Abstract
Free full text

Membrane-Anchored Serine Proteases in Vertebrate Cell and Developmental Biology*
Abstract
Analysis of vertebrate genome sequences at the turn of the millennium revealed that a vastly larger repertoire of enzymes execute proteolytic cleavage reactions within the pericellular and extracellular environments than was anticipated from biochemical and molecular analysis. Most unexpected was the unveiling of an entire new family of structurally unique multidomain serine proteases that are anchored directly to the plasma membrane. Unlike secreted serine proteases, which function primarily in tissue repair, immunity, and nutrient uptake, these membrane-anchored serine proteases regulate fundamental cellular and developmental processes, including tissue morphogenesis, epithelial barrier function, ion and water transport, cellular iron export, and fertilization. Here the cellular and developmental biology of this fascinating new group of proteases is reviewed. Particularly highlighted is how the study of membrane-anchored serine proteases has expanded our knowledge of the range of physiological processes that require regulated proteolysis at the cell surface.
INTRODUCTION
Proteases were originally discovered as enzymes that generate free amino acids during nutrient digestion. The range of biological processes in which proteases are known to participate expanded gradually during the past century to include tissue remodeling, hemostasis, and immunity. A parallel expansion took place in the number of known proteases, which culminated with the complete sequencing and mining of the human genome at the turn of the millennium and the associated realization that 561 proteases execute proteolysis in humans (reviewed in López-Otín & Bond 2008). The most dramatic repertoire expansion resulting from genome mining efforts occurred in the class of serine proteases, which has 175 predicted members in humans and dozens more in rodents (Puente et al. 2003). Much of this increase was due to the unveiling of an entirely new family of membrane-anchored serine proteases that often had an unprecedented complex modular structure (Figure 1; Supplemental Table 1, follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). All but one of the 20 members of the new family were orphan proteases at the time of their discovery; that is, they had no known biological function or target substrate. The pace, however, with which the functions of individual family members have been unraveled has been very fast and aided in particular by the recent advances in the fields of human and animal genetics. Thus, within just a single decade, distinct cellular and developmental functions have been assigned to approximately half of the membrane-anchored serine proteases, and target substrates have been identified for several. Interestingly, membrane-anchored serine proteases frequently turned out to have essential functions in already well-studied life processes that were not known, or were anticipated, to involve a pericellular proteolytic component. The new protease family, therefore, has emerged as not only structurally unique but also functionally different from its well-characterized secreted cousins.

The human complement of membrane-anchored serine proteases. The single serine protease domain of tryptase γ1, prostasin, and testisin is attached to the membrane by a C-terminal type I transmembrane domain or glycophosphatidylinositol (GPI) anchor. Type II transmembrane serine proteases are attached to the membrane by a signal anchor (SA) located close to the N terminus. The stem region between the SA and the C-terminal serine protease domain contains an assortment of domains including low-density lipoprotein receptor class A (LDLA); sea urchin sperm protein, enteropeptidase, and agrin (SEA); Cls/Clr, urchin embryonic growth factor, and bone morphogenic protein-1 (CUB); meprin, A5 antigen, and receptor protein phosphatase μ (MAM); frizzled; and group A scavenger receptor. Type II transmembrane proteases are phylogenetically divided into four subfamilies (Bugge et al. 2009, Szabo et al. 2003): (1) human airway trypsin-like (HAT)/differentially expressed in squamous cell carcinoma gene (DESC), which comprises HAT, DESC1, TMPRSS11A, HAT-like 4, and HAT-like 5 (blue shading); (2) hepsin/transmembrane protease, serine (TMPRSS), which comprises hepsin, TMPRSS2, TMPRSS3, TMPRSS4, mosaic serine protease large-form (MSPL), spinesin, and enteropeptidase ( yellow shading); (3) Matriptase, which consists of matriptase, matriptase-2, matriptase-3, and polyserase-1 ( green shading); and (4) corin, which contains only corin ( purple shading).
In this review, first the biochemical features of membrane-anchored serine proteases are briefly discussed, and then their participation in selected cell and developmental processes is described in depth. We highlight in particular how the past decade of studies of this protease family has expanded the knowledge of the range of cellular processes that require regulated proteolysis at the cell surface, and we identify some of the most important gaps remaining in our current knowledge.
STRUCTURAL FEATURES AND BIOCHEMISTRY OF MEMBRANE-ANCHORED SERINE PROTEASES
Life Cycle
Membrane-anchored serine proteases are synthesized as catalytically inactive or near-inactive proenzymes (zymogens) that are converted into active proteases by autocatalytic or heterocatalytic cleavage after an arginine or lysine residue that is located in a conserved activation motif within the catalytic domain. Activation cleavage severs the bond between the catalytic domain and upstream accessory domains, but the activated protease domain remains connected to upstream accessory domains by a disulfide bond. Zymogen activation is irreversible, and proteolytic activity typically is terminated either by proteolytic shedding of the protease from the cell surface or by its internalization and lysosomal degradation. This frequently occurs after the formation of high-affinity complexes between the protease and cognate membrane-associated or secreted serine protease inhibitors that bind the catalytic site and accessory domains (reviewed in Bugge et al. 2009).
Catalysis and Substrate Selection
The catalytic domain of all membrane-anchored serine proteases belongs to the S1 peptidase family (Rawlings & Barrett 1993). The biochemistry of this family, which includes the prototypic proteases chymotrypsin and trypsin, is exceptionally well characterized, and the mechanism of catalysis is understood in great detail (for reviews, see Hedstrom 2002, Page & Di Cera 2008). A catalytic triad of serine, aspartate, and histidine residues with a conserved spatial orientation executes peptide bond hydrolysis. All membrane-anchored serine proteases show a strong preference for cleavage of substrates after arginine or lysine residues. Target substrate selection in vivo, however, is determined by a host of additional factors, including substrate binding to other parts of the catalytic domain and to accessory protein domains, control of proenzyme activation, protease and substrate compartmentalization, macromolecular and small-molecule cofactors, and general and specific protease inhibitors.
Membrane Anchorage
Membrane anchorage is achieved by one of three means (Figure 1): by a single-pass transmembrane domain located close to the C terminus that orients the protease as a type I transmembrane protein (as in tryptase γ1), by a glycosylphosphatidylinositol (GPI) anchor that is added posttranslationally to the C terminus and attaches the protease to the outer leaflet of the plasma membrane (as in prostasin and testisin), or by a signal anchor that is located near the N terminus and orients the protease in the membrane as a type II transmembrane protein with a large extracellular C terminus containing the serine protease domain (all type II transmembrane serine proteases).
Accessory Domains
Tryptase γ1, prostasin, and testisin are composed of a single protease domain linked to a transmembrane domain or GPI anchor at the C terminus. The type II transmembrane serine proteases are much more complex, as they include a so-called stem region between the signal anchor and serine protease domain; this stem varies widely in length and may contain an assortment of from one to eleven protein domains of six different types (Figure 1). This profusion of accessory protein domains in the stem region of type II transmembrane serine proteases is unique among the S1 peptidases, and these domains are not frequently found in other proteases that function in the extracellular environment. These accessory domains have multiple functions that facilitate the proper spatial and temporal execution of the intended cleavage reactions. They regulate cellular trafficking of the protease to the appropriate plasma membrane domain (Kim et al. 2005), zymogen activation (Altamura et al. 2010, Lee et al. 2007, Oberst et al. 2003, Ramsay et al. 2009, Wang et al. 2008), target substrate recognition (Altamura et al. 2010, Knappe et al. 2004), interactions with cofactors, and the binding or modulation of the activity of cognate serine protease inhibitors (Inouye et al. 2010). Other functions of accessory domains, although not yet formally demonstrated, most likely include substrate repulsion to prevent pathogenic cleavage of inappropriate substrates and shielding of the catalytic domain from inhibition by high-abundance, nonselective serine protease inhibitors.
CELLULAR AND DEVELOPMENTAL FUNCTIONS OF MEMBRANE-ANCHORED SERINE PROTEASES
This section covers the newly discovered and often quite unexpected functions of membrane-anchored serine proteases. As the role of enteropeptidase in initiating the activation of the digestive enzyme cascade was established more than a century ago, it will not be described further (for an excellent review, see Zheng et al. 2009).
Orchestrating Neural Tube Closure
The neural tube forms from a thickened region of ectoderm that is termed the neural plate or neuroepithelium. From this structure, bilateral neural folds form at the junction with the surface ectoderm. These folds elevate, come into contact, and fuse at the midline to create the neural tube (Figure 2). Failure to complete the closure of the neural tube is among the most common and serious human birth defects, affecting approximately 1 in 1,000 established pregnancies and causing anencephaly and spina bifida (reviewed in Copp et al. 2003). Owing to the clinical importance of this birth defect, considerable effort has been put into determining the molecular basis of neural tube closure. Genetic analysis has identified a diverse array of molecules as critical to the process, including cytoskeleton-associated proteins, cell cycle and neurogenesis proteins, cell death–associated proteins, cell signaling molecules, extracellular matrix proteins and their receptors, and transcription factors. Of the more than 150 genes identified over the years by loss-of-function studies as critical to neural tube closure, not one has encoded an extracellular protease, which suggests that pericellular proteolysis might not play a role in this process (reviewed in Copp & Greene 2010, Zohn & Sarkar 2008). However, a recent landmark study by Camerer et al. (2010) changed this perception by showing that protease-activated receptor (PAR)-1 or PAR-2 activation in the nonneural surface ectoderm that directly overlies the forming neural tube is essential for late events of neural tube closure. Proteolytic activation of either of the PARs in this tissue induces fusion of the neural folds by activating a cell motility-associated molecule, Rac1, through the Gi/o/z subfamily of G proteins (Figure 2). Thrombin, the principal activator of PAR-1, is widely expressed in the developing central nervous system (Dihanich et al. 1991, Szabo et al. 2009a) and likely accounts for PAR-1 activation during neural tube closure. PAR-2, however, is not efficiently activated by thrombin, but systematic profiling revealed that a surprisingly large number of membrane-anchored serine proteases with PAR-2-activating potential are expressed at the site of neural tube closure. These include matriptase; matriptase-3; transmembrane protease, serine 2 (TMPRSS2); TMRPSS4; hepsin; and prostasin (Camerer et al. 2010, Szabo et al. 2009a). Of these, matriptase is a particularly potent activator of PAR-2 and, interestingly, can sensitize cultured cells to PAR-2 activation by prostasin and hepsin, which suggests that a local membrane-anchored serine protease cascade mediates PAR-2 activation in the context of neural tube closure (Camerer et al. 2010).

Membrane-anchored serine protease signaling in the surface ectoderm facilitates neural tube closure. Protease-activated receptor (PAR)-dependent Rac1 activation in the surface ectoderm through Gi/o/z is required for the fusion of the neural folds. PAR-1 is activated by thrombin. PAR-2 may be activated by prostasin, acting through matriptase, or by other locally expressed membrane-anchored serine proteases including hepsin; transmembrane protease, serine 2 (TMPRSS2); TMPRSS4; and matriptase-3. Membrane-anchored serine protease activity in surface ectoderm cells is negatively regulated by hepatocyte growth factor activator inhibitor 2 (HAI-2).
Independent support for a membrane-anchored serine protease signaling axis acting in neural tube closure arose from studies of the effect of ablation of hepatocyte growth factor activator inhibitor 2 (HAI-2) (Szabo et al. 2009a). This transmembrane serine protease inhibitor is expressed in neural and nonneural ectoderm precisely at the future point of contact between the neural folds, and its deletion from mice causes neural tube closure defects that are strikingly similar to those observed after combined PAR-1 and PAR-2 ablation. Furthermore, this phenotype was partially rescued by simultaneous ablation of matriptase, indicating that both insufficient and excessive local protease-initiated cell signaling derails the closing of the neural tube, with the latter possibly due to the well-established desensitization of PARs that occurs after constitutive stimulation.
Excess local membrane-anchored serine protease activity is detrimental not only to neural tube closure but also to mouse placental labyrinth formation. This structure, which is vital to the fetomaternal exchange of nutrients and oxygen, is formed by branching morphogenesis of chorionic trophoblasts. Matriptase, HAI-2, and the related inhibitor HAI-1 all are expressed by this epithelial cell population. Ablation of either HAI-1 or HAI-2 or the combined haploinsufficiency for HAI-1 and HAI-2 leads to the failure of the placental labyrinth to form. However, when matriptase is simultaneously reduced or eliminated from the placenta, placental development proceeds normally (Fan et al. 2007; Szabo et al. 2007, 2009a,b; Tanaka et al. 2005), which indicates that matriptase is not necessary for placental development but that its activity in the placenta nevertheless needs to be regulated by both HAI-1 and HAI-2.
HAI-2 deficiency in humans does not cause placental or neural tube defects but rather a wide spectrum of other developmental abnormalities such as duplication of internal organs, duplication and abnormal location of digits, craniofacial dysmorphisms, anal and choanal atresia, fistulas, and hamartoma (Heinz-Erian et al. 2009). It remains to be established if these are caused by excess matriptase activity or by the loss of inhibition of other membrane-anchored or secreted serine proteases.
Erecting Barriers Between Cells
Sealing the epidermis
Prior to birth, terrestrial mammals develop an external permeability barrier that prevents excessive water loss and the entry of noxious chemicals and microbes. This barrier function is provided by the epidermis, a multilayered epithelium in which keratinocytes arise from proliferating basal cells, move outward, and undergo a series of distinct differentiation events to form the stratum corneum, a two-compartment structure consisting of a lipid-enriched extracellular matrix in which an interlocking meshwork of dead keratinocytes (corneocytes) is embedded. The stratum corneum and the tight junctions formed between the underlying living keratinocytes constitute the epidermal barrier. The thickness of the stratum corneum is regulated by the shedding (desquamation) of the outermost layer of corneocytes through proteolytic degradation of their desmosomal junctions by epidermal kallikrein-related peptidases (Fuchs & Raghavan 2002, Furuse et al. 2002, Nemes & Steinert 1999, Presland & Dale 2000, Roop 1995, Segre 2003).
The epidermis has long been studied as a convenient model of epithelial cell differentiation, but the essential role of membrane-anchored serine proteases in epidermal development was uncovered only recently by the fortuitous discovery that mice deficient in either matriptase or prostasin fail to develop epidermal barrier function sufficient for postnatal survival (Leyvraz et al. 2005, List et al. 2002). Subsequent studies showed that both proteases are selectively expressed in terminally differentiating keratinocytes and act as part of a single proteolytic cascade in which the autoactivating matriptase serves to activate the prostasin zymogen (Netzel-Arnett et al. 2006). This proteolytic cascade regulates at least four key steps of terminal epidermal differentiation that are shown schematically in Figure 3: (a) formation of claudin-1-based tight junctions between living suprabasal keratinocytes (Furuse et al. 2002, Leyvraz et al. 2005, List et al. 2009); (b) activation of an intracellular μ-calpain-caspase-14-bleomycin hydrolase proteolytic and hydrolytic cascade that converts profilaggrin into modified free hygroscopic amino acids that hydrate the skin and absorb UV light (Denecker et al. 2007, Kamata et al. 2009, Leyvraz et al. 2005, List et al. 2003, Yamazaki et al. 1997); (c) initiation of intercorneocyte lipid manufacturing and deposition, possibly by enhancing keratinocyte sodium uptake by activation of the epithelial sodium channel (ENaC) (see below) (Charles et al. 2008, Leyvraz et al. 2005, List et al. 2003); and (d ) induction of desquamation by kallikrein-related peptidases by promoting their expression or activation (List et al. 2003, Sales et al. 2010). The matriptase-prostasin cascade also plays a critical role in the development of hair follicles and in hair growth, but very little as yet is known about the differentiation and growth pathways upon which the protease cascade acts (Alef et al. 2009; Avrahami et al. 2008; Basel-Vanagaite et al. 2007; List et al. 2002, 2006, 2007a, 2009; Spacek et al. 2010).
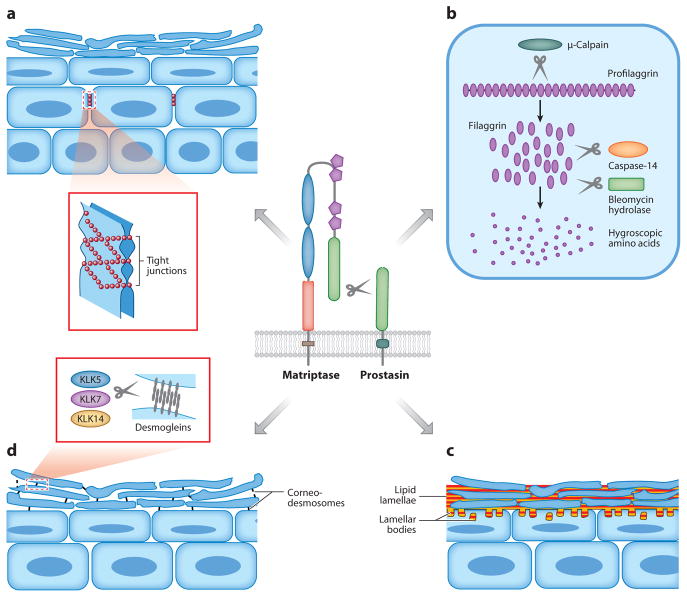
Epidermal differentiation processes controlled by the matriptase-prostasin proteolytic cascade. (a) Formation of tight junctions between granular-layer keratinocytes. (b) Cytoplasmic processing of profilaggrin to filaggrin monomers by μ-calpain and complete processing to free hygroscopic and UV light–absorbing amino acids by caspase-14 and bleomycin hydrolase. (c) Epidermal lipid synthesis by transitional cells and its deposition into the intercellular space between corneocytes as lipid lamellae. (d ) Proteolytic shedding (desquamation) of the stratum corneum by kallikrein (KLK)-mediated degradation of desmogleins in desmosomes linking corneocytes. If the four processes are independent or interrelated, and what target substrate(s) is cleaved in each process by the matriptase-prostasin cascade, are unknown.
As demonstrated by recent genetic interaction studies, the local action of cognate serine protease inhibitors must tightly regulate membrane-anchored epidermal serine proteases. HAI-1 is coexpressed with matriptase and prostasin in the terminally differentiating layers of the epidermis as well as in hair cortex and cuticle cells of the hair follicle. HAI-1 ablation from keratinocytes leads to fatal ichthyosis, a hallmark of compromised epidermal barrier function, and to defects in hair structure and growth (Nagaike et al. 2008). However, both barrier and hair follicle defects are eliminated completely by simultaneous reduction in the level of epidermal matriptase (Nagaike et al. 2008, Szabo et al. 2009b). Netherton syndrome, a second example of the detrimental effects of dysregulated epidermal membrane-anchored serine protease activity, is characterized by premature stratum corneum shedding with direct exposure of the living layers of the epidermis to the external environment. It is caused by genetic deficiency of the lympho-epithelial Kazal-type-related serine protease inhibitor (LEKTI) (Chavanas et al. 2000a,b; Deraison et al. 2007; Descargues et al. 2005, 2006; Hachem et al. 2006; Komatsu et al. 2002; Magert et al. 1999; Schechter et al. 2005; Suzuki et al. 1994). At the molecular level, LEKTI deficiency causes a matriptase-initiated runaway kallikrein-related peptidase cascade, which leads to premature desmosome degradation (Sales et al. 2010).
A global role for membrane-anchored serine proteases in tight junction function?
Polarized epithelia must be able to limit and regulate the diffusion of molecules between cells. This is achieved by formation of intercellular tight junctions that are composed of interacting protein strands comprised of members of the claudin family of integral membrane proteins (Shin et al. 2006). The unexpected failure of matriptase and prostasin-ablated epidermis to assemble functional tight junctions between suprabasal keratinocytes suggested that the two membrane-anchored serine proteases could have a global role in tight junction formation. Indeed, anecdotal evidence for a proteolysis component in tight junction assembly was provided decades ago by electron microscopy studies showing that tight junction strands are formed after exposure of cultured epithelial cells to trypsin or other nonspecific serine proteases (Cohen et al. 1985 and references therein). The capacity of trypsin to stimulate tight junction function was later confirmed using more sophisticated assays, and importantly, the capacity of the broad-spectrum serine protease inhibitor, aprotinin, to suppress tight junction formation through inhibition of endogenous proteases was demonstrated (Liu et al. 2002). Matriptase and prostasin are both near-ubiquitously expressed in simple epithelia with low paracellular permeability (List et al. 2007b) and potentially could account for the tight junction–stimulating proteolytic activity mimicked by trypsin and inhibited by aprotinin. Indeed, two recent studies have provided direct evidence for a critical role of at least matriptase in tight junction formation. Buzza et al. (2010) used siRNA to silence matriptase in polarized Caco-2 intestinal cell monolayers, whereas List et al. (2009) used a tamoxifen-inducible Cre recombinase to acutely ablate matriptase from adult mouse intestinal epithelium. In both cases, loss of matriptase dramatically increased paracellular permeability, as measured by transepithelial electrical resistance, and diffusion of macromolecules. Matriptase ablation from several other mouse tissues also lead to an epithelial dysfunction compatible with impaired tight junction integrity (List et al. 2009).
It is as yet unclear if matriptase also promotes tight junction function through the activation of prostasin in these nonepidermal tissues. Matriptase is located on the basolateral plasma membrane, and prostasin is located on the apical plasma membrane of polarized epithelia (Buzza et al. 2010; List et al. 2007b, 2009; Steensgaard et al. 2010; Tsuzuki et al. 2005). Newly synthesized prostasin is routed initially to the basolateral plasma membrane and only thereafter is transported to the apical membrane by transcytosis. This complex cellular itinerary provides a plausible mechanism for how matriptase could activate prostasin in polarized epithelial cells (Friis et al. 2010). Selective inhibition of apical serine protease activity suffices to decrease tight junction function, which would tentatively argue for matriptase acting through prostasin (Steensgaard et al. 2010). The molecular mechanism by which matriptase promotes tight junction function is also unclear. Studies in Caco-2 cells indicate that it may do so, at least in part, by suppressing the incorporation of leaky claudins via a posttranslational mechanism that involves activation of atypical protein kinase Cζ, but does not involve direct cleavage of claudins (Buzza et al. 2010).
Developing the Inner Ear
At least two and likely three membrane-anchored serine proteases participate in auditory development, which was realized recently when mutations in the TMPRSS3 gene were identified as a cause of congenital and childhood onset autosomal recessive deafness (Ben-Yosef et al. 2001, Scott et al. 2001). Spurred by these findings, subsequent systematic screening of human deafness-associated loci and functional analysis of mouse knockouts additionally identified hepsin and likely spinesin as critical for hearing function (Guipponi et al. 2007, 2008). The cause of spinesin- and TMPRSS3-associated deafness has not been explored. Hepsin deficiency, however, has been linked to aberrant tectorial membrane development and decreased myelination of the auditory nerve. These defects appear to be secondary to either low circulating levels of thyroid hormone or low responsiveness of inner ear tissues to thyroid hormone, but how hepsin would control thyroid hormone action is not evident and as yet unstudied (Guipponi et al. 2007, 2008; Hanifa et al. 2010).
Regulating Apical Sodium Entry and Fluid Volume
The highly amiloride-sensitive epithelial sodium channel (ENaC) has a central role in determining extracellular fluid composition and volume by regulating the apical entry of sodium into polarized epithelial cells. ENaC is expressed in high-resistance epithelia of the distal colon, renal collecting tubes, airway epithelium, suprabasal epidermis, and sweat glands. Loss of ENaC function reduces fluid clearance from the alveolar space, causes renal sodium loss, and causes failure to develop epidermal barrier function (Barker et al. 1998, Hummler et al. 1996, Mauro et al. 2002, McDonald et al. 1999, Rossier et al. 2002). In its most active form, ENaC is a heterotetramer composed of homologous α, β, and γ subunits that combine to form a channel (Firsov et al. 1998, Garty & Palmer 1997, Rossier et al. 2002). The potential relevance of proteolysis to apical sodium transport became evident with the discovery that exposure of epithelial cells to trypsin or aprotinin increased or decreased, respectively, the open probability of ENaC. Later studies showed that proteolysis indeed provides one of the principal means to rapidly increase cellular sodium transport in response to a homeostatic challenge such as dietary sodium restriction or pulmonary edema (Ergonul et al. 2006, Masilamani et al. 1999, Narikiyo et al. 2002, Planès et al. 2009).
The mechanism by which serine proteases regulate ENaC activity is complex and far from fully elucidated. A tentative model is shown in Figure 4. During biosynthesis of the channel, furin cleaves the α subunit after the sequences RSTR205 and RSAR231 and the γ chain after RKRR144 (Hughey et al. 2003, 2004). α-Chain cleavage increases channel activity, but these furin cleavage events are constitutive and therefore unlikely to be the point of action for homeostatic regulation of ENaC by proteases. A second cleavage event in the ENaC γ subunit, which can enhance sodium transport, occurs after the sequence RKRK186 (Bruns et al. 2007). Cleavage at RKRK186, when combined with the preceding furin cleavage after RKRR144, increases ENaC activity several-fold by removing an inhibitory loop of the γ chain (Bruns et al. 2007). Importantly, cleavage at this site in the γ chain is enhanced by sodium restriction in vivo (Masilamani et al. 1999), and is consistent with the activation of ENaC by serine proteases with trypsin-like specificity in various model systems, such as Xenopus oocytes reconstituted with all three ENaC subunits and cell lines expressing endogenous ENaC (Garcia-Caballero et al. 2008; Vallet et al. 1997; Vuagniaux et al. 2000, 2002; Wakida et al. 2006).

Proteolytic regulation of epithelial sodium channel (ENaC) activity. Only one α subunit is shown, and the β subunit is omitted for simplicity. (a) The unprocessed channel has low sodium currents. (b) Furin removes an autoinhibitory disulfide-bonded loop in the α subunit during ENaC trafficking to the plasma membrane to increase the open probability. Furin also partially removes a second autoinhibitory loop in the γ subunit. (c) Full ENaC activation is achieved by complete removal of the γ subunit autoinhibitory loop by prostasin. Other membrane-anchored serine proteases may activate furin-processed ENaC in a similar manner.
TMPRSS3, TMPRSS4, matriptase, and prostasin can activate ENaC in model systems (Guipponi et al. 2002, Vallet et al. 1997, Vuagniaux et al. 2002; reviewed in Rossier & Stutts 2009). However, evidence for a physiological role in channel activation is limited to prostasin thus far. Most compelling in this regard, ENaC activity is reduced in prostasin-silenced cultured epithelial cells, and fluid clearance from the lungs is impaired in mice with alveolar epithelium-specific prostasin ablation (Narikiyo et al. 2002, Planès et al. 2009, Tong et al. 2004). ENaC activation by prostasin requires membrane anchorage, is inhibited by aprotinin, and requires an intact RKRK186 γ chain sequence. Paradoxically, however, the catalytic activity of prostasin appears to be dispensable for its capacity to activate ENaC, at least in the Xenopus model system, where catalytically inactive prostasin can induce both ENaC γ subunit cleavage and channel activation (Andreasen et al. 2006, Bruns et al. 2007). Tentatively, inactive prostasin may activate ENaC by titrating out a membrane-associated serine protease inhibitor, thereby increasing the activity of an endogenous ENaC-activating protease. Alternatively, inactive prostasin may serve as a scaffold for assembly of an ENaC-activating protease complex on the cell surface. Whichever the case, the above findings make it clear that mechanistic insight into the complex association between membrane-anchored serine proteases and epithelial sodium transport is still limited.
Controlling the Natriuretic Peptide System
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are polypeptide hormones released from secretory storage granules of atrial cardiocytes in response to high blood pressure and volume overload. They promote urine production, urinary sodium excretion, and widening of blood vessels by exerting pleiotropic effects on kidneys, adrenals, vasculature, and the peripheral and central nervous systems through binding and activation of a guanylyl cyclase-coupled receptor, natriuretic peptide receptor-A. ANP and BNP also display antiproliferative effects on vascular smooth muscle cells, fibroblasts, and cardiocytes (reviewed in de Bold 1985, McGrath et al. 2005). Both hormones are synthesized as inactive precursors, proANP and proBNP, that are converted to their biologically active forms by proteolytic removal of 98 and 76 amino acids, respectively, from their N termini. Furin constituitively activates proBNP during biosynthesis (Semenov et al. 2010) and it is stored in secretory granules in its biologically active form. ProANP, however, lacks the RXXR furin cleavage motif found in proBNP. It is proteolytically converted to ANP only during secretion from storage granules (reviewed in McGrath et al. 2005, Wu et al. 2009). The identity of the proANP-converting protease was long uncertain and was finally revealed to be the complex cardiac-specific membrane-anchored serine protease, corin (Hooper et al. 2000, Yan et al. 1999). In model systems, corin proteolytically processes proANP to ANP by cleavage after R98 (Knappe et al. 2003, Yan et al. 2000). In Corin-ablated mice, ANP is undetectable, and proANP is elevated. Furthermore, these mice display spontaneous and salt-sensitive hypertension similar to proANP-and natriuretic peptide receptor-A-deficient mice, as well as cardiac hypertrophy and reduced cardiac function in later life (Chan et al. 2005 and references therein).
Corin is found on the surface of proANP-producing atrial cardiocytes as a mixture of zymogen and active forms (Gladysheva et al. 2008). Productive interaction of corin with proANP requires at least the first frizzled domain and the first four LDLR repeats, which likely bind directly to proANP (Knappe et al. 2004). The corin zymogen itself is activated by an unidentified serine protease in a process that involves the second frizzled domain and N-linked glycans within the serine protease domain (Liao et al. 2007, Wang et al. 2008). Importantly, corin zymogen activation is a rate-limiting step for the entire natriuretic peptide system, as revealed by recent genome-wide association studies. Thus, a minor CORIN allele that is characterized by two nonsynonymous nonconservative single nucleotide polymorphisms is present in approximately 10% of surveyed African Americans and gives rise to a corin variant protein with T555I and Q568P substitutions in the second frizzled domain (Dries et al. 2005). ProANP processing by this corin variant is unaltered, but zymogen activation is 60% reduced (Wang et al. 2008). This reduction of corin zymogen activation puts carriers of the variant CORIN allele at increased risk for both hypertension and cardiac hypertrophy (Dries et al. 2005, Rame et al. 2007).
Adjusting Cellular Iron Export to Iron Need
Iron homeostasis is maintained by regulating intestinal iron absorption and the release from macrophages of iron recycled from erythrocytes (Figure 5). Both processes are controlled systemically by a hepatic peptide hormone, hepcidin (reviewed in Andrews 2008, Nemeth & Ganz 2006). Hepcidin binds directly to the major iron export protein, ferroportin, which is located on macrophages and on the basolateral membrane of iron-absorbing enterocytes of the duodenum. This binding triggers the internalization and ubiquitin-mediated lysosomal degradation of ferroportin. Loss of ferroportin thus prevents both intestinal iron uptake and release of iron from intracellular stores of iron-recycling macrophages, with the net result of decreased serum iron (De Domenico et al. 2007, 2008; Nemeth et al. 2004b, 2006). Hepcidin is regulated at the level of transcription, as it is induced by iron overload and suppressed by iron deficiency, anemia, and hypoxia (Nemeth et al. 2004a, Nicolas et al. 2002). The GPI-anchored protein hemojuvelin is a major regulator of hepcidin production that functions as a coreceptor for bone morphogenetic protein (BMP)6, which signals through BMP receptors and SMAD proteins to stimulate hepcidin gene (HAMP) transcription (Figure 5; Andriopoulos et al. 2009, Babitt et al. 2006, Huang et al. 2005, Meynard et al. 2009, Niederkofler et al. 2005, Roetto et al. 2003, Wang et al. 2005).
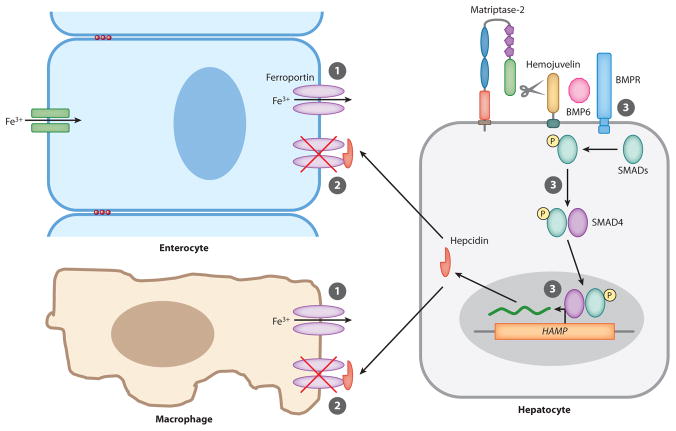
Matriptase-2 regulates cellular iron export. ➀ Dietary iron and iron recycled from erythrocytes are stored in enterocytes and splenic macrophages and are released to the circulation through ferroportin. ➁ Hepcidin produced by hepatocytes binds ferroportin and targets the channel for degradation. ➂ Hepcidin gene (HAMP) expression is positively regulated by bone morphogenic protein (BMP)6, which signals through the BMP receptor (BMPR)-SMAD pathway in a hemojuvelin-dependent manner. Matriptase-2 increases cellular iron export by degrading hemojuvelin to reduce hepcidin production and thus increase ferroportin levels.
The homeostatic circuit outlined above, although extremely elegant, does not account for how iron levels regulate hepcidin synthesis. A surprising explanation involving cell surface proteolysis emerged recently from two independent lines of research: the study of individuals with the rare autosomal recessive disorder, iron-refractory, iron-deficiency anemia (IRIDA), and the generation of mice deficient in Tmprss6, which encodes matriptase-2. Individuals with IRIDA present with an iron deficiency anemia that is unresponsive to oral iron administration. Paradoxically, hepcidin levels are high in IRIDA patients, indicating a systemic failure to suppress hepcidin gene transcription in response to iron deficiency. Tmprss6-deficient mice displayed a strikingly similar phenotype, which prompted the identification of TMPRSS6 loss-of-function mutations in IRIDA patients (Du et al. 2008, Finberg et al. 2008, Folgueras et al. 2008, Hooper et al. 2003, Velasco et al. 2002). Independent support for a role of matriptase-2 in regulating iron homeostasis was gained subsequently from several genome-wide association studies that all strongly linked serum iron, erythrocyte volume, and hemoglobin levels to common nonsynonymous coding single nucleotide polymorphisms in TMPRSS6 (Benyamin et al. 2009, Chambers et al. 2009, Ganesh et al. 2009, Tanaka et al. 2010).
Matriptase-2 is hepatocyte specific, and its expression is negatively regulated by iron, most likely at the level of mRNA translation (Hooper et al. 2003, Velasco et al. 2002, Zhang et al. 2010). This location and mode of regulation implies that the protease regulates hepcidin production by cleavage and inactivation of a substrate on the hepatocyte plasma membrane that is part of the BMP/SMAD pathway. Three lines of evidence strongly suggest that this substrate is hemojuvelin: (a) Hemojuvelin binds to the stem region of both the matriptase-2 zymogen and the active protease, (b) forced overexpression of matriptase-2 dramatically reduces cell surface hemojuvelin levels (Altamura et al. 2010, Silvestri et al. 2008), and (c) iron overload disease and low hepcidin levels are observed in hemojuvelin/matriptase-2 double-deficient mice as well as in BMP6/matriptase-2 double-deficient mice (Lenoir et al. 2011, Truksa et al. 2009).
Completing the Cycle of Life: Fertilization
Mammalian fertilization is a complex process that involves sequential sperm penetration of the cumulus cell layer surrounding the oocyte, binding to and invasion of the tough zona pellucida extracellular matrix, and, finally, the fusion of the sperm and oocyte plasma membranes (Primakoff & Myles 2002). An involvement of trypsin-like serine proteases in these processes was long suspected because of the inhibitory effect of benzamidine-derived active site inhibitors on zona pellucida penetration during in vitro fertilization (Fraser 1982). Two trypsin-like serine proteases are expressed specifically in mature sperm: the secreted protease, acrosin, which is located in a large secretory granule of the sperm head and is released by exocytosis during fertilization, and the GPI-anchored protease testisin, which is located on the sperm head membrane and in other parts of the sperm (Honda et al. 2002, Hooper et al. 1999, Klemm et al. 1991, Netzel-Arnett et al. 2009). Notably, the combined activity of the two proteases accounts for almost all measurable serine protease activity in sperm extracts (Kawano et al. 2010).
Ablation of acrosin or testisin affects only marginally the reproductive success of male mice, although distinct in vitro fertilization defects are evident (Adham et al. 1997, Baba et al. 1994, Netzel-Arnett et al. 2009, Yamashita et al. 2008). Combined deficiency of acrosin and testisin, however, causes complete loss of in vitro fertilization capacity by exacerbating the defects noted in single-deficient sperm (Kawano et al. 2010), thus revealing an interesting case of functional redundancy between a secreted serine protease and a membrane-anchored serine protease. Double-deficient sperm fail to penetrate and disperse the outer cumulus cell layer, erode and invade the zona pellucida, and fuse with the oocyte plasma membrane when added to intact oocytes, cumulus-free oocytes, and zona pellucida–denuded oocytes in vitro, respectively. Remarkably, however, when mated with females, the reproductive success of acrosin and testisin double-deficient male mice is reduced only by approximately 50%, owing to the acquisition by the double-deficient sperm of an unknown maternal factor during uterine transit (presumably an acrosin/testisin-like serine protease) (Kawano et al. 2010). Because active site serine protease inhibitors cause similar fertilization defects (Fraser 1982, Kawano et al. 2010), it is reasonable to assume that testisin and acrosin impart fertilizing capacity to sperm by proteolytic events, and their functional redundancy would indicate that both cleave the same substrate(s). Also, the multiplicity of fertilization defects seen in double-deficient mice suggests that acrosin and testisin regulate a fundamental signaling pathway in sperm rather than simply execute the cleavage of one or more structural proteins in the oocyte matrix.
SIGNIFICANT GAPS IN KNOWLEDGE
Regulation of Substrate Cleavage
As is clear from the preceding sections, membrane-anchored serine proteases frequently sense changes in the extracellular milieu and initiate a rapid response to restore cell and tissue homeostasis through cleavage and activation or destruction of specific target substrates. Knowledge of how specific environmental changes are translated into an altered rate of target substrate cleavage is fragmentary and represents a key challenge for future research. The absence of crystal structures of the entire extracellular domains of these proteases is a particularly lamentable obstacle to closing this knowledge gap. By inference from other proteolytic systems, this process is likely to be specific for individual proteases and may include conformational changes in the protease itself, its cognate inhibitors, cofactors, or the target substrates that lead to altered zymogen activation, catalytic activity, protease-substrate compartmentalization, or a spatially favorable orientation of the substrate for cleavage.
Orphan Membrane-Anchored Serine Proteases
The unraveling of the cellular and developmental functions of membrane-anchored serine proteases has been rapid. Nevertheless, approximately half of the family members (HAT, DESC1, TMPRSS11A, HAT-like 4, HAT-like 5, TMPRSS2, TMPRSS4, MSPL, matriptase-3, polyserase-1, and tryptase γ1) remain orphans in the sense that no functions or substrates are known. HAT, TMPRSS11A, or TMPRSS2 deficiency in mice is not associated with an obvious spontaneous phenotype, which suggests subtle or partially redundant functions (Kim et al. 2006; K. Sales & T.H. Bugge, unpublished data). The effects of loss-of-function mutations still have not been determined for DESC1, HAT-like 4, HAT-like 5, TM-PRSS4, MSPL, matriptase-3, polyserase-1, and tryptase γ1, and these may prove immediately insightful. On the basis of structural similarities and overlapping expression, partial functional redundancies may be anticipated between members of the TMPRSS subfamily and between members of the HAT/DESC subfamily, but these redundancies may be challenging to delineate genetically owing to the tight clustering of their cognate genes.
Target Substrates
Some membrane-anchored serine proteases (hepsin, TMPRSS3, spinesin, testisin) have established physiological functions, but the substrates that are cleaved to exert these functions are as yet unknown. For still other proteases (prostasin and matriptase), multiple functions have been identified, but target substrates are known for only some of these. Connecting the cellular and developmental functions of this protease family to the cleavage of specific target substrates will be a particularly challenging enterprise because of the marked reliance of membrane-anchored serine proteases on microenvironmental factors for target substrate selection, the general promiscuity of proteases in purified systems, and the lack of stringent ex vivo or cell-based assays for protease substrate identification.
Species-Specific Functions
Functional information on the cellular and developmental roles of HAT, TMPRSS11A, hepsin, TMPRSS2, prostasin, and testisin is currently available only for mice, and caution must be exerted when extrapolating findings obtained from animal experiments to humans. This is especially true as regards proteases because of the remarkable divergence of the human and mouse protease gene complements (Puente et al. 2003, 2005; Quesada et al. 2010). Of some comfort is that matriptase-2 and corin have conserved functions in the two species. However, matriptase-deficient humans present with epidermal barrier, hair, tooth, and possibly tear and sweat gland defects, but the pervasive loss of intestinal epithelial function characteristic of matriptase-ablated mice is not apparent in these individuals (Alef et al. 2009, Avrahami et al. 2008, Basel-Vanagaite et al. 2007). Whether this is due to fundamentally different roles of matriptase in the human and mouse gastrointestinal tract or to species-specific functional redundancies (for example, in the activation of the prostasin zymogen) is unclear. Clearly, species differences also exist in the regulation of membrane-anchored serine proteases. Thus, the absence of a matriptase inhibitor, HAI-2, in humans and mice causes a spectacular, but essentially nonoverlapping, set of developmental abnormalities in the two species (Heinz-Erian et al. 2009, Szabo et al. 2009a).
Acknowledgments
The authors thank Drs. Mary Jo Danton, Silvio Gutkind, Kelly Ten Hagen, Karin List, and Larry Wahl for critically reviewing this manuscript. The National Institute of Dental and Craniofacial Research intramural program supported this work. We apologize to our many colleagues whose research could not be appropriately cited owing to space constraints.
Glossary
| S1 peptidase family | phylogenetically related group of serine proteases with a common catalytic domain structure and mechanism of catalysis |
| Ectoderm | outer layer of the early embryo that is formed by the outer layer of germ cells |
| Surface ectoderm | the part of the ectoderm that forms the epidermis, epithelium of oral and nasal cavity, lens, cornea, and tooth enamel |
| Anencephaly and spina bifida | inborn absence of a large part of the brain and failure of the spinal cord to close before birth, respectively |
| Protease-activated receptor (PAR) | G protein–coupled signaling receptor that is activated by site-specific cleavage of the extracellular domain by serine proteases |
| Thrombin | secreted trypsin-like serine protease that cleaves multiple target substrates to first promote and subsequently inhibit hemostasis; activates PARs |
| Hepatocyte growth factor activator inhibitor (HAI)-1 and -2 | membrane-anchored Kunitz-type serine protease inhibitors that are physiological regulators of matriptase and prostasin, and likely other membrane-anchored serine proteases |
| Placental labyrinth | placental structure where fetal-maternal exchange of nutrients and oxygen takes place |
| Kallikrein-related peptidases | a large family of secreted trypsin- or chymotrypsin-like serine proteases encoded by a contiguous noninterrupted gene cluster |
| μ-Calpain | calcium-dependent cytoplasmic cysteine protease of the calpain family that proteolytically processes profilaggrin to filaggrin monomers |
| Caspase-14 | caspase-type cytoplasmic cysteine protease expressed in upper epidermal layers that converts filaggrin monomers into free amino acids |
| Bleomycin hydrolase | hydrolase related to cysteine proteases that converts filaggrin monomers with deiminated side chains into free amino acids |
| Profilaggrin | large polyprotein abundant in terminally differentiating keratinocytes that is a source of moisturizing and UV light–absorbing stratum corneum amino acids |
| Ichthyosis (fish skin) | a heterogeneous group of skin disorders characterized by dry, thickened, scaly, or flaky epidermis; they range from mild to life threatening |
| Aprotinin | basic/ bovine pancreatic trypsin inhibitor (BPTI, trade name Trasylol); a broad specificity Kunitz-type serine protease inhibitor |
| Paracellular permeability | the ability of substances to diffuse between cells within a confluent cell layer |
| Transepithelial electrical resistance | the degree to which a confluent cell monolayer limits the passage of ions. Used as measure of paracellular permeability |
| Transcytosis | process by which macromolecules are transported across the interior of a cell in a vesicle |
| Tectorial membrane | acellular connecting tissue membrane that covers auditory hair cells in the cochlea |
| Furin | membrane-anchored serine protease of the proprotein convertase family located in the secretory pathway and on the cell surface; it cleaves and matures multiple proteins |
| Cumulus cell layer | an aggregate of granulosa cells that surrounds and nourishes the oocyte |
| Zona pellucida | thick glycoprotein membrane that surrounds the oocyte plasma membrane |
Footnotes
*This is a work of the U.S. Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adham IM, Nayernia K, Engel W. Spermatozoa lacking acrosin protein show delayed fertilization. Mol Reprod Dev. 1997;46:370–76. [Abstract] [Google Scholar]
- Alef T, Torres S, Hausser I, Metze D, Tursen U, et al. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Investig Dermatol. 2009;129:862–69. [Abstract] [Google Scholar]
- Altamura S, D’Alessio F, Selle B, Muckenthaler M. A novel TMPRSS6 mutation that prevents protease auto-activation causes IRIDA. Biochem J. 2010;431:363–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol. 2006;17:968–76. [Abstract] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30. [Europe PMC free article] [Abstract] [Google Scholar]
- Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–87. [Europe PMC free article] [Abstract] [Google Scholar]
- Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, et al. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet. 2008;74:47–53. [Abstract] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845–49. [Abstract] [Google Scholar]
- Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–39. [Abstract] [Google Scholar]
- Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, et al. Role of γENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoal-dosteronism. J Clin Investig. 1998;102:1634–40. [Europe PMC free article] [Abstract] [Google Scholar]
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–77. [Europe PMC free article] [Abstract] [Google Scholar]
- Ben-Yosef T, Wattenhofer M, Riazuddin S, Ahmed ZM, Scott HS, et al. Novel mutations of TMPRSS3 in four DFNB8/B10 families segregating congenital autosomal recessive deafness. J Med Genet. 2001;38:396–400. [Europe PMC free article] [Abstract] [Google Scholar]
- Benyamin B, Ferreira MA, Willemsen G, Gordon S, Middelberg RP, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41:1173–75. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, et al. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem. 2007;282:6153–60. Proposes a specific molecular mechanism for the homeostatic regulation of ENaC activity by membrane-anchored serine proteases. [Abstract] [Google Scholar]
- Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–81. [Europe PMC free article] [Abstract] [Google Scholar]
- Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, et al. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA. 2010;107:4200–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, et al. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18:25–38. Uncovered an essential role of membrane-anchored serine proteases in the induction of neural tube closure through PAR-2 activation. [Europe PMC free article] [Abstract] [Google Scholar]
- Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41:1170–72. [Europe PMC free article] [Abstract] [Google Scholar]
- Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci USA. 2005;102:785–90. Definitively identifies corin as the long sought-after physiological pro-ANP convertase and demonstrates hypertension and cardiac hypertrophy after corin ablation. [Europe PMC free article] [Abstract] [Google Scholar]
- Charles RP, Guitard M, Leyvraz C, Breiden B, Haftek M, et al. Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J Biol Chem. 2008;283:2622–30. [Abstract] [Google Scholar]
- Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000a;25:141–42. [Abstract] [Google Scholar]
- Chavanas S, Garner C, Bodemer C, Ali M, Teillac DH, et al. Localization of the Netherton syndrome gene to chromosome 5q32, by linkage analysis and homozygosity mapping. Am J Hum Genet. 2000b;66:914–21. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen E, Talmon A, Faff O, Bacher A, Ben-Shaul Y. Formation of tight junctions in epithelial cells. I Induction by proteases in a human colon carcinoma cell line. Exp Cell Res. 1985;156:103–16. [Abstract] [Google Scholar]
- Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217–30. [Abstract] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. [Abstract] [Google Scholar]
- de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–70. [Abstract] [Google Scholar]
- De Domenico I, Nemeth E, Nelson JM, Phillips JD, Ajioka RS, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8:146–56. [Europe PMC free article] [Abstract] [Google Scholar] Retracted
- De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–78. [Europe PMC free article] [Abstract] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–74. [Abstract] [Google Scholar]
- Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell. 2007;18:3607–19. [Europe PMC free article] [Abstract] [Google Scholar]
- Descargues P, Deraison C, Bonnart C, Kreft M, Kishibe M, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. [Abstract] [Google Scholar]
- Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, et al. Corneodesmosomal cadherins are preferential targets of stratum corneum trypsin- and chymotrypsin-like hyperactivity in Netherton syndrome. J Investig Dermatol. 2006;126:1622–32. [Abstract] [Google Scholar]
- Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–81. [Abstract] [Google Scholar]
- Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. [Abstract] [Google Scholar]
- Du X, She E, Gelbart T, Truksa J, Lee P, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–92. [Europe PMC free article] [Abstract] [Google Scholar]
- Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–93. [Abstract] [Google Scholar]
- Fan B, Brennan J, Grant D, Peale F, Rangell L, Kirchhofer D. Hepatocyte growth factor activator inhibitor-1 (HAI-1) is essential for the integrity of basement membranes in the developing placental labyrinth. Dev Biol. 2007;303:222–30. [Abstract] [Google Scholar]
- Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–71. The first study to describe the essential role of matriptase-2 in the regulation of iron homeostasis. [Europe PMC free article] [Abstract] [Google Scholar]
- Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO J. 1998;17:344–52. [Europe PMC free article] [Abstract] [Google Scholar]
- Folgueras AR, de Lara FM, Pendas AM, Garabaya C, Rodriguez F, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–45. [Abstract] [Google Scholar]
- Fraser LR. p-Aminobenzamidine, an acrosin inhibitor, inhibits mouse sperm penetration of the zona pellucida but not the acrosome reaction. J Reprod Fertil. 1982;65:185–94. [Abstract] [Google Scholar]
- Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, et al. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem. 2010;286:5793–802. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. [Abstract] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. [Europe PMC free article] [Abstract] [Google Scholar]
- Ganesh SK, Zakai NA, van Rooij FJ, Soranzo N, Smith AV, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41:1191–98. [Europe PMC free article] [Abstract] [Google Scholar]
- García-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol. 2008;132:521–35. [Europe PMC free article] [Abstract] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–96. [Abstract] [Google Scholar]
- Gladysheva IP, Robinson BR, Houng AK, Kovats T, King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. 2008;44:131–42. [Abstract] [Google Scholar]
- Guipponi M, Tan J, Cannon PZ, Donley L, Crewther P, et al. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol. 2007;171:608–16. [Europe PMC free article] [Abstract] [Google Scholar]
- Guipponi M, Toh MY, Tan J, Park D, Hanson K, et al. An integrated genetic and functional analysis of the role of type II transmembrane serine proteases (TMPRSSs) in hearing loss. Hum Mutat. 2008;29:130–41. [Abstract] [Google Scholar]
- Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, et al. The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet. 2002;11:2829–36. [Abstract] [Google Scholar]
- Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Investig Dermatol. 2006;126:1609–21. [Abstract] [Google Scholar]
- Hanifa S, Scott HS, Crewther P, Guipponi M, Tan J. Thyroxine treatments do not correct inner ear defects in tmprss1 mutant mice. NeuroReport. 2010;21:897–901. [Europe PMC free article] [Abstract] [Google Scholar]
- Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102:4501–24. [Abstract] [Google Scholar]
- Heinz-Erian P, Müller T, Krabichler B, Schranz M, Becker C, et al. Mutations in SPINT2 cause a syndromic form of congenital sodium diarrhea. Am J Hum Genet. 2009;84:188–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Honda A, Yamagata K, Sugiura S, Watanabe K, Baba T. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2002;277:16976–84. [Abstract] [Google Scholar]
- Hooper JD, Campagnolo L, Goodarzi G, Truong TN, Stuhlmann H, Quigley JP. Mouse matriptase-2: identification, characterization and comparative mRNA expression analysis with mouse hepsin in adult and embryonic tissues. Biochem J. 2003;373:689–702. [Europe PMC free article] [Abstract] [Google Scholar]
- Hooper JD, Nicol DL, Dickinson JL, Eyre HJ, Scarman AL, et al. Testisin, a new human serine proteinase expressed by premeiotic testicular germ cells and lost in testicular germ cell tumors. Cancer Res. 1999;59:3199–205. [Abstract] [Google Scholar]
- Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–37. [Abstract] [Google Scholar]
- Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Investig. 2005;115:2187–91. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–14. [Abstract] [Google Scholar]
- Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem. 2003;278:37073–82. [Abstract] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, et al. Early death due to defective neonatal lung liquid clearance in α-ENaC-deficient mice. Nat Genet. 1996;12:325–28. [Abstract] [Google Scholar]
- Inouye K, Tsuzuki S, Yasumoto M, Kojima K, Mochida S, Fushiki T. Identification of the matriptase second CUB domain as the secondary site for interaction with hepatocyte growth factor activator inhibitor type-1. J Biol Chem. 2010;285:33394–403. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamata Y, Taniguchi A, Yamamoto M, Nomura J, Ishihara K, et al. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem. 2009;284:12829–36. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawano N, Kang W, Yamashita M, Koga Y, Yamazaki T, et al. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol Reprod. 2010;83:359–69. A comprehensive study revealing redundant roles of testisin in multiple steps of fertilization. [Abstract] [Google Scholar]
- Kim C, Cho Y, Kang CH, Kim MG, Lee H, et al. Filamin is essential for shedding of the transmembrane serine protease, epithin. EMBO Rep. 2005;6:1045–51. [Europe PMC free article] [Abstract] [Google Scholar]
- Kim TS, Heinlein C, Hackman RC, Nelson PS. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol. 2006;26:965–75. [Europe PMC free article] [Abstract] [Google Scholar]
- Klemm U, Müller-Esterl W, Engel W. Acrosin, the peculiar sperm-specific serine protease. Hum Genet. 1991;87:635–41. [Abstract] [Google Scholar]
- Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–71. [Abstract] [Google Scholar]
- Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278:52363–70. [Abstract] [Google Scholar]
- Komatsu N, Takata M, Otsuki N, Ohka R, Amano O, et al. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5-derived peptides. J Investig Dermatol. 2002;118:436–43. [Abstract] [Google Scholar]
- Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, et al. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–105. [Abstract] [Google Scholar]
- Lenoir A, Deschemin JC, Kautz L, Ramsay AJ, Roth MP, et al. Iron deficiency anemia due to matriptase-2 inactivation is dependent upon the presence of functional Bmp6. Blood. 2011;117:647–50. [Europe PMC free article] [Abstract] [Google Scholar]
- Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–35. [Abstract] [Google Scholar]
- List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, et al. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007a;282:36714–23. [Abstract] [Google Scholar]
- List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, et al. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–79. First identification of an essential role of membrane-anchored serine proteases in terminal epidermal differentiation. [Abstract] [Google Scholar]
- List K, Hobson JP, Molinolo A, Bugge TH. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol. 2007b;213:237–45. [Abstract] [Google Scholar]
- List K, Kosa P, Szabo R, Bey AL, Wang CB, et al. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–63. [Europe PMC free article] [Abstract] [Google Scholar]
- List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–25. [Europe PMC free article] [Abstract] [Google Scholar]
- List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, et al. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–10. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu L, Hering-Smith KS, Schiro FR, Hamm LL. Serine protease activity in M-1 cortical collecting duct cells. Hypertension. 2002;39:860–64. [Abstract] [Google Scholar]
- López-Otín C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008;283:30433–37. [Europe PMC free article] [Abstract] [Google Scholar]
- Magert HJ, Standker L, Kreutzmann P, Zucht HD, Reinecke M, et al. LEKTI, a novel 15-domain type of human serine proteinase inhibitor. J Biol Chem. 1999;274:21499–502. [Abstract] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Investig. 1999;104:R19–23. [Europe PMC free article] [Abstract] [Google Scholar]
- Mauro T, Guitard M, Behne M, Oda Y, Crumrine D, et al. The ENaC channel is required for normal epidermal differentiation. J Investig Dermatol. 2002;118:589–94. [Abstract] [Google Scholar]
- McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, et al. Disruption of the β subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci USA. 1999;96:1727–31. [Europe PMC free article] [Abstract] [Google Scholar]
- McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–77. [Abstract] [Google Scholar]
- Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81. [Abstract] [Google Scholar]
- Nagaike K, Kawaguchi M, Takeda N, Fukoshima T, Sawaguchi A, et al. Defect of hepatocyte growth factor activator inhibitor type 1/serine protease inhibior, Kunitz type 1 (Hai-1/Spint1) leads to ichthyosis-like condition and abnormal hair development in mice. Am J Pathol. 2008;173:1–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, et al. Regulation of prostasin by aldosterone in the kidney. J Clin Investig. 2002;109:401–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. [Abstract] [Google Scholar]
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–42. [Abstract] [Google Scholar]
- Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328–33. [Europe PMC free article] [Abstract] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig. 2004a;113:1271–76. [Europe PMC free article] [Abstract] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004b;306:2090–93. [Abstract] [Google Scholar]
- Netzel-Arnett S, Bugge TH, Hess RA, Carnes K, Stringer BW, et al. The glycosylphosphatidylinositol-anchored serine protease PRSS21 (testisin) imparts murine epididymal sperm cell maturation and fertilizing ability. Biol Reprod. 2009;81:921–32. [Europe PMC free article] [Abstract] [Google Scholar]
- Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, et al. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–45. Proposes that matriptase and prostasin are part of a single membrane-anchored serine protease cascade initiated by matriptase autoactivation. [Abstract] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Investig. 2002;110:1037–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Investig. 2005;115:2180–86. [Europe PMC free article] [Abstract] [Google Scholar]
- Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278:26773–79. [Abstract] [Google Scholar]
- Page MJ, Di Cera E. Serine peptidases: classification, structure and function. Cell Mol Life Sci. 2008;65:1220–36. [Abstract] [Google Scholar]
- Planès C, Randrianarison NH, Charles R-P, Frateschi S, Cluzeaud F, et al. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med. 2009;2:26–37. First study to provide a definitive demonstration of the homeostatic regulation of ENaC by a membrane-anchored serine protease in vivo. Provides a molecular mechanism for the regulation of iron homeostasis by matriptase-2. [Europe PMC free article] [Abstract] [Google Scholar]
- Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. [Abstract] [Google Scholar]
- Primakoff P, Myles DG. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science. 2002;296:2183–85. [Abstract] [Google Scholar]
- Puente XS, Sánchez LM, Gutiérrez-Fernández A, Velasco G, López-Otín C. A genomic view of the complexity of mammalian proteolytic systems. Biochem Soc Trans. 2005;33:331–34. [Abstract] [Google Scholar]
- Puente XS, Sánchez LM, Overall CM, López-Otín C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–58. [Abstract] [Google Scholar]
- Quesada V, Velasco G, Puente XS, Warren WC, López-Otín C. Comparative genomic analysis of the zebra finch degradome provides new insights into evolution of proteases in birds and mammals. BMC Genomics. 2010;11:220. [Europe PMC free article] [Abstract] [Google Scholar]
- Rame JE, Drazner MH, Post W, Peshock R, Lima J, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–64. [Abstract] [Google Scholar]
- Ramsay AJ, Quesada V, Sanchez M, Garabaya C, Sardá MP, et al. Matriptase-2 mutations in iron-refractory iron deficiency anemia patients provide new insights into protease activation mechanisms. Hum Mol Genet. 2009;18:3673–83. [Abstract] [Google Scholar]
- Rawlings ND, Barrett AJ. Evolutionary families of peptidases. Biochem J. 1993;290(Pt. 1):205–18. [Europe PMC free article] [Abstract] [Google Scholar]
- Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. [Abstract] [Google Scholar]
- Roop D. Defects in the barrier. Science. 1995;267:474–75. [Abstract] [Google Scholar]
- Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–97. [Abstract] [Google Scholar]
- Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–79. [Abstract] [Google Scholar]
- Sales KU, Masedunskas A, Bey AL, Rasmussen AL, Weigert R, et al. Matriptase initiates activation of epidermal prokallikrein and disease onset in a mouse model of Netherton syndrome. Nat Genet. 2010;42:676–83. [Europe PMC free article] [Abstract] [Google Scholar]
- Schechter NM, Choi EJ, Wang ZM, Hanakawa Y, Stanley JR, et al. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI) Biol Chem. 2005;386:1173–84. [Abstract] [Google Scholar]
- Scott HS, Kudoh J, Wattenhofer M, Shibuya K, Berry A, et al. Insertion of β-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat Genet. 2001;27:59–63. [Abstract] [Google Scholar]
- Segre J. Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol. 2003;15:776–82. [Abstract] [Google Scholar]
- Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56:1166–76. [Abstract] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. [Abstract] [Google Scholar]
- Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–11. Provides a molecular mechanism for the regulation of iron homeostasis by matriptase-2. [Europe PMC free article] [Abstract] [Google Scholar]
- Spacek DV, Perez AF, Ferranti KM, Wu LK, Moy DM, et al. The mouse frizzy ( fr) and rat ‘hairless’ ( frCR) mutations are natural variants of protease serine S1 family member 8 (Prss8) Exp Dermatol. 2010;19:527–32. [Abstract] [Google Scholar]
- Steensgaard M, Svenningsen P, Tinning AR, Nielsen TD, Jorgensen F, et al. Apical serine protease activity is necessary for assembly of a high-resistance renal collecting duct epithelium. Acta Physiol. 2010;200:347–59. [Abstract] [Google Scholar]
- Suzuki Y, Nomura J, Koyama J, Horii I. The role of proteases in stratum corneum: involvement in stratum corneum desquamation. Arch Dermatol Res. 1994;286:249–53. [Abstract] [Google Scholar]
- Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009a;136:2653–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies Spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol. 2009b;174:2015–22. [Europe PMC free article] [Abstract] [Google Scholar]
- Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–56. [Abstract] [Google Scholar]
- Szabo R, Wu Q, Dickson RB, Netzel-Arnett S, Antalis TM, Bugge TH. Type II transmembrane serine proteases. Thromb Haemost. 2003;90:185–93. [Abstract] [Google Scholar]
- Tanaka H, Nagaike K, Takeda N, Itoh H, Kohama K, et al. Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is required for branching morphogenesis in the chorioallantoic placenta. Mol Cell Biol. 2005;25:5687–98. [Europe PMC free article] [Abstract] [Google Scholar]
- Tanaka T, Roy CN, Yao W, Matteini A, Semba RD, et al. A genome-wide association analysis of serum iron concentrations. Blood. 2010;115:94–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;287:L928–35. [Abstract] [Google Scholar]
- Truksa J, Gelbart T, Peng H, Beutler E, Beutler B, Lee P. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br J Haematol. 2009;147:571–81. [Abstract] [Google Scholar]
- Tsuzuki S, Murai N, Miyake Y, Inouye K, Hirayasu H, et al. Evidence for the occurrence of membrane-type serine protease 1/matriptase on the basolateral sides of enterocytes. Biochem J. 2005;388:679–87. [Europe PMC free article] [Abstract] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–10. [Abstract] [Google Scholar]
- Velasco G, Cal S, Quesada V, Sánchez LM, López-Otín C. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem. 2002;277:37637–46. [Abstract] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol. 2002;120:191–201. [Europe PMC free article] [Abstract] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, et al. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–34. [Abstract] [Google Scholar]
- Wakida N, Kitamura K, Tuyen DG, Maekawa A, Miyoshi T, et al. Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-β1 and aldosterone. Kidney Int. 2006;70:1432–38. [Abstract] [Google Scholar]
- Wang RH, Li C, Xu X, Zheng Y, Xiao C, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. [Abstract] [Google Scholar]
- Wang W, Liao X, Fukuda K, Knappe S, Wu F, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–46. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamashita M, Honda A, Ogura A, Kashiwabara S, Fukami K, Baba T. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes Cells. 2008;13:1001–13. [Abstract] [Google Scholar]
- Yamazaki M, Ishidoh K, Suga Y, Saido TC, Kawashima S, et al. Cytoplasmic processing of human profilaggrin by active μ-calpain. Biochem Biophys Res Commun. 1997;235:652–56. [Abstract] [Google Scholar]
- Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. [Abstract] [Google Scholar]
- Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–29. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang AS, Anderson SA, Wang J, Yang F, DeMaster K, et al. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2010;117:1687–99. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng XL, Kitamoto Y, Sadler JE. Enteropeptidase, a type II transmembrane serine protease. Front Biosci (Elite Ed) 2009;1:242–49. [Abstract] [Google Scholar]
- Zohn IE, Sarkar AA. Modeling neural tube defects in the mouse. Curr Top Dev Biol. 2008;84:1–35. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1146/annurev-cellbio-092910-154247
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3391589?pdf=render
Subscription required at AnnualReviews
http://arjournals.annualreviews.org/doi/full/10.1146/annurev-cellbio-092910-154247
Subscription required at AnnualReviews
http://arjournals.annualreviews.org/doi/pdf/10.1146/annurev-cellbio-092910-154247
Free to read at AnnualReviews
http://arjournals.annualreviews.org/doi/abs/10.1146/annurev-cellbio-092910-154247
Citations & impact
Impact metrics
Citations of article over time
Article citations
Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID.
NPJ Sci Food, 8(1):19, 30 Mar 2024
Cited by: 2 articles | PMID: 38555403 | PMCID: PMC10981760
Review Free full text in Europe PMC
Type II Transmembrane Serine Proteases as Modulators in Adipose Tissue Phenotype and Function.
Biomedicines, 11(7):1794, 23 Jun 2023
Cited by: 3 articles | PMID: 37509434 | PMCID: PMC10376093
Review Free full text in Europe PMC
A Machine Learning Approach to Identify Potential miRNA-Gene Regulatory Network Contributing to the Pathogenesis of SARS-CoV-2 Infection.
Biochem Genet, 62(2):987-1006, 29 Jul 2023
Cited by: 1 article | PMID: 37515735
Meta-analysis of age-related cognitive decline reveals a novel locus for the attention domain and implicates a COVID-19-related gene for global cognitive function.
Alzheimers Dement, 19(11):5010-5022, 23 Apr 2023
Cited by: 1 article | PMID: 37089073 | PMCID: PMC10590825
Competition for dominance within replicating quasispecies during prolonged SARS-CoV-2 infection in an immunocompromised host.
Virus Evol, 8(1):veac042, 21 May 2022
Cited by: 16 articles | PMID: 35706980 | PMCID: PMC9129230
Go to all (89) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Membrane-anchored proteases in endothelial cell biology.
Curr Opin Hematol, 23(3):243-252, 01 May 2016
Cited by: 12 articles | PMID: 26906027 | PMCID: PMC4882107
Review Free full text in Europe PMC
Membrane-anchored serine proteases as regulators of epithelial function.
Biochem Soc Trans, 48(2):517-528, 01 Apr 2020
Cited by: 17 articles | PMID: 32196551 | PMCID: PMC9869603
Review Free full text in Europe PMC
Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer.
Cancer Metastasis Rev, 22(2-3):237-258, 01 Jun 2003
Cited by: 183 articles | PMID: 12784999
Review
The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment.
Biochem J, 428(3):325-346, 01 Jun 2010
Cited by: 89 articles | PMID: 20507279 | PMCID: PMC3680374
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: ZIA DE000699-12




