Abstract
Free full text

Typhoid fever
Abstract
The host restricts dissemination of invasive enteric pathogens, such as non-typhoidal Salmonella serovars, by mounting acute inflammatory responses characterized by the recruitment of neutrophils. However, some enteric pathogens, such as Salmonella enterica serovar Typhi (S. typhi), can bypass these defenses and cause an invasive bloodstream infection known as typhoid fever. Recent studies on virulence mechanisms of S. typhi suggest that tight regulation of virulence gene expression during the transition from the intestinal lumen into the intestinal mucosa enables this pathogen to evade detection by the innate immune system, thereby penetrating defenses that prevent bacterial dissemination. This example illustrates how the outcome of host pathogen interaction at the intestinal mucosal interface can alter the clinical presentation and dictate the disease outcome.
In immunocompetent individuals non-typhoidal Salmonella serovars are associated with gastroenteritis, a localized infection of the terminal ileum, colon and mesenteric lymph nodes.1 Upon ingestion, non-typhoidal Salmonella serovars use flagella-mediated motility and the invasion-associated type III secretion system (T3SS-1) to enter the ileal and colonic mucosa.2,3 Subsequently, a second type III secretion system (T3SS-2) is deployed to mediate survival in tissue macrophages.4 The presence of bacteria in tissue can be sensed by the host innate immune surveillance system through detection of conserved molecular patterns.5 For example, bacterial flagellin is a conserved molecular pattern recognized by Toll-like receptor 5 (TLR5)6 the lipid A moiety of lipopolysaccharide (LPS) is recognized by the TLR4/MD2/CD14 receptor complex7 and the O-antigen moiety of LPS is recognized by complement (Fig. 1), which involves covalent attachment of complement component 3 fragment b (C3b) by an ester bond formed with free hydroxyl-groups in LPS sugar moieties.8 In addition, the host can gauge the pathogenic potential of microbes by detecting pathogen-induced processes.9 A pathogen-induced process that marks non-typhoidal Salmonella serovars for detection by the host is the deployment of T3SS-1, sensed through NLRC4 (nucleotide-binding oligomerization domain-like receptor family caspase-associated recruitment domain-containing protein 4).10-12
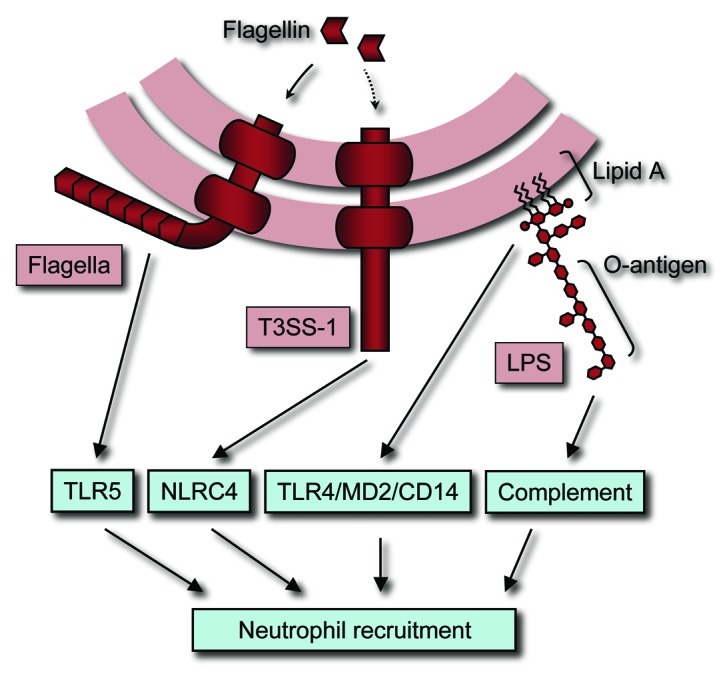
Figure 1. Detection of S. typhimurium by the innate immune system results in neutrophil recruitment. Complement, TLR5 and TLR4/MD2/CD14 detect conserved molecular patterns of S. typhimurium, including LPS and flagella. A pathogen-induced process detected by the host innate immune system is the deployment of T3SS-1, which is sensed through NLRC4. Signals generated by activation of complement, TLR5, TLR4/MD2/CD14 and NLRC4 cooperate in orchestrating neutrophil recruitment.
The detection of conserved molecular patterns and pathogen-induced processes by the innate immune system induces pro-inflammatory responses that culminate in the recruitment of neutrophils, the pathological hallmark of gastroenteritis (reviewed in ref. 13). Macrophages are the preferred intracellular niche of non-typhoidal Salmonella serovars and might serve as a vehicle for their dissemination. Intracellular survival in macrophages shelters non-typhoidal Salmonella serovars from neutrophil attack. However, macrophages can detect the vacuolar pathogen through NLRC4 and react by releasing non-typhoidal Salmonella serovars through pyroptosis (reviewed in ref. 14), a process that exposes the microbe to neutrophils.15 Clearance of bacteria from systemic sites makes neutrophils an effective defense against disseminated bacterial infection as illustrated by the fact that neutropenia is a risk factor for developing bloodstream infections with non-typhoidal Salmonella serovars.16,17
Similar to non-typhoidal Salmonella serovars, S. typhi employs its flagella and T3SS-1 to invade the intestinal epithelium,18,19 followed by T3SS-2-mediated survival inside macrophages.20 Akin to non-typhoidal Salmonella serovars, purified S. typhi flagellin signals through TLR5,21 the lipid A moiety of purified S. typhi LPS is a potent TLR4/MD2/CD14 agonist,22 the O-antigen of purified S. typhi LPS activates complement23 and macrophages detect the deployment of the S. typhi T3SS-1 as pathogen-induced process in vitro24 (Fig. 1). However, in humans S. typhi is associated with typhoid fever, which differs dramatically in its clinical presentation from gastroenteritis caused by non-typhoidal Salmonella serovars (reviewed in ref. 25). While rapid recruitment of neutrophils leads to symptoms of gastroenteritis within 24 h after ingestion of non-typhoidal Salmonella serovars,26 S. typhi does not evoke overt responses during the initial invasion of the intestinal mucosa, as indicated by an average incubation period of two weeks.27 Although S. typhi causes intestinal inflammation, the inflammatory infiltrates are dominated by mononuclear cells and neutrophils are scarce.28-32 Finally, S. typhi causes a disseminated bloodstream infection in immunocompetent individuals, while non-typhoidal Salmonella serovars are associated with a localized gastroenteritis. These clinical observations raise the question of how pathogens that are so similar in their basic repertoire of virulence factors can cause so different host responses and disease manifestations in humans. Surprisingly, inconspicuous changes in the control of virulence gene expression in S. typhi might hold the answer to this question.
A first clue on how gene regulation in S. typhi differs from that in non-typhoidal Salmonella serovars came from studies by Eleanor Metcalf and coworkers on invasion of epithelial cell lines. This work revealed that, unlike non-typhoidal Salmonella serovars, S. typhi needs to be grown under conditions of high-osmolarity (300 mM NaCl) for optimal invasion33 and maximum expression of T3SS-1 genes.34 The second clue was provided by the observation that S. typhi grown at low-osmolarity is poorly invasive, but entry into epithelial cells is markedly increased in strains lacking expression of the virulence (Vi) capsular polysaccharide, also known as Vi-antigen.35 Production of the Vi-antigen is encoded by the viaB (Vi-antigen B) locus, a 14 kb DNA region on the S. typhi chromosome that is absent from the genomes of non-typhoidal Salmonella serovars associated with human gastroenteritis.36,37 Expression of the Vi-antigen at low-osmolarity correlates with reduced secretion of flagellin and impaired invasiveness. In contrast, growth of S. typhi at high-osmolarity conditions suppresses Vi-antigen expression, which is accompanied by increased invasiveness and elevated flagellin secretion.38,39 It turns out that the first gene within the viaB locus, tviA, encodes the regulatory protein responsible for these phenotypic changes.40
The tviA gene is not expressed under high-osmolarity conditions, but the EnvZ/OmpR two-component regulatory system induces tviA expression under conditions of low-osmolarity.40,41 TviA forms heterodimers with RcsB, the response regulator of the RcsBCD phosphorelay system, to activate expression of genes in the viaB locus, which encode proteins involved in the Vi-antigen biosynthesis (tviBCDE) and Vi-antigen export (vexABCDE).37,42 Furthermore, TviA in conjunction with RcsB represses expression of the flhDC genes (Fig. 2).40,43 FlhDC is the master regulator of flagellin gene expression.44 One FlhDC-regulated gene, fliZ, encodes a regulatory protein that induces expression of the hilD gene, encoding an activator of the hilA gene.45,46 Finally, HilA positively regulates T3SS-1 gene expression.47,48 Through this mechanism, TviA suppresses invasion and flagella production, while activating Vi-antigen expression at low-osmolarity conditions (Fig. 2).40 But how can this unassuming change in virulence gene expression alter the outcome of host pathogen interaction?
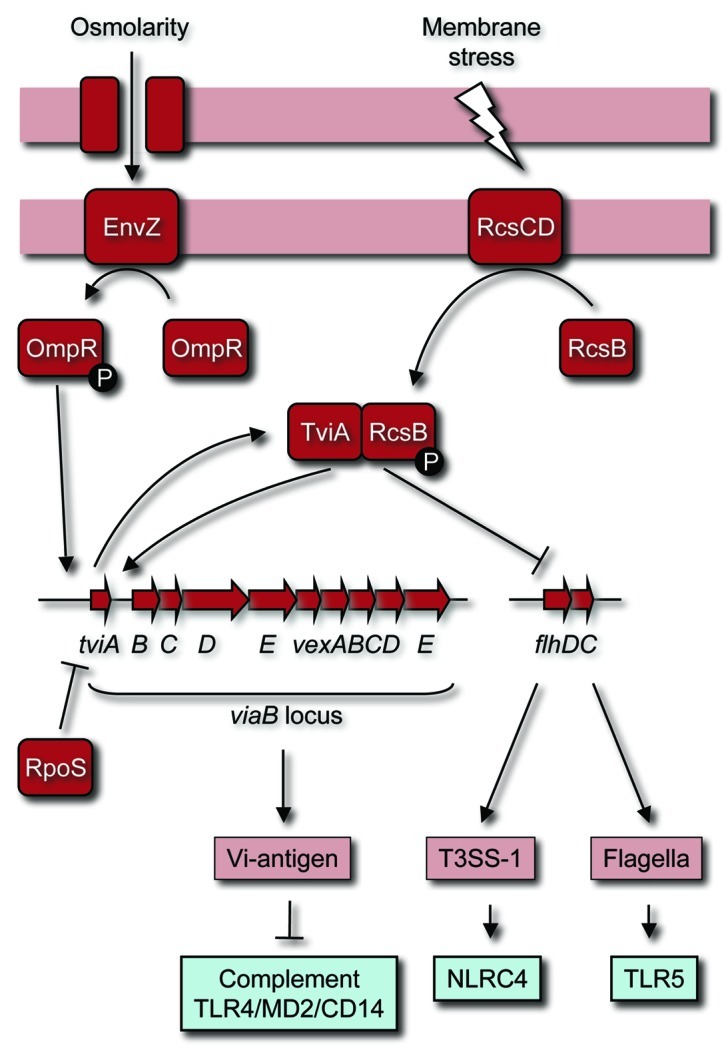
Figure 2. TviA-mediated gene regulation conceals conserved molecular patterns and pathogen-induced processes during S. typhi infection. Expression of TviA is induced at tissue osmolarity by the OmpR/EnvZ two-component system. TviA in conjunction with RcsB activates expression of the tviBCDEvexABCDE operon and represses expression of the flhDC operon. As a result, S. typhi expresses the Vi-antigen while suppressing flagella and T3SS-1 expression when entering the intestinal mucosa. The Vi-antigen interferes with the detection of LPS as a conserved molecular pattern. Thus, TviA-mediated gene regulation prevents detection of a pathogen-induced process (i.e., the deployment of T3SS-1) and detection of conserved molecular patterns (i.e., LPS and flagella) by the innate immune surveillance system in the intestinal mucosa.
Full suppression of tviA expression occurs at conditions that resemble the osmolarity encountered in the intestinal lumen.49 This regulation ensures that S. typhi is motile and invasive as it approaches the mucosal surface. However, upon invasion of the intestinal epithelium, S. typhi is exposed to conditions of lower osmolarity, which results in a rapid induction of TviA expression.49 In turn, TviA in conjunction with RcsB induces expression of the Vi-antigen, while repressing flagella and T3SS-1 expression as the pathogen transits through the epithelium (Fig. 2).49,50 Once S. typhi emerges in the lamina propria, several pathogen-induced processes and conserved molecular patterns are no longer detectable by the innate immune surveillance system.
Specifically, TviA/RcsB turns off expression of flagella, a conserved molecular pattern whose detection by TLR5 expressed on the basolateral surface of epithelial cells serves as an indicator of microbial translocation from the gut.43 When TviA expression in S. typhi is induced in vitro by inactivating rpoS, a gene encoding a negative regulator of tviA,51 macrophages exhibit a reduced ability to release intracellular bacteria through pyroptosis.52 These data suggest that the ability of macrophages to detect a pathogen-induced process of S. typhi is impaired, presumably because TviA-regulation suppresses T3SS-1 expression. Finally, expression of the Vi-antigen by S. typhi prevents complement activation through the alternative pathway,53,54 most likely because this capsular polysaccharide does not contain free hydroxyl groups that could form ester bonds with C3b,55 a process necessary for formation of C3 convertase on the bacterial surface. Complement activation leads to the generation of the anaphylatoxins C3a and C5a, two potent enhancers of cytokine responses elicited by endotoxin (lipid A) (reviewed in ref. 56). Thus, reduced complement activation might explain the apparent inhibition of TLR4/CD14/MD2 signaling by the Vi-antigen.57-60 In summary, when S. typhi enters the intestinal mucosa, TviA-regulation helps the microbe to conceal pathogen-induced processes and conserved molecular patterns (Fig. 1) from its host. Through this “stealth” mechanism, S. typhi eludes detection by the innate immune system, a “you can’t hit what you can’t see*”-strategy that makes it difficult for the host to contain the dissemination of the intruding microbe.
By evading detection by the innate immune system, the viaB locus impairs neutrophil recruitment in the intestinal mucosa,61,62 which helps explain the scarcity of neutrophils in intestinal infiltrates28-32 and the long incubation period of typhoid fever.27 A viaB-mediated inhibition of pyroptosis might also reduce exposure of S. typhi to neutrophil attack.52 Furthermore, inhibition of C3b deposition on the bacterial surface prevents phagocytosis of S. typhi through complement receptor (CR) 3,53,54 a process coupled to a respiratory burst in neutrophils.8 Through this mechanism, S. typhi prevents phagocytosis and the generation of a respiratory burst when it encounters neutrophils,63-65 a cell type crucial for preventing dissemination of non-typhoidal Salmonella serovars in humans, as indicated by the increased risk of neutropenic individuals to develop bloodstream infections.16,17 Finally, TviA-mediated repression of flagella expression increases bacterial dissemination in an animal model, presumably because detection of this conserved molecular pattern by the innate immune system helps to orchestrate host responses that enhance bacterial clearance from systemic sites and/or prevent bacterial dissemination49 (Fig. 1). Collectively, these mechanisms help explain why S. typhi slips past the host’s defenses to cause an invasive bloodstream infection, even in immunocompetent individuals.
The picture emerging from these studies is that subtle changes in the regulation of virulence gene expression can influence the outcome of host pathogen interaction in the intestinal mucosa. Common approaches for studying innate immunity, such as stimulating host cells with purified conserved molecular patterns, cannot model such interactions accurately, which is one of the reasons why TviA-mediated innate immune evasion has eluded us until recently. Furthermore, studies on the interaction of S. typhi with tissue culture cells can be misleading, unless care is taken that bacteria are cultured under conditions that mimic gene expression in vivo. Although TviA-mediated innate immune evasion is easily overlooked, its influence on the outcome of host pathogen interaction is profound, as vividly illustrated by the different clinical presentations of typhoid fever and gastroenteritis.
Acknowledgments
Work in A.J.B.’s laboratory is supported by Public Health Service Grants AI040124, AI044170, AI076246, AI088122 and AI096528.
Footnotes
*Walter Johnson
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/18602
References
Articles from Gut Microbes are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.4161/gmic.18602
Read article for free, from open access legal sources, via Unpaywall:
https://www.tandfonline.com/doi/pdf/10.4161/gmic.18602?needAccess=true
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4161/gmic.18602
Article citations
Differential regulatory control of curli (csg) gene expression in Salmonella enterica serovar Typhi requires more than a functional CsgD regulator.
Sci Rep, 13(1):14905, 09 Sep 2023
Cited by: 3 articles | PMID: 37689734 | PMCID: PMC10492818
Intracellular Salmonella Paratyphi A is motile and differs in the expression of flagella-chemotaxis, SPI-1 and carbon utilization pathways in comparison to intracellular S. Typhimurium.
PLoS Pathog, 18(4):e1010425, 05 Apr 2022
Cited by: 1 article | PMID: 35381053 | PMCID: PMC9012535
Review on the Recent Advances on Typhoid Vaccine Development and Challenges Ahead.
Clin Infect Dis, 71(suppl 2):S141-S150, 01 Jul 2020
Cited by: 30 articles | PMID: 32725225 | PMCID: PMC7388714
Review Free full text in Europe PMC
Persistent Infection and Long-Term Carriage of Typhoidal and Nontyphoidal Salmonellae.
Clin Microbiol Rev, 32(1):e00088-18, 28 Nov 2018
Cited by: 80 articles | PMID: 30487167 | PMCID: PMC6302356
Review Free full text in Europe PMC
Typhoidal Salmonella serovars: ecological opportunity and the evolution of a new pathovar.
FEMS Microbiol Rev, 42(4):527-541, 01 Jul 2018
Cited by: 21 articles | PMID: 29790924
Review
Go to all (26) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical pathogenesis of typhoid fever.
J Infect Dev Ctries, 2(4):260-266, 30 Aug 2008
Cited by: 59 articles | PMID: 19741286
Review
Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi.
mBio, 4(4):e00232-13, 16 Jul 2013
Cited by: 32 articles | PMID: 23860765 | PMCID: PMC3735119
Now you see me, now you don't: the interaction of Salmonella with innate immune receptors.
Nat Rev Microbiol, 13(4):206-216, 09 Mar 2015
Cited by: 92 articles | PMID: 25749454
Review
A Salmonella Typhi RNA thermosensor regulates virulence factors and innate immune evasion in response to host temperature.
PLoS Pathog, 17(3):e1009345, 02 Mar 2021
Cited by: 15 articles | PMID: 33651854 | PMCID: PMC7954313
Funding
Funders who supported this work.
NIAID NIH HHS (11)
Grant ID: R01 AI076246
Grant ID: AI040124
Grant ID: AI096528
Grant ID: R01 AI096528
Grant ID: AI044170
Grant ID: AI088122
Grant ID: R29 AI040124
Grant ID: AI076246
Grant ID: R01 AI040124
Grant ID: R01 AI044170
Grant ID: R21 AI088122




