Abstract
Free full text

Fas Ligand Enhances Malignant Behavior of Tumor Cells through Interaction with Met, Hepatocyte Growth Factor Receptor, in Lipid Rafts*
Abstract
Many late-stage cancer cells express Fas ligand (FasL) and show high malignancy with metastatic potential. We report here a novel signaling mechanism for FasL that hijacks the Met signal pathway to promote tumor metastasis. FasL-expressing human tumor cells express a significant amount of phosphorylated Met. The down-regulation of FasL in these cells led to decreased Met activity and reduced cell motility. Ectopic expression of human FasL in NIH3T3 cells significantly stimulated their migration and invasion. The inhibition of Met and Stat3 activities reverted the FasL-associated phenotype. Notably, FasL variants activated the Met pathway, even though most of their intracellular domain or Fas binding sites were deleted. FasL interacted with Met through the FasL(105–130) extracellular region in lipid rafts, which consequently led to Met activation. Knocking down Met gene expression by RNAi technology reverted the FasL-associated motility to basal levels. Furthermore, treatment with synthetic peptides corresponding to FasL(117–126) significantly reduced the FasL/Met interaction, Met phosphorylation, and cell motility of FasL+ transfectants and tumor cells. Finally, the transfectants of truncated FasL showed strong anchorage-independent growth and lung metastasis potential in null mice. Collectively, our results establish the FasL-Met-Stat3 signaling pathway and explains the metastatic phenotype of FasL-expressing tumors.
Introduction
Fas ligand (FasL, CD95L, CD178)2 is a 40-kDa type II transmembrane protein belonging to the tumor necrosis factor (TNF) family. It is expressed on a variety of cells including activated T cells, natural killer cells, tumor cells, and cells of immune-privileged sites (1, 2). When FasL binds to Fas, the authentic receptor for FasL, on the target cells, it activates the Fas-associated death program under physiological conditions (3–5). In addition to initiating the Fas signal, FasL can also transmit a costimulatory signal in the reverse direction. For instance, whereas CD4+ T cells show reduced cell cycle progression and undergo apoptosis upon Fas/FasL engagement, CD8+ T cells require the reverse signal of FasL for rapid proliferation and maturation (6, 7).
The FasL cytoplasmic region is the longest one in the TNF family and highly conserved across species (8, 9). It contains a casein kinase I binding motif (amino acids 17–21 (-SSASS-)) and a proline-rich domain (amino acids 45–70) (10, 11). Upon T cell activation, FasL is recruited into lipid rafts where the serine residue on the casein kinase I motif is phosphorylated. The proline-rich domain of FasL binds to Src homology 3 domain-containing proteins, such as Fyn, Grb2, and PI3K, and subsequently enhances the phosphorylation of Akt, Erk1/2, and JNK (12). Besides, FasL can be cleaved by ADAM metallopeptidase domain 10 and signal peptide peptidase-like 2A, to release soluble FasL (sFasL) (13). Soluble FasL is less effective to initiate Fas-mediated apoptosis than its membrane-bound counterpart (14, 15).
FasL overexpression is acquired early during malignant transformation of epithelial cells. FasL-bearing melanoma or hepatoma cells induce apoptosis of T cells in vitro (6, 7). The tumorigenesis of melanoma cells is delayed in Fas-deficient lpr mutant mice, in which FasL-mediated antitumor immunity is missing (6). Among patients with colorectal carcinoma, metastatic tumors express a higher level of FasL than primary tumors (8). In addition, the expression of FasL is positively correlated to lymph node metastasis and increased mortality in breast carcinoma (9). These findings have been interpreted as suggesting a Fas counterattack mechanism for immune erosion in which FasL+ tumor cells actively kill infiltrating immune cells (10). However, conflicting data about the action of FasL exists. Ectopic expression of FasL may trigger profound inflammation resulting in rapid tissue rejection in organ transplantation models (16–18). At the moment, the signal of FasL in tumor cells correlated with the malignant transformation is still undecided.
Regarding the escape of Fas/FasL-mediated cytotocixity, the hepatocyte growth factor (HGF) receptor (Met) has been reported to sequester Fas and thus inhibits Fas activation and apoptosis in hepatocytes (19). Unexpectedly, we found that Met and FasL were co-immunoprecipitated by monoclonal anti-FasL antibody G247-4. Met is a tyrosine kinase receptor expressed by embryo cells, hepatocytes, and cancer cells (20). Met activation strongly promotes metastasis and invasiveness in tumor cells and fibroblasts (21, 22). Moreover, Met has been activated to interact with a variety of membrane proteins in cancer or embryonic cells (20, 23). For example, Met associates with CD44 upon HGF stimulation, leading to MEK and ERK activation and interacts with integrins in the presence or absence of ligand (24, 25). The RET receptor tyrosine kinase promotes Met phosphorylation through a Src-dependent mechanism (26). Given the importance of the Met pathway in tumor metastasis, we hypothesized that Met contributes to FasL-associated tumor malignancy. In this study, we provide evidence to show that FasL interacts with Met to activate the Met signaling pathway.
EXPERIMENTAL PROCEDURES
Cells and Reagents
A human lung adenocarcinoma cell line (A549), a human hepatoma cell line (PLC/PRF/5), and a human glioma cell line (U118MG) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM). Mouse NIH3T3 fibroblasts, kindly provided by Dr. M. D. Lai (National Cheng Kung University, Tainan, Taiwan), were cultured in high-glucose DMEM. FasL was detected either with monoclonal antibody G247-4, recognizing the C-terminal of FasL (BD Biosciences, Pharmingen) or with polyclonal antibody N-20, recognizing the N-terminal of FasL (Santa Cruz Biotechnology, San Diego, CA). ZB4 (Santa Cruz), a Fas-neutralizing antibody, was used to block the Fas signal. Met was inhibited by PHA665752 (Tocris Cookson, Bristol, UK). Stat3 was inhibited by AG490 (Calbiochem, La Jolla, CA). FasL(117–126) short peptides, stsqmhtass, were synthesized by MDBio, Taipei, Taiwan.
FasL Constructs and Transfection
The cDNA clones for human full-length FasL and Fas were obtained from Open Biosystems (Open Biosystems, Huntsville, AL). Truncated human FasL was generated from a full-length FasL cDNA clone by polymerase chain reaction (PCR) using various primer sets (Table 1), subcloned into EGFP-N1 vector, and confirmed by DNA sequencing (MB Mission Biotech, Taipei, Taiwan).
TABLE 1
The primer sets for FasL truncation
| Constructs | Primer sets | Oligonucleotide sequences (5′-3′) |
|---|---|---|
| FasLΔ33 (31) | Forward | ATGACCTCTGTGCCCAGAAGGCC |
| Reverse | CTCTTAGAGCTTATATAAGCCG | |
| FasLΔ70 (31) | Forward | ATGCTGAAGAAGAGAGGGAACCACAGC |
| Reverse | CTCTTAGAGCTTATATAAGCCG | |
| FasL-270 | Forward | ATGCAGCAGCCCTTCAATTACCCA |
| Reverse | TTACTCAAAATTGACCAGAGAGAGCT | |
| FasL-185 | Forward | ATGCAGCAGCCCTTCAATTACCCA |
| Reverse | TTATTCATTGATCACAAGGCCACC | |
| FasL-130 | Forward | ATGCAGCAGCCCTTCAATTACCCA |
| Reverse | TATTTGCTTCTCCAAAGATGATGCTG | |
| FasL-105 | Forward | ATGCAGCAGCCCTTCAATTACCCA |
| Reverse | TTAGAAGAGCTGAAACATCCCCAGGC |
Lentivirus Production and Cell Transduction
To knockdown the expression of FasL and Met, tumor cells were transducted with lentivirus carrying shRNA (RNAi core of Academia Sinica, Taipei, Taiwan). To produce lentivirus, 15 μg of the transfer plasmid, 9 μg of the package plasmid (psPAX2), and 6 μg of the envelope plasmid (pMD2.G) were cotransfected into 293T cells by the calcium phosphate method. After 24 h of transfection, the cells were cultured in 15 ml of fresh serum-free medium for another 48 h. The culture medium with virions was then collected, filtered through a 0.45-μm filter, and centrifuged at 90,000 × g for 90 min. For transduction, tumor cells or NIH3T3 cells were infected for 16 h with 10 μl of virus suspension containing 8 μg/ml of Polybrene (Sigma).
Western Blot Analysis and Immunoprecipitation
Total extracted proteins were heat-denatured with sample buffer containing β-mercaptoethanol, separated in 10% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane. After being blocked with 5% skim milk, the membrane was probed with the primary antibody for targeted proteins followed by horseradish peroxidase-conjugated secondary antibody. Targeted proteins were made visible by fluorography using an enhanced chemiluminescence detection kit (Millipore, Bedford, MA). For immunoprecipitation, 0.5–2 mg of protein lysates were precleared with protein G-agarose beads (Upstate Biotechnology, Lake Placid, NY) and then incubated with FasL-specific antibody and protein G-agarose beads. In the short peptides blocking experiment, transfectants of the cells were treated with short peptides at the indicated concentrations for 2 h. Immunocomplex binding on the beads was eluted and subjected to Western blot analysis for FasL and Met.
Lipid Raft Isolation
Cells were lysed in buffer containing 1 mm Na3VO4 and protease inhibitor mixture (Calbiochem). Equal amounts of lysates were brought to 600 μl with 40% OptiPrep (Sigma) and covered with 2,400 μl of 30% OptiPrep and 600 μl of 5% OptiPrep. Protein lysates were ultracentrifuged at 485,000 × g for 20 h to separate the lipid rafts. 600 μl of each fraction were collected for further analysis.
Colony Formation Assay
A six-well culture plate was coated with 2 ml of medium containing 0.5% Noble-agarose. After the preloaded medium was gelled at room temperature, 5 × 103 cells suspended in 400 μl of medium containing 0.5% Noble-agarose and 10% fetal bovine serum (FBS) were then added. Cell colonies were counted after 2 weeks of culture.
In Vitro Wound Healing Assay and Invasion Assay
About 2 × 105 cells were seeded in 24-well plates and grown to confluence. A wound on the cell monolayer was created by scraping. After the scraped cells were washed away, fresh medium was added. The migration of cells into the denuded areas was monitored at the time described. For invasion assessment, Matrigel containing laminin, collagen IV, and heparin sulfate proteoglycans were thawed on ice and diluted to 1 mg/ml with cold serum-free medium. The membrane filter of the upper chamber of a transwell was coated with 50 μl of the diluted Matrigel and incubated at 37 °C for gelling. About 2 × 105 cells suspended in 200 μl of serum-free medium containing 0.1% bovine serum albumin were added to a well of a 12-well transmigration plate. The lower chamber was loaded with 600 μl of medium containing 10% FBS as the nutritional attractant. After 16 h of incubation, the invading cells attached to the underside of the membrane filter were stained with Giemsa and counted.
Single-cell Motility Assay
About 5 × 104 cells were seeded in a 40-mm culture dish and incubated for 6 h to allow cell attachment. Cell migration was captured every 5 min for up to 6 h using a time-lapse microscope system and Image-Pro ExpressTM (Media Cybernetics, Bethesda, MD). Cell motility was tracked and measured by ImageJ software (NIH). Proliferating cells were excluded from analysis.
Confocal Imaging
Cells grown on coverslips for 24 h were incubated with cholera toxin subunit B-Alexa Fluor 647 (CTxB-Alexa Fluor 647, Molecular Probes, Carlsbad, CA) to stain lipid rafts for 20 min on ice, washed with PBS, and then fixed in 3.7% paraformaldehyde in PBS at room temperature for 20 min. Cells were permeabilized with 0.05% Triton X-100 prepared in PBS and probed with monoclonal anti-FasL antibody G247-4 (BD Biosciences, Pharmingen) and anti-Met (Santa Cruz) antibodies overnight at 4 °C. After being washed with PBS, the cells were further probed with the appropriate Alexa Fluor-conjugated secondary antibodies (Molecular Probes). Nuclei were stained with Hoechst 33342 dye. Images were taken using a FV1000 confocal microscope (Olympus, Tokyo, Japan). Colocalization of Met, FasL, and lipid raft was analyzed using FV10-ASW software (Olympus).
Experimental Lung Metastasis
Eight-week-old BALB/cAnN.Cg-Foxn1nu/CrlNarl nude mice were obtained from the National Laboratory Animal Breeding and Research Center, Tainan, Taiwan, and maintained under specific pathogen-free conditions. All animal experiments were carried out with the approval of the Ethics Committee of the Animal Research Center, National Cheng Kung University. Mice received 5 × 105 cells suspended in 0.1 ml of PBS via tail vein injection. Tumor nodules in the lungs of inoculated mice were counted under a dissecting microscope. The lungs, kidneys, liver, and spleen were removed from tumor-bearing mice, fixed in 10% buffered formalin, and then embedded in paraffin. Tissue sections (5 μm) were placed on poly-l-lysine-coated glass slides, and stained with hematoxylin and eosin. Tumor tissues were captured by a dotSlide-digital virtual microscope system (Olympus).
Flow Cytometry Analysis
Transfectant cells were detached from culture plate with 10 mm EDTA solution, then fixed in 3.7% formaldehyde solution. These cells were stained with anti-FasL antibody (G247-4) for 60 min at room temperature followed by anti-mouse IgG-conjugated Alexa Fluor 488 for another 60 min. The fluorescence intensity was recorded by flow cytometry, and analyzed by WinMDI software.
Membrane Protein Extraction
Membrane proteins were prepared by the centrifugal protein extraction method. Transfectant cells were homogenized by sonication in a buffer containing 150 mm KCl, 1 mm Na3VO4, and a protease inhibitor mixture. The homogenate was centrifuged at 11,000 × g for 30 min at 4 °C. The supernatant was collected and centrifuged at 170,000 × g for 1 h at 4 °C. The pellet was dissolved in KCl buffer and centrifuged again at 170,000 × g for 1 h at 4 °C. The membrane pellet was solubilized in a sample buffer containing 2-mercaptoethanol for Western blotting analysis.
Statistical Analysis
Data are expressed as mean ± S.E. Student's t test was used, with p < 0.05 considered as significant.
RESULTS
A549, PLC/PRF/5, and U118 cells expressed various amounts of FasL and the expressions, both at transcriptional and translational levels, were significantly inhibited by a lentivirus carrying shRNA against FasL (Fig. 1, A and B). Along with knockdown of FasL, the migration ability of transfectants was reduced by 20–70% (Fig. 1C). Notably, the phosphorylation of Met in A549, PLC/PRF/5, and U118 cells was also reduced (Fig. 1B). We further pulled down FasL protein by the monoclonal antibody (G247-4), recognizing its extracellular domain, and unexpectedly found that the Met protein was co-immunoprecipitated with FasL (Fig. 1D).
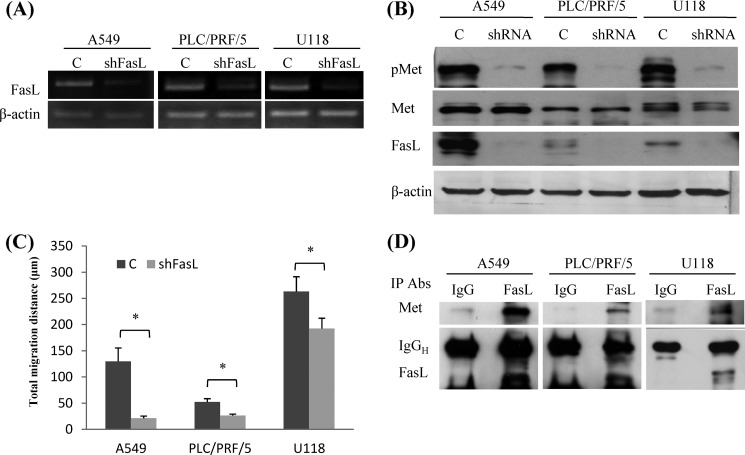
FasL and Met-associated cell motility. A, A549, PLC/PRF/5, and U118 cells were transduced with lentivirus carrying FasL shRNA or control RNA. The FasL transcripts of transduced cells were detected by RT-PCR. B, the phospho-Met (pMet), Met, FasL, and β-actin in transfectants were analyzed by Western blot analysis. C, cell migration was recorded using time-lapse videomicroscopy and the total migration distance in 6 h was calculated by ImageJ software. Shown are the averages (mean ± S.E.) of three independent experiments (*, p < 0.05 compared with N1). D, to detect the Met-FasL complexes of tumor cells, total proteins were extracted and subjected to immunoprecipitation (IP) using monoclonal anti-FasL antibody (G247-4). Mouse IgG was used as the antibody isotype control for the immunoprecipitation assay. FasL and Met proteins in the immunocomplexes were detected by Western blot analysis.
FasL has been shown to transduce a reverse signal in CD8+ T lymphocytes by recruiting effector proteins to its cytoplasmic domain (6, 12). We wondered whether a similar signaling mechanism is used by FasL in tumor cells. To explore possible binding proteins for the FasL cytoplasmic domain, we constructed truncated versions of human FasL, called Δ70 and Δ33 (Fig. 2A). The expression level of full-length FasL in NIH3T3 cells was low, probably due to a degradation process, as reported in a previous study (27). Although high levels of Δ70 and Δ33 variants were detected in NIH3T3 cells (Fig. 2B), they did not bring about the endoplasmic reticulum stress-response protein, Bip. Cell-surface FasL expression was detected on those transfectants by flow cytometry analysis (Fig. 2C). In addition, the growth rate and viability of NIH3T3 cells were not affected by FasL variants, regardless of the length of their intracellular domain (Fig. 2, D and E).

Enhancement of tumorigenicity and invasiveness in NIH3T3 cells by FasL. A, schematic diagram of FasL constructs adapted from Jodo et al. (31). Δ33, deleting the N-terminal 2–33 aa; Δ70, deleting the N-terminal 2–70 amino acids; PRD, proline-rich domain; TM, transmembrane domain; SA, self-assembly domain; THD, TNF family homology domain. NIH3T3 cells were transfected with plasmids encoding various FasL fragments. B, the FasL variants (arrows) and Bip were detected by Western blot analysis. β-Actin served as a protein loading control. Parental NIH3T3 cells treated with tunicamycin at the indicated doses for 24 h served as positive control for ER stress. C, cells were fixed in 3.7% formaldehyde solution. FasL was stained with anti-FasL antibody (G247-4) followed by antibody conjugated with Alexa Fluor-488. The cell population that stained positively for FasL or the negative control are indicated by the gray area or dark black line, respectively. D, cell proliferation was determined at 72 h by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide proliferation assay. E, apoptotic cells were stained by propidium iodine and detected by flow cytometric analysis, appearing as the sub-G0 population. F, polarized cell migration at 10 h was analyzed by a wound healing assay with or without 500 ng/ml of ZB4 antibody. G, cell motility was captured with time-lapse photography and the total migration distance in 6 h was compared with or without ZB4 antibody treatment. H, the number of cells transmigrating across the transwell membrane were counted at 16 h. Shown are the averages (mean ± S.E.) of three independent experiments (*, p < 0.05 compared with N1).
In the wound healing assay (Fig. 2F), transfectants of full-length FasL and Δ33 showed about 85% healing in 10 h. The Δ70 transfectant reached about 60% healing in 10 h, which is slower than full-length FasL and Δ33, but still faster than the control cells (45% healing). Similar to a previous report (28), overexpression of Fas in NIH3T3 cells elevated the wound healing capacity to 60% healing in 10 h. To exclude the potential of the Fas signal being activated by the ectopic expression of FasL, we used the blocking antibody ZB4 to prevent interaction of Fas and FasL. The ZB4 antibody effectively reverted the wound healing capacity of the Fas transfectant from 60 to 45%. However, the elevated wound healing ability of the FasL transfectants was not affected by the ZB4 antibody, indicating a Fas-independent pathway in our study system. We further measured the motility of individual cells by time-lapse videomicroscopy (Fig. 2G). Control NIH3T3 cells moved about 100 μm in 6 h. Transfectants of Fas, full-length FasL, Δ70, and Δ33 moved significantly further (170–250 μm). In addition, treatment with ZB4-neutralized antibody effectively blocked the Fas-enhanced motility, but not the FasL-mediated migration. Similarly, transfectants of full-length FasL, Δ70, and Δ33 showed a higher cell invasion degree in Matrigel than did the control group (Fig. 2H). We have also measured MMP2 and MMP9, but their expressions did not change in cells with different levels of FasL (data not shown).
Collectively, these results reveal that FasL increases the metastatic potential in the absence of a cytoplasmic domain (e.g. Δ70). This finding goes against the conventional receptor-mediated signaling concept, in which a cytoplasmic protein domain is required for adaptor protein recruitment.
As mentioned earlier, while searching for FasL-binding proteins, we found that Met was co-immunoprecipitated with FasL (Fig. 1D). Colocalization of FasL and Met was observed in confocal microscopy images (Fig. 3A). FasL, with or without intracellular domains, not only appeared on the surface membrane but also showed a punctate pattern in the cytoplasm. In control NIH3T3 cells, about 30% of Met was localized in lipid rafts (Fig. 3B, ML/L). Ectopic expression of FasL did not significantly change the distribution of Met. In addition, 30–50% of the total Met was colocalized with FasL (Fig. 3B, MF/M). To verify the interaction of FasL and Met, we investigated their cellular distribution. By fractionation in the density gradient, FasL, full-length or truncated, was present both in lipid rafts (fraction 3) and nonraft regions (fractions 5 and 6). Caveolin-1, a lipid raft marker, was detected only in fraction 3. In agreement with confocal microscopy images, overexpression of FasL variants did not alter the distribution of Met. However, the Met in lipid rafts became highly phosphorylated (Fig. 3C). Furthermore, we pulled down the FasL of transfectants by immunoprecipitation. The amount of Met protein detected in the precipitates was positively correlated with FasL (Fig. 3D). To determine whether lipid rafts are required for the formation of FasL-Met complexes, we extracted cholesterol from the plasma membranes with MβCD to disrupt the integrity of the rafts (29). Treatment with 10 μm MβCD for 1 h had no effect on the expression of Met and FasL, but severely reduced the formation of complexes (Fig. 3D).
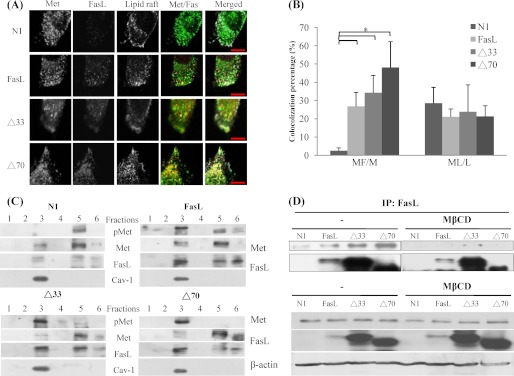
Interaction of FasL and Met in lipid raft. A, cells were grown on coverslides and stained with anti-FasL (Alexa Fluor-488) and anti-Met (Alexa Fluor-594) antibodies. Plasma membrane cholesterol in lipid raft was stained with cholera toxin subunit B conjugated with Alexa Fluor-647. Nuclei were stained with Hoechst 33342 dye. Representative confocal images are shown. Scale bars represent 10 μm. B, pixel-by-pixel colocalization analysis of Met, FasL, and lipid rafts was done using FV10-ASW software. L, M, and F represent the total image pixels of lipid raft, Met, and FasL, respectively. Numerators were the colocalized pixels of two corresponding molecules (*, p < 0.05 compared with N1 control). C, cell extracts were separated in a sucrose density gradient and collected by fractionation. Proteins in the fraction were separated on SDS-PAGE. FasL, phospho-Met (pMet), Met, and caveolin-1 were detected by Western blot analysis. D, to disrupt the integrity of the lipid raft, cells were treated with 10 μm MβCD for 1 h and subjected to immunoprecipitation assay using monoclonal anti-FasL antibody G247-4.
The Met-Stat3 signal axis is essential for the invasive behavior of many tumors (22). Similarly, we found that ectopic expression of FasL increased the phosphorylation of both Met and Stat3 (Fig. 4A). The full-length FasL transfectant showed moderate induction of phospho-Met and phospho-Stat3. When the Met activity was pharmacologically inhibited by PHA665752, both FasL-associated Stat3 phosphorylation and cell migration were decreased (Fig. 4, A and B). In addition, inhibition of Stat3 activity by AG490 treatment completely reverted FasL-induced cell migration to basal levels, just as the Met inhibitor had done (Fig. 4B). Furthermore, we employed the adenovirus-mediated RNA interference technique to knockdown c-Met (Fig. 4C). In parallel with a decrease in Met expression, FasL-enhanced cell motility was diminished (Fig. 4D). These data indicate that Met plays an important role in FasL-mediated motility.

Activation of the Met-Stat3 signaling pathway by FasL. A, cells were grown in 1% FBS/DMEM for 16 h to reduce background phosphorylation. Inhibition of Met activity was done by pretreatment with 100 nm PHA 665752 for 1 h. Phospho-Met (pMet), Met, pStat3, and Stat3 were detected by Western blot analysis. B, single-cell migration was measured as described in the legend to Fig. 1. Cells were pretreated with 100 nm PHA 665752 or 20 μm AG490 for 6 h to inhibit Met or Stat3, respectively. Shown are the averages (mean ± S.E.) obtained from three independent experiments. C, knockdown of Met by lentivirus-shMet. D, transfection of shMet reduced the migration of NIH3T3 transfectants expressing different FasL variants. *, p < 0.05 compared with N1 control; #, p < 0.05 compared with shRNA control group.
To determine the Met binding site of FasL, we constructed FasL mutants encoding various lengths of the extracellular domains; the mutants were named FasL-270, FasL-185, FasL-130, and FasL-105 (Fig. 5A). The antibody-recognizing intracellular domain of FasL (N-20) cross-reacted with unknown proteins in NIH3T3 cells, which made the interpretation of results difficult. We thus expressed the extracellular domain-truncated FasL variants in human MRC5 cells, which is a FasL-negative and Met-positive fibroblast cell line. These FasL variants were well expressed in MRC5 cells, and they were effectively pulled down by the N-20 antibody (Fig. 5B). Met co-immunoprecipitated with FasL-270, FasL-185, and FasL-130. In parallel with the detection of FasL-Met complexes, MRC5 cells expressing FasL-270, FasL-185, and FasL-130 showed higher degrees of Met phosphorylation and migration than control cells (Fig. 5, C and D). The binding of FasL-105 and Met was very weak; FasL-105-expressing cells showed an accordingly low level of Met phosphorylation and slow movement. Based on these results, we concluded that the FasL(105–130) region, in the vicinity of the cytoplasmic membrane, is essential for interaction of Met and FasL. Regarding the lack of any known protein-protein interaction motifs in Δ70, Met is probably autoactivated by the binding of FasL. This is further supported by the results of an inhibition experiment that used a synthetic peptide corresponding to the FasL(117–126) sequence. In addition, the membrane protein was extracted and it was found that the FasL-105 protein was located in the membrane fraction similar to the FasL-270 protein (Fig. 5E). The interaction between FasL/Met was disrupted using the FasL(117–126) short peptide. Under the experimental conditions, the FasL-Met complex in NIH3T3 transfectant cells was reduced to about 30% in the presence of the FasL(117–126) short peptide, as determined in the immunoprecipitation assay (Fig. 5F).

Domain of FasL for Met interaction. A, schematic diagram of FasL constructs deleting various extracellular regions. B, plasmids encoding for FasL-270 (deleting the C-terminal 270–284 amino acids), FasL-185 (deleting the C-terminal 185–284 amino acids), FasL-130 (deleting the C-terminal 130–284 amino acids), or FasL-105 (deleting the C-terminal 105–284 amino acids) were transfected into MRC5 human lung fibroblast cells. FasL of these transfectants were pulled down by anti-FasL antibody (N-20). The FasL and Met in immunocomplexes were detected by Western blot analysis. C, the expression of phospho-Met (pMet), Met, and truncated FasL of these transfectants. D, single cell migration was measured, as described in the legend to Fig. 1. E, detection of FasL variant in the cell membrane or cytoplasm. F, the FasL/Met interaction in NIH3T3 expressing FasLΔ33 cells treated with short peptides was analyzed by immunoprecipitation assay. G and H, to block the FasL and Met interaction, NIH3T3 transfectants and tumor cells were treated with 200 μg/ml of synthesized FasL(117–126) short peptides for 24 h followed by the addition of the same peptides (200 μg/ml) for another 12 h. I, tumor cells were treated with 200 μg/ml of short peptides for 6 h. Single cell migration was measured. Shown are the averages (mean ± S.E.) obtained from three independent experiments (*, p < 0.05 compared with control).
Treatment with FasL(117–126) peptides effectively decreased the levels of phospho-Met and phospho-Stat3 in FasL-expressing NIH3T3 transfectants and human tumor cells (Fig. 5, G and H). In addition, FasL(117–126) peptides effectively reduced tumor cell motility (Fig. 5I).
To evaluate the effect of FasL on anchorage-independent growth, we used a colony formation assay. About 5 × 103 cells of the full-length FasL transfectant formed 40 colonies with an average diameter of 150 μm in soft agar in 2 weeks, which was similar to the behavior exhibited by the control NIH3T3 cells. Notably, Δ33 and Δ70 transfectants showed high anchorage-independent growth; 5 × 103 cells produced about 80 colonies that were 200–230 μm in diameter (Fig. 6, A and B). Correlated with the enhanced anchorage-independent growth of Δ33 and Δ70 transfectants, these cells had an elevated expression of the cyclin D1 protein, which is a downstream target gene after Stat3 activation and mediates anchorage-independent growth (Fig. 6C). We further confirmed the metastasis-promoting effect of FasL in an experimental metastasis animal model. Control NIH3T3 cells spontaneously transform and form small lung tumor nodules when injected intravenously into BALB/c nude mice (30). The full-length FasL did not increase lung tumor formation of NIH3T3 cells in terms of tumor size and number. A dramatic increase in tumor volume and number in the lung was observed for Δ70 and Δ33 transfectants (Fig. 6, D and E).
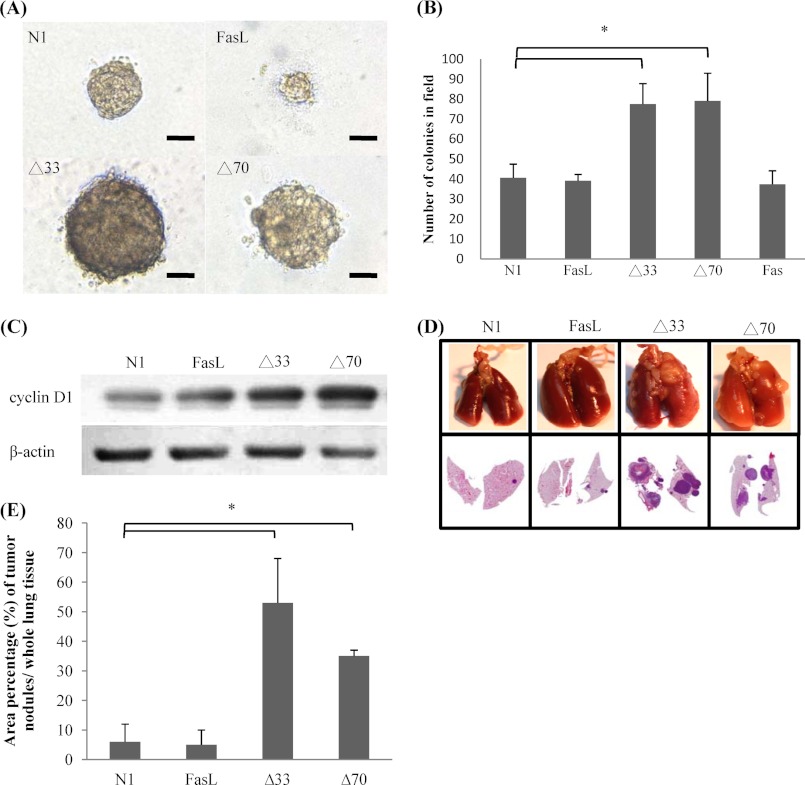
Increases in anchorage-independent growth and lung metastasis by FasL. A, to determine anchorage-independent growth, transfectants were grown in 0.5% Nobel-agarose. Representative images of cell foci formed in 2 weeks are shown. B, the foci with a diameter greater than 200 μm were counted. Shown are the average values (mean ± S.E.) obtained from three independent experiments. C, cyclin D1 expression of transfectants was detected by Western blot analysis. D, about 5 × 105 cells of FasL transfectants were injected into BALB/c nude mice via the tail vein (n = 8). After 4 weeks, tumor nodules in the lung were surgically removed and stained with hematoxylin and eosin. E, the area of tumor nodules on a section was quantified by ImageJ software (*, p < 0.05 compared with N1 control).
DISCUSSION
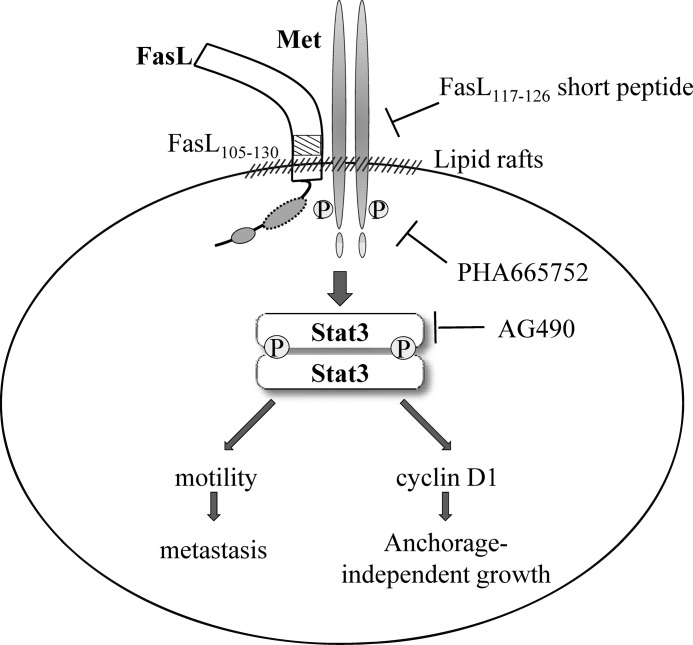
In this study, we describe a unique signaling mechanism for the action of FasL coupled with high tumor grade. According to this mechanism, FasL forms complexes with Met in lipid rafts and thus hijacks the Met signal pathway.
Elevated cell migrations are positively correlated with tumor malignancy. It is known that Fas can promote cell migration through the activation of NF-κB, caspase-8, and ERK1/2 under nonapoptotic conditions (28). We excluded the conventional Fas pathway by interrupting the engagement of Fas and FasL with the blocking antibody ZB4 (Fig. 2E). FasL without a Fas binding site still formed complexes with Met and effectively activated Met pathway in FasL transfectants, which further indicates that the Fas signal is not required under this condition (Fig. 5D). In addition, the Δ70 FasL variant lacks most of the cytoplasmic domain, which precludes a reverse signal, as has been documented in CD8+ T cells that requires the cytoplasmic domain for other protein engagements to enhance phosphorylations of Akt, ERK, JNK, and FasL itself (6, 12). Moreover, the migration-enhancing effect of FasL requires Met and Stat3, which is clearly different from the Fas-counterattack mechanism, the conventional Fas pathway, and the FasL reverse signaling.
In normal tissue, Met is mainly expressed in epithelial and embryonic cells. Although HGF, the ligand for Met, is produced by surrounding mesenchymal cells. Regulating the HGF expression of mesenchymal cells warrants a well controlled migration and proliferation of normal epithelial cells (20). Overexpression of Met has been reported in several tumors and can be activated in the absence of HGF by interacting with a variety of membrane proteins (20, 23). Met associates with CD44 upon HGF stimulation, leading to MEK and ERK activation. Met can interact with integrins in the presence or absence of ligand (24, 25). The RET receptor tyrosine kinase promotes Met phosphorylation through a Src-dependent mechanism (26). Yet, neither of the cases mentioned above can directly activate the authentic Met pathway. As mentioned earlier, Met can bind to the extracellular portion of Fas and act as a potent FasL antagonist and inhibitor of Fas trimerization (19). In this study, we showed that the binding of FasL with Met in lipid rafts via a narrow domain adjacent to the cell membrane activates the Met signaling pathway. Met-knockdown transfectants show only basal cell motility and it cannot be induced by ectopic expression of FasL (Fig. 4D). Furthermore, disrupting the interaction of FasL and Met with a FasL(117–126) short peptide resulted in reduced phosphorylation of Met and impairment of cell migration.
It is worth noting that transfectants of Δ33 and Δ70, but not full-length FasL, developed lung tumor nodules in immune intact mice. This may be due to the different expression levels of FasL variants in cells. Although full-length FasL slightly activated Met, it was probably still below the threshold of Met activity for tumor induction. Alternatively, full-length FasL might transduce an unknown signal to inhibit tumor formation.
In summary, our data indicate that FasL can hijack the Met signaling pathway to confer cells with an invasive phenotype. The activation of Met by FasL deletion mutants without intracellular domains represents a new paradigm for FasL signal transduction. This novel signaling mechanism explains the observation that high-grade FasL-positive tumors show elevated metastasis potential in a Fas-independent pathway (Fig. 7). Our findings suggest that disrupting the FasL and Met interaction is a potential therapeutic target for FasL-positive metastatic tumors.
*This work was supported in part by grants (to B. C. Y) from the National Science Council of Taiwan (NSC 96-2320-B-006-021-MY3) and the Infectious Disease and Signaling Research Center of National Cheng Kung University and Aim for the Top University Project.
2The abbreviations used are:
- FasL
- Fas ligand
- HGF
- hepatocyte growth factor
- MβCD
- methyl-β-cyclodextrin.
REFERENCES
Articles from The Journal of Biological Chemistry are provided here courtesy of American Society for Biochemistry and Molecular Biology
Full text links
Read article at publisher's site: https://doi.org/10.1074/jbc.m111.326058
Read article for free, from open access legal sources, via Unpaywall:
http://www.jbc.org/content/287/24/20664.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
CD95/Fas protects triple negative breast cancer from anti-tumor activity of NK cells.
iScience, 24(11):103348, 29 Oct 2021
Cited by: 10 articles | PMID: 34816102 | PMCID: PMC8593563
Hematological Malignancy-Derived Small Extracellular Vesicles and Tumor Microenvironment: The Art of Turning Foes into Friends.
Cells, 8(5):E511, 27 May 2019
Cited by: 19 articles | PMID: 31137912 | PMCID: PMC6562645
Review Free full text in Europe PMC
Apoptosis of tumor-infiltrating T lymphocytes: a new immune checkpoint mechanism.
Cancer Immunol Immunother, 68(5):835-847, 07 Nov 2018
Cited by: 63 articles | PMID: 30406374 | PMCID: PMC11028327
Review Free full text in Europe PMC
Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma.
Hepatology, 64(5):1637-1651, 23 Sep 2016
Cited by: 66 articles | PMID: 27530187
Regulation of the MET oncogene: molecular mechanisms.
Carcinogenesis, 37(4):345-355, 10 Feb 2016
Cited by: 45 articles | PMID: 26905592
Review
Go to all (12) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Deletion of the ectodomain unleashes the transforming, invasive, and tumorigenic potential of the MET oncogene.
Cancer Sci, 100(4):633-638, 21 Jan 2009
Cited by: 19 articles | PMID: 19175607
HGF/c-met/Stat3 signaling during skin tumor cell invasion: indications for a positive feedback loop.
BMC Cancer, 11:180, 19 May 2011
Cited by: 40 articles | PMID: 21595927 | PMCID: PMC3112164
Activation of the c-Met receptor complex in fibroblasts drives invasive cell behavior by signaling through transcription factor STAT3.
J Cell Biochem, 95(4):805-816, 01 Jul 2005
Cited by: 20 articles | PMID: 15838885
The Fas/FasL Signaling Pathway: Its Role in the Metastatic Process and as a Target for Treating Osteosarcoma Lung Metastases.
Adv Exp Med Biol, 1258:177-187, 01 Jan 2020
Cited by: 9 articles | PMID: 32767242
Review