1.8 | 28.7 ± 2.5 | 1.4 | −0.1–3.0 | 30.1 ± 2.9 |
| OPL | 25.0 ± 1.5 | 0.4 | −0.6–1.4 | 24.4 ± 2.0 | 1.0 | −0.1–2.2 | 25.4 ± 2.1 |
| ONL + IS | 74.6 ± 7.1 | 1.3 | −2.2–4.8 | 73.2 ± 5.8 | 2.7 | −1.1–6.6 | 75.9 ± 6.2 |
| OS | 23.5 ± 1.9 | 0.3 | −0.7–1.1 | 23.7 ± 2.2 | 0.1 | −1.1–1.3 | 23.8 ± 2.0 |
| RPE | 39.3 ± 1.7 | 0.0 | −1.0–1.0 | 39.5 ± 2.0 | −0.2 | −1.4–0.9 | 39.3 ± 1.9 |
Values are the mean ± standard deviation for all subjects in each group. The bold values indicate a statistically significant difference between patients and controls.
A multivariable regression analysis including diabetes duration, DR status, age, sex, and HbA1c showed that DR status was the only significant explanatory variable (R =
= 0.31, P
0.31, P =
= 0.03) for the thinning of the inner retinal layers (RNFL, GCL, IPL) in both the pericentral and the peripheral area of the macula.
0.03) for the thinning of the inner retinal layers (RNFL, GCL, IPL) in both the pericentral and the peripheral area of the macula.
Discussion
The results of this study demonstrated that the inner retinal layers—RNFL, GCL, and IPL—in the macula were thinner in patients with minimal DR compared to controls in an unselected population of patients with type 2 diabetes. In multiple linear regression analysis, DR status—that is, the presence of minimal DR—was the only significant explanatory variable for this retinal thinning, with age, sex, duration of DM, and HbA1c corrected for.
A previous study using identical methods, showed selective loss of thickness of the GCL in the pericentral area and corresponding loss of RNFL thickness in the peripheral area of the macula in type 1 diabetic patients with minimal DR compared to normal controls.17 The changes in retinal thickness were comparable in type 1 and 2 diabetes (Figs. 1, ,2).2). This finding is not self-evident, as there are obvious pathophysiological differences between these two types of diabetes. Type 1 diabetic patients have lower plasma insulin concentrations compared to hyperinsulinemic type 2 patients. In the retina, insulin action stimulates neuronal development, differentiation, growth, and survival. Retinal apoptosis can be reversed by systemic insulin therapy, and insulin provides trophic support for retinal neurons.22–24
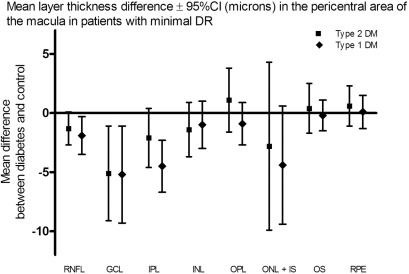
Mean layer thickness difference ± 95%CI (microns) in the pericentral area of the macula in patients with minimal DR. DR, diabetic retinopathy; CI, confidence interval; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium.
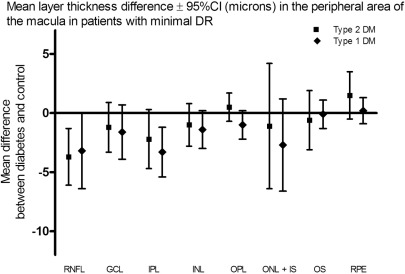
Mean layer thickness difference ± 95%CI (microns) in the peripheral area of the macula in patients with minimal DR. DR, diabetic retinopathy; CI, confidence interval; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium.
In the previous study with type 1 diabetic patients, there was a significant linear correlation (R = 0.53, P value < 0.01) between GCL thickness and the duration of diabetes.17 In patients with type 2 DM, glucose metabolism can be disturbed years before the diagnosis is made. The duration of the disease process is therefore uncertain in patients with type 2 diabetes, and the assessment of a possible correlation between thinning of the inner retinal layers and duration of disease is therefore less precise.
The present study had some limitations. The grading of the severity of DR was done by a single reader through ophthalmologic examination, including indirect fundoscopy and slit-lamp stereo biomicroscopy, instead of the gold standard, namely, seven-field stereoscopic fundus photography assessment by independent trained graders.25 However, the patients certainly did not have advanced retinopathy, indicating that the results do apply to the earliest stage of DR.
Type 2 diabetes is often subclinical. It cannot be excluded that some of the controls also had diabetes type 2, because the HbA1c of the controls was not known. The presence of undiagnosed diabetes would most likely lead to underestimation of the difference in retinal layer thickness between patients and controls instead of overestimation.
In a previous study, the present authors' algorithm showed an excellent reproducibility of intraretinal layer thickness. After segmentation of intraretinal layers from repeated OCT scans, the overall mean regional thickness difference was 1.16 ± 0.84 μm.18 This indicates that the resulting mean retinal layer thickness differences in the RNFL, GCL, and IPL between patients and controls in this study exceeded expected differences in intrasubject repeat automated layer thickness measurements. In individual patients, however, repeated retinal layer thickness measurements may not be suitable for detecting small differences in the inner retinal layers. Future studies need to address whether prolonged follow-up measurements of individual patients can show statistically significant thinning over time due to neurodegeneration.
The findings in this study provide further evidence for a neurodegenerative component in early DR. Previous studies have demonstrated neurodegenerative changes including neural apoptosis, loss of ganglion cell bodies, glial reactivity, and reduction in thickness of the inner retinal layers in DR.1,3–14,17 These findings of structural neuropathy may explain the neuroretinal functional deficits that are known in patients with diabetes.1–7,11 The relationship of this retinal loss to other neural damage in diabetes, such as white matter loss, cognitive decline, peripheral neuropathy, and diabetic autonomic neuropathy, remains to be determined and is the subject of future studies.
In summary, this study provided proof of concept that the inner retinal layers in the pericentral and peripheral area of the macula in type 2 diabetic patients with minimal DR are thinner compared to normal controls. These results support the concept that early DR includes a neurodegenerative component.
Footnotes
Supported by Netherlands Organization for Health Research and Development; National Institutes of Health Grants R01-EY017066, EY018853, and EB004640; Research to Prevent Blindness; and The Edward en Marianne Blaauwfonds.
Disclosure: H.W. van Dijk, None; F.D. Verbraak, None; P.H.B. Kok, None; M. Stehouwer, None; M.K. Garvin, None; M. Sonka, None; J.H. DeVries, None; R.O. Schlingemann, None; M.D. Abràmoff, P
References
Articles from Investigative Ophthalmology & Visual Science are provided here courtesy of Association for Research in Vision and Ophthalmology
Full text links
Read article at publisher's site: https://doi.org/10.1167/iovs.11-8997
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3366721?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1167/iovs.11-8997
Article citations
The Role of Immune Cells and Signaling Pathways in Diabetic Eye Disease: A Comprehensive Review.
Biomedicines, 12(10):2346, 15 Oct 2024
Cited by: 0 articles | PMID: 39457658 | PMCID: PMC11505591
Review Free full text in Europe PMC
Evaluation of thickness of individual macular retinal layers in diabetic eyes from optical coherence tomography.
Sci Rep, 14(1):17909, 02 Aug 2024
Cited by: 0 articles | PMID: 39095380 | PMCID: PMC11297304
Advances in Structural and Functional Retinal Imaging and Biomarkers for Early Detection of Diabetic Retinopathy.
Biomedicines, 12(7):1405, 25 Jun 2024
Cited by: 0 articles | PMID: 39061979 | PMCID: PMC11274328
Review Free full text in Europe PMC
Associations of retinal neurovascular dysfunction with inner retinal layer thickness in non-proliferative diabetic retinopathy.
Graefes Arch Clin Exp Ophthalmol, 15 Jun 2024
Cited by: 0 articles | PMID: 38878068
Changes in foveal avascular zone parameters in individuals with prediabetes compared to normoglycemic controls: a systematic review and meta-analysis.
Eye (Lond), 38(10):1855-1860, 08 Apr 2024
Cited by: 0 articles | PMID: 38589460
Review
Go to all (182) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes.
Invest Ophthalmol Vis Sci, 51(7):3660-3665, 03 Feb 2010
Cited by: 175 articles | PMID: 20130282 | PMCID: PMC2904016
Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy.
Invest Ophthalmol Vis Sci, 50(7):3404-3409, 17 Jan 2009
Cited by: 193 articles | PMID: 19151397 | PMCID: PMC2937215
The spatial relation of diabetic retinal neurodegeneration with diabetic retinopathy.
PLoS One, 15(4):e0231552, 16 Apr 2020
Cited by: 9 articles | PMID: 32298369 | PMCID: PMC7161968
Vascular Changes and Neurodegeneration in the Early Stages of Diabetic Retinopathy: Which Comes First?
Ophthalmic Res, 56(1):1-9, 02 Apr 2016
Cited by: 53 articles | PMID: 27035578
Review
Funding
Funders who supported this work.
NEI NIH HHS (8)
Grant ID: R01 EY019112-03
Grant ID: R01 EY017066
Grant ID: EY018853
Grant ID: R01-EY017066
Grant ID: R01 EY018853
Grant ID: R01 EY019112
Grant ID: R01 EY019112-01A2
Grant ID: R01 EY019112-02
NIBIB NIH HHS (2)
Grant ID: R01 EB004640
Grant ID: EB004640





