Abstract
Background
Angiomyolipomas in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis are associated with mutations in tuberous sclerosis genes resulting in constitutive activation of the mammalian target of rapamycin (mTOR). The drug sirolimus suppresses mTOR signaling.Methods
We conducted a 24-month, nonrandomized, open-label trial to determine whether sirolimus reduces the angiomyolipoma volume in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. Sirolimus was administered for the first 12 months only. Serial magnetic resonance imaging of angiomyolipomas and brain lesions, computed tomography of lung cysts, and pulmonary-function tests were performed.Results
Of the 25 patients enrolled, 20 completed the 12-month evaluation, and 18 completed the 24-month evaluation. The mean (+/-SD) angiomyolipoma volume at 12 months was 53.2+/-26.6% of the baseline value (P<0.001) and at 24 months was 85.9+/-28.5% of the baseline value (P=0.005). At 24 months, five patients had a persistent reduction in the angiomyolipoma volume of 30% or more. During the period of sirolimus therapy, among patients with lymphangioleiomyomatosis, the mean forced expiratory volume in 1 second (FEV1) increased by 118+/-330 ml (P=0.06), the forced vital capacity (FVC) increased by 390+/-570 ml (P<0.001), and the residual volume decreased by 439+/-493 ml (P=0.02), as compared with baseline values. One year after sirolimus was discontinued, the FEV1 was 62+/-411 ml above the baseline value, the FVC was 346+/-712 ml above the baseline value, and the residual volume was 333+/-570 ml below the baseline value; cerebral lesions were unchanged. Five patients had six serious adverse events while receiving sirolimus, including diarrhea, pyelonephritis, stomatitis, and respiratory infections.Conclusions
Angiomyolipomas regressed somewhat during sirolimus therapy but tended to increase in volume after the therapy was stopped. Some patients with lymphangioleiomyomatosis had improvement in spirometric measurements and gas trapping that persisted after treatment. Suppression of mTOR signaling might constitute an ameliorative treatment in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. (ClinicalTrials.gov number, NCT00457808.)Free full text

Sirolimus for Angiomyolipoma in Tuberous Sclerosis Complex or Lymphangioleiomyomatosis
Abstract
Background
Angiomyolipomas in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis are associated with mutations in tuberous sclerosis genes resulting in constitutive activation of the mammalian target of rapamycin (mTOR). The drug sirolimus suppresses mTOR signaling.
Methods
We conducted a 24-month, nonrandomized, open-label trial to determine whether sirolimus reduces the angiomyolipoma volume in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. Sirolimus was administered for the first 12 months only. Serial magnetic resonance imaging of angiomyolipomas and brain lesions, computed tomography of lung cysts, and pulmonary-function tests were performed.
Results
Of the 25 patients enrolled, 20 completed the 12-month evaluation, and 18 completed the 24-month evaluation. The mean (±SD) angiomyolipoma volume at 12 months was 53.2±26.6% of the baseline value (P<0.001) and at 24 months was 85.9±28.5% of the baseline value (P = 0.005). At 24 months, five patients had a persistent reduction in the angiomyolipoma volume of 30% or more. During the period of sirolimus therapy, among patients with lymphangioleiomyomatosis, the mean forced expiratory volume in 1 second (FEV1) increased by 118±330 ml (P = 0.06), the forced vital capacity (FVC) increased by 390±570 ml (P<0.001), and the residual volume decreased by 439±493 ml (P = 0.02), as compared with baseline values. One year after sirolimus was discontinued, the FEV1 was 62±411 ml above the baseline value, the FVC was 346±712 ml above the baseline value, and the residual volume was 333±570 ml below the baseline value; cerebral lesions were unchanged. Five patients had six serious adverse events while receiving sirolimus, including diarrhea, pyelonephritis, stomatitis, and respiratory infections.
Conclusions
Angiomyolipomas regressed somewhat during sirolimus therapy but tended to increase in volume after the therapy was stopped. Some patients with lymphangioleiomyomatosis had improvement in spirometric measurements and gas trapping that persisted after treatment. Suppression of mTOR signaling might constitute an ameliorative treatment in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. (ClinicalTrials.gov number, NCT00457808.)
The tuberous sclerosis complex, a tumor-suppressor syndrome caused by mutations in the tuberin gene (TSC2) or the hamartin gene (TSC1), is characterized by hamartomas in organs including the brain, kidney, lung, skin, and heart.1 Angiomyolipomas — tumors rich in fat, muscle, and blood vessels that can hemorrhage or infiltrate the kidney, leading to renal failure — develop in approximately 80% of patients.2 Lymphangioleiomyomatosis, the major pulmonary manifestation in women with the tuberous sclerosis complex, is a progressive lung disease characterized by infiltration of smooth-muscle cells and formation of parenchymal cysts.3 Sporadic lymphangioleiomyomatosis can develop in women without the tuberous sclerosis complex, owing to somatic mutations in tuberous sclerosis genes.4 The cells comprising the lymphangioleiomyomatosis lesions and angiomyolipomas appear to arise from a common source.5
The hamartin–tuberin complex regulates the activity of the target of rapamycin complex 1, which lies downstream of cellular pathways controlling cell growth and proliferation. Abnormal signaling through the target of rapamycin complex 1 is involved in a number of tumor-suppressor syndromes6-8 and cancers.9 Sirolimus, an immunosuppressive agent approved by the Food and Drug Administration, forms a complex with FK binding protein 12 and inactivates the target of rapamycin complex 1, abrogating the signaling. Sirolimus corrects size defects in tuberin-deficient Drosophila melanogaster cells and induces the apoptosis of renal cystadenomas and hepatic hemangiomas in rodent models of the tuberous sclerosis complex.10,11 In our phase 1–2, proof-of-concept study, we aimed to determine whether sirolimus has an effect on the volume of angiomyolipomas in patients with the tuberous sclerosis complex, sporadic lymphangioleiomyomatosis, or both.
Methods
Selection and Enrollment of Patients
The consecutive enrollment of male and female patients, 18 to 65 years of age, began in May 2003 and continued until November 2004, when the target of 25 patients was reached. The voluntary provision of written informed consent, a confirmed diagnosis of the tuberous sclerosis complex12 or sporadic lymphangioleiomyomatosis,13,14 the use of contraception (for female patients), and the presence of at least one angiomyolipoma 1 cm or more in the largest dimension were required for participation. Exclusion criteria were the use of continuous supplemental oxygen; concurrent infection; surgery within 8 weeks before the start of sirolimus therapy; current or planned pregnancy; lactation; substantial hematologic, renal, hepatic, or metabolic abnormalities; and the use of an investigational drug within 30 days before entrance into the study. All female patients who could conceive were administered a pregnancy test before enrollment and at each visit.
Study Design
The study was conducted at the General Clinical Research Center of the Cincinnati Children's Hospital Medical Center. Patients were recruited from the Tuberous Sclerosis Clinic and the patient registry of the LAM Foundation. The institutional review board approved the study protocol, and a data and safety monitoring board reviewed trial progress semiannually.
In our open-label, phase 1–2 trial, all patients received sirolimus for 1 year and were then followed for an additional year after the therapy was stopped. The primary end point was angiomyolipoma volume at 1 year, and secondary end points included angiomyolipoma volume at 2 years and spirometric measurements, lung volumes, diffusing capacity, results of the 6-minute walk test, and the percentage of the cyst volume at 1 and 2 years. Patients were seen at baseline; at week 2, 3, or 4; and at months 2, 4, 6, 9, 12, 18, and 24. The angiomyolipomas were imaged at all visits except the visit at week 2, 3, or 4 and the visit at month 9. Sirolimus dosing was based on serum target levels that would prevent rejection in patients who had renal transplants. The initial sirolimus dose was 0.25 mg per square meter of body-surface area. Sirolimus levels were measured at 2 weeks, and the dosage was adjusted to achieve a blood sirolimus level between 1 and 5 ng per milliliter. If the target angiomyolipoma lesions had not decreased by 10% of the baseline value in the longest coronal-plane dimension at the 2-month visit, the dose was increased to achieve a blood sirolimus level of 5 to 10 ng per milliliter. At the 4-month visit, if the threshold of a 10% reduction from the baseline value had not been reached, the dose was increased to achieve a blood sirolimus level of 10 to 15 ng per milliliter. The dose chosen at the 4-month visit was continued through 12 months.
Imaging
Cross-sectional brain and abdominal evaluations were performed by means of magnetic resonance imaging (MRI) with the use of a clinical 1.5-Tesla system (General Electric Medical Systems). Coronal and axial fast spin–echo T2-weighted sequences were performed with and without fat suppression. At baseline, up to five lesions per patient were identified for volume measurement throughout the study. The volumes for all lesions from a given time point were averaged for use in analyses. Computed tomography (CT) of the lungs was performed during full inspiration and during full expiration, with the use of thin-section images. Brain MRI was performed with the use of an 8-channel phased-array head coil.
Tumor volume is typically estimated with the use of orthogonal measurements, which assume that the masses are ellipsoid.15 However, angiomyolipomas often have complex shapes, resulting from asymmetrical growth or the coalescence of multiple lesions.2 MRI and CT are easily adapted for volumetric analysis.16,17 Because volumetric techniques are superior to diameter-based approaches for measuring the size of tumors with complex shapes,18 we used a standardized validated software program,19 similar to software used for other renal-volume determinations,20 for volumetric analyses of angiomyolipomas and cystic lung lesions. One investigator measured angiomyolipoma volumes. For validation, another investigator independently measured the lesion volumes by measuring the three orthogonal diameters.
Pulmonary Function and 6-Minute Walk Testing
Testing was performed according to guidelines of the American Thoracic Society.21,22 Patients with lymphangioleiomyomatosis underwent complete pulmonary-function testing — including the measurement of spirometric variables, lung volumes, diffusing capacity, and the 6-minute walk distance — at baseline, 6 or 9 months, 12 months, and 24 months; simple spirometric measurements were obtained at other study visits. Assessment of reversible airflow obstruction was performed at baseline; if the results were positive, during subsequent visits, spirometric variables were measured after the administration of a bronchodilator.
Laboratory Studies
To assess safety, at each visit we measured the levels of electrolytes, blood urea nitrogen, creatinine, glucose, hepatic enzymes, bilirubin, serum lipids, and sirolimus, and performed a complete blood count and urinalysis.
Statistical Analysis
We estimated that 25 patients would be needed for our study to have a statistical power of 95% to detect the difference between a 0% reduction in the mean angiomyolipoma volume from the baseline value (the null hypothesis) and a 30% reduction (the alternative hypothesis). A one-sided test was used, since angiomyolipomas do not spontaneously regress. An independent interim analysis was undertaken by the Cincinnati Children's Hospital Biostatistical Core, after 10 patients had completed 1 year of sirolimus therapy, to determine whether there was evidence of a reduction in lesion volume and of adequate safety.
Analyses were performed with the use of the Proc Mixed procedure with the Kenward–Roger correction (SAS software, version 9.1). To avoid bias due to missing data, least-square means (±SD) are reported. These are model-based estimates calculated from parameter estimates. Separate analyses were performed for the angiomyolipoma volumes alone, those expressed as percentages of the baseline value, and the percentages of the predicted values for the pulmonary-function outcomes, which are comparisons of the patients' pulmonary-function values with normative values derived from population studies.
For variables for which there was a significant difference between the means at baseline and follow-up, we also compared the least-square means at these time points. To estimate the slopes and determine whether the least-square means had changed significantly by 12 months, a random-coefficient model involving a spline function was applied. All tests reflected in Tables 1 and and22 were conducted a priori. P values of less than 0.05 were considered to indicate statistical significance. Reported P values were not adjusted for multiple testing.
Table 1
| Value | Baseline (N = 20) | 12 Mo (N = 20) | 18 Mo (N = 19) | 24 Mo (N = 18) |
|---|---|---|---|---|
| Least-square mean (95% CI) — ml | 71.6±105.3 (24.9 to 118.2) | 36.5±105.3 (-10.2 to 83.2) | 64.8±106.1 (18.1 to 111.6) | 74.9±108.0 (27.8 to 121.9) |
| Range — ml | 1.0 to 389.0 | 0.7 to 169.9 | 0.7 to 357.2 | 1.1 to 462.4 |
| Least-square mean (95% CI) — % of baseline value | 53.2±26.6 (46.3 to 60.2) | 76.8±27.5 (69.7 to 83.9) | 85.9±28.5 (78.7 to 93.2) | |
| P value for change from baseline value | P<0.001 | P<0.001 | P = 0.005 |
Table 2
| Value | Baseline (N = 11) | 12 Mo (N = 11) | 24 Mo (N = 10) | Change from Baseline | |
|---|---|---|---|---|---|
| 12 Mo (N = 11) | 24 Mo (N = 10) | ||||
| FEV1 | |||||
| Least-square mean (95% CI) — liters | 1.77±0.79 (1.23 to 2.32) | 1.89±0.79 (1.34 to 2.44) | 1.83±0.79 (1.29 to 2.38) | 0.12±0.33 (-0.01 to 0.24) | 0.06±0.41 (-0.09 to 0.22) |
| Percent of predicted value (95% CI) | 57.2±22.57 (42.31 to 73.15) | 62.00±22.57 (46.58 to 77.42) | 60.37±22.73 (44.93 to 75.81) | 4.27±11.07 (0.11 to 8.44) | 2.64±13.84 (-2.52 to 7.80) |
| FVC | |||||
| Least-square mean (95% CI) — liters | 2.99±0.85 (2.42 to 3.56) | 3.38±0.85 (2.81 to 3.95) | 3.34±0.86 (2.77 to 3.90) | 0.39±0.57 (0.18 to 0.60)† | 0.35±0.71 (0.08 to 0.61)§ |
| Percent of predicted value (95% CI) | 78.82±19.72 (65.81 to 91.83) | 89.18±19.72 (76.17 to 102.19) | 88.65±19.99 (75.60 to 101.70) | 10.36±14.19 (5.07 to 15.65)† | 9.83±17.81 (3.32 to 16.35)¶ |
| Total lung capacity | |||||
| Least-square mean (95% CI) — liters | 5.25±0.86 (4.68 to 5.82) | 5.22±0.86 (4.64 to 5.79) | 5.23±0.87 (4.66 to 5.81) | -0.04±0.40 (-0.23 to 0.16) | -0.02±0.59 (-0.30 to 0.26) |
| Percent of predicted value (95% CI) | 99.09±15.33 (89.02 to 109.15) | 98.45±15.33 (88.39 to 108.52) | 98.48±15.65 (88.37 to 108.59) | -0.64±7.68 (-4.34 to 3.06) | -0.61±11.31 (-5.87 to 4.65) |
| Residual volume | |||||
| Least-square mean (95% CI) — liters | 2.30±0.84 (1.72 to 2.89) | 1.87±0.48 (1.53 to 2.20) | 1.97±0.55 (1.58 to 2.36) | -0.44±0.49 (-0.79 to -0.09)‡ | -0.33±0.57 (-0.73 to 0.07) |
| Percent of predicted value (95% CI) | 127.64±46.52 (94.86 to 160.41) | 100.64±25.17 (82.90 to 118.37) | 104.78±28.42 (84.76 to 124.79) | -27.00±27.06 (-46.07 to -7.94)§ | -22.86±31.45 (-44.74 to -0.98)‖ |
| DlCO | |||||
| Least-square mean (95% CI) — ml/mm Hg/min | 13.08±4.34 (10.02 to 16.14) | 13.16±4.48 (10.01 to 16.32) | 13.82±7.35 (8.67 to 18.97) | -0.08±2.67 (-1.80 to 1.96) | -0.74±4.02 (-2.06 to 3.53) |
| Percent of predicted value (95% CI) | 53.18±14.12 (43.24 to 63.12) | 53.27±14.62 (42.97 to 63.57) | 55.95±23.95 (39.18 to 72.73) | 0.09±11.66 (-8.12 to 8.30) | 2.77±15.49 (-8.00 to 13.55) |
| Cyst volume (95% CI) — % of lung volume | 21.63±12.32 (13.50 to 29.77) | 18.79±12.32 (10.66 to 26.93) | 18.17±12.49 (10.01 to 26.34) | -2.84±5.56 (-5.50 to -0.18) | -3.46±8.15 (-7.26 to 0.34) |
| 6-Minute walk distance (95% CI) — m | 485.30±82.87 (426.91 to 543.69) | 509.21±100.31 (438.53 to 579.89) | 465.88±160.65 (352.69 to 579.07) | 23.91±60.23 (-18.53 to 66.35) | -19.42±111.42 (-97.93 to 59.08) |
Results
Characteristics of the Patients
The 25 study patients consisted of 5 men and 2 women with the tuberous sclerosis complex only and 18 women with lymphangioleiomyomatosis, 12 of whom had the tuberous sclerosis complex with lymphangioleiomyomatosis and 6 of whom had sporadic lymphangioleiomyomatosis only. Five patients with the tuberous sclerosis complex (four who also had lymphangioleiomyomatosis) left the study during the first year: two withdrew consent, one had pyelonephritis and recurrent diarrhea, one had a unilateral renal hemorrhage, and one did not comply with the protocol (Fig. 1).
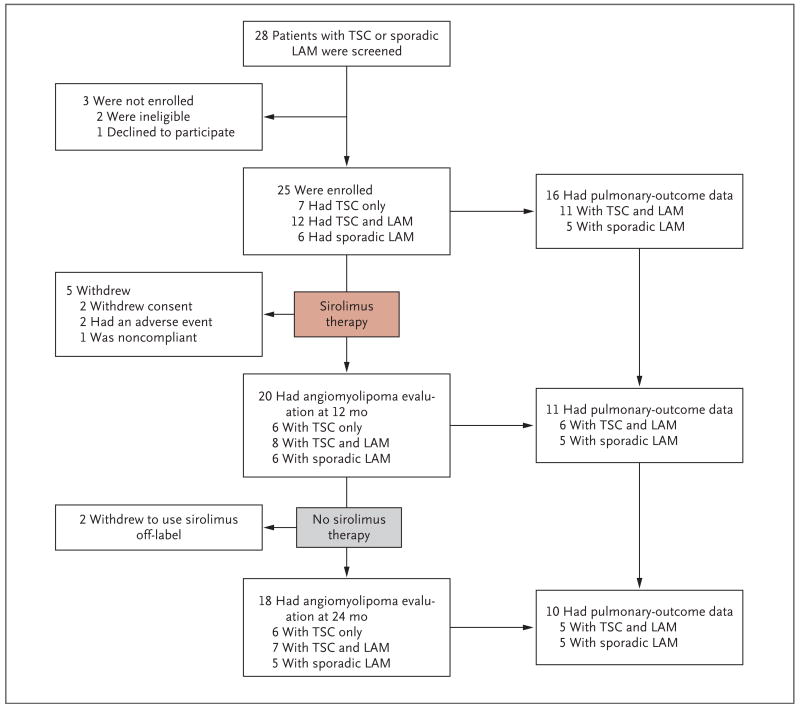
Angiomyolipoma volumes and pulmonary-function tests in patients with LAM were assessed during the period of sirolimus treatment, from baseline to 12 months, and during a post-treatment observation period, from 12 to 24 months. At baseline, 18 patients had LAM, but pulmonary data were uninterpretable in 2 patients because of chylothorax or pneumothorax. At the 12-month time point, one patient declined to undergo pulmonary-function tests, and four patients had withdrawn from the study. One patient withdrew during the second year.
In one patient with sporadic lymphangioleiomyomatosis, the target serum sirolimus range of 1 to 5 ng per milliliter was maintained, but in all other patients the dose was increased to the highest range (10 to 15 ng per milliliter) on the basis of imaging results at 2 months and 4 months.
Twenty patients underwent the 12-month evaluation. Two patients withdrew from the study after the 12-month visit to continue sirolimus therapy off-label because of self-perceived benefit, leaving 18 patients at the 24-month assessment of angiomyolipoma. Fourteen patients with lymphangioleiomyomatosis remained in the study for the second year, but chylothorax or pneumothorax at baseline precluded pulmonary-outcome assessments in two patients, and one patient who had the tuberous sclerosis complex with lymphangioleiomyomatosis declined the 12-month pulmonary-function tests.
Pulmonary end points after 1 year of receiving sirolimus were available for 11 patients with lymphangioleiomyomatosis (6 with the tuberous sclerosis complex with lymphangioleiomyomatosis and 5 with sporadic lymphangioleiomyomatosis). During the second year, 1 patient with lymphangioleiomyomatosis withdrew from the study to take the drug off-label; therefore, data for 10 patients with lymphangioleiomyomatosis were available for the 24-month analysis.
Angiomyolipoma Burden
The targeted renal angiomyolipoma lesions were bilateral in 12 of the 20 patients (60%) and unilateral in 6 of the 20 patients (30%). Hepatic angiomyolipoma lesions were targeted in the remaining two patients (10%). The mean (±SD) angiomyolipoma volume at baseline was 71.6±105.3 ml (Table 1). After 12 months of therapy, the mean volume decreased to 53.2±26.6% of the baseline volume (P<0.001). At 12 months, 16 of the 20 patients for whom we had data for the first-year follow-up period (80%) had at least a 30% reduction in angiomyolipoma volume (Fig. 2). At 6 and 12 months after stopping sirolimus, the mean angiomyolipoma volume had increased to 76.8±27.5% of the baseline volume (P<0.001) and 85.9±28.5% of the baseline volume (P = 0.005), respectively (Table 1 and Fig. 2). Angiomyolipomas in 5 of the 18 patients (28%) remained at least 30% smaller 1 year after therapy than they were at baseline. There was no correlation between statin use or lesion size at baseline and the response to sirolimus (see the Supplementary Appendix, available with the full text of this article at www.nejm.org). The response of a renal angiomyolipoma after 12 months of sirolimus therapy, visualized on MRI, is shown in Figure 3.
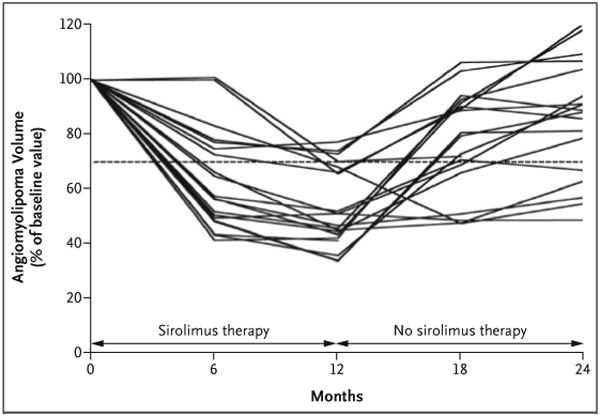
Angiomyolipomas were visualized with the use of abdominal magnetic resonance imaging, and volumetric analysis was performed at baseline and at 2, 4, 6, 12, 18, and 24 months. The angiomyolipoma volume at each visit is expressed as a percentage of the baseline size. The dashed line represents 70% of the baseline value; data below the line indicate that the mean angiomyolipoma volume was reduced by 30% or more.
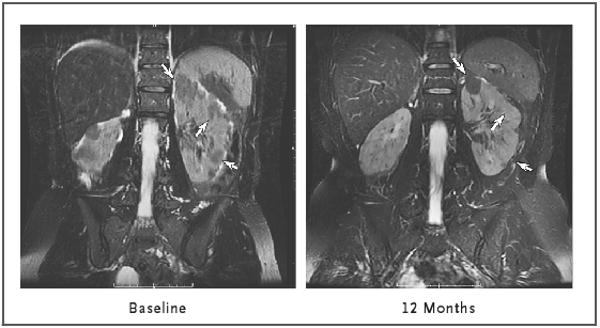
Bilateral angiomyolipomas are shown at baseline and after 12 months of sirolimus therapy. Three lesions in the left kidney are identified by arrows; at 12 months, the top lesion had become reduced in size and the bottom two had become imperceptible. The images were obtained with the use of fast spin–echo T2-weighted magnetic resonance imaging with fat suppression.
The angiomyolipoma volumes were divided into three categories: small (<6.5 ml), medium (6.5–85.0 ml), and large (>85.0 ml). The size assessments obtained with the use of volumetric techniques and those obtained by means of measuring the orthogonal diameters were correlated for each category. The intraclass correlation coefficients for the two methods ranged from 0.76 to 0.86 (mean, 0.81; P<0.001) across the visits. The statistical significance of the measurements obtained through either method was similar.
Pulmonary Studies
Pulmonary structural and functional data for 11 female patients with lymphangioleiomyomatosis are listed in Table 2. One patient was a current smoker, three were former smokers, and seven had never been smokers. At enrollment, spirometric measurements were normal in four patients, revealed moderate airflow obstruction (forced expiratory volume in 1 second [FEV1], 50 to 70% of the predicted value) in three patients, and indicated severe airflow obstruction (FEV1 <50% of the predicted value) in four patients. During sirolimus therapy, the mean FEV1 increased from the baseline mean by 120±230 ml at 6 months (P = 0.009) and by 118±330 ml at 12 months (P=0.06), with 5 of the 11 patients gaining 100 ml or more in volume during therapy (Fig. 4A and 4C). After 1 year of sirolimus therapy, the FEV1 in these patients was significantly improved (P=0.002) as compared with the expected decline in FEV1 for patients with lymphangioleiomyomatosis of 75 ml per year.23 Twelve months after stopping sirolimus, the mean FEV1 was 62±411 ml greater than the mean baseline value.
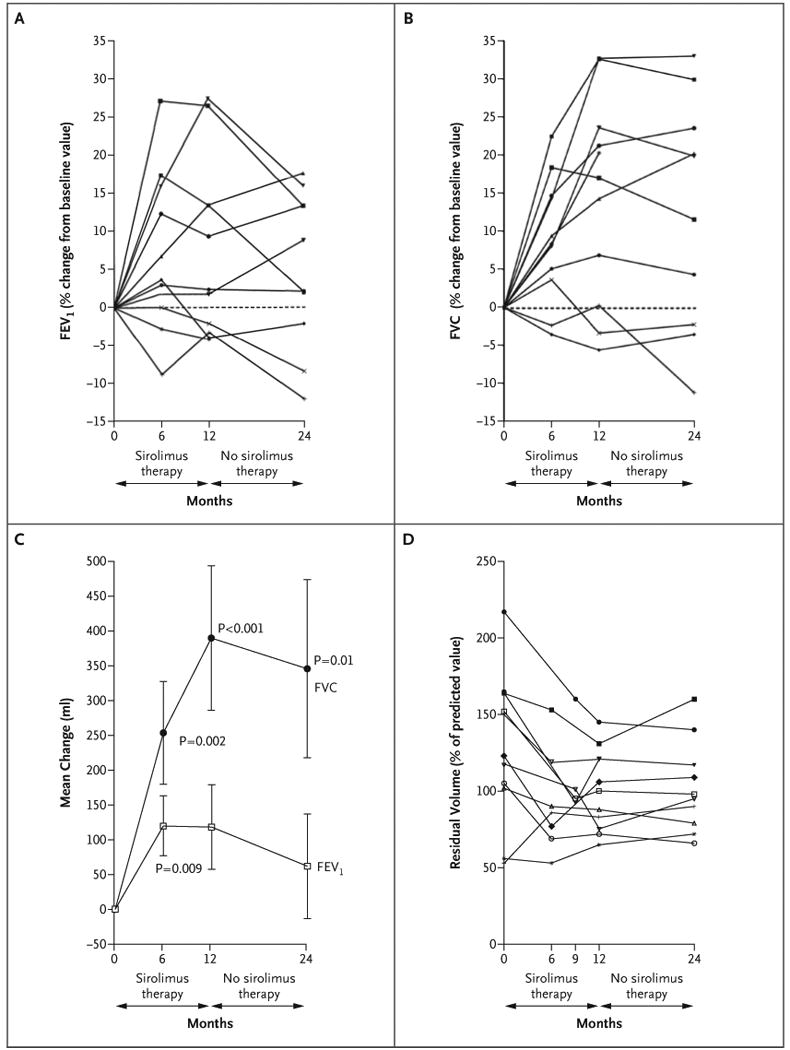
Panel A shows the forced expiratory volume in 1 second (FEV1) for each patient. Panel B shows the forced vital capacity (FVC) for each patient. Panel C shows the mean change (in milliliters) from the baseline values for FEV1 and for FVC. I bars indicate the standard errors. Panel D shows the residual volume for each patient.
The forced vital capacity (FVC) at 12 months was increased by 390±570 ml over the baseline value (range, -190 to 1100 ml) (P<0.001) (Fig. 4B and 4C). Eight of the 11 patients had an increase of at least 250 ml in FVC during the year of sirolimus therapy. The mean FVC remained 346±712 ml above the baseline mean at 24 months (P = 0.01), and the mean percent of the predicted FVC value was significantly improved at 12 months (P<0.001) and at 24 months (P = 0.004).
The mean residual volume fell by 439±493 ml after 1 year of sirolimus therapy, as compared with the baseline value (P = 0.02). The six patients who had substantial air trapping at baseline (residual volume >120% of the predicted value) had a reduction in the residual volume after 12 months of receiving sirolimus (Fig. 4D). The improvement in the percent of the predicted residual volume remained significant at 24 months (P = 0.04), whereas the improvement for the absolute residual volume did not (P = 0.09).
The mean diffusing capacity of the lung for carbon monoxide (DlCO) was reduced in all but one patient at baseline (overall mean, 53.2±14.1% of the predicted value). Neither the DlCO nor the 6-minute walk distance changed significantly during the study (Table 2).
CT volumetric analysis of lung-cyst volume did not reveal a significant change (Table 2). For the year of sirolimus therapy, the correlation coefficient between residual volume and FVC was -0.75 (P = 0.01) and that between residual volume and cyst volume was 0.76 (P<0.001). There was no clear relationship between the reduction in angiomyolipoma volume and the pulmonary response to sirolimus in the study population. The changes in FEV1, FVC, and residual volume associated with sirolimus therapy were similar in the patients who had the tuberous sclerosis complex with lymphangioleiomyomatosis and in those who had sporadic lymphangioleiomyomatosis, but there may have been too few patients in each subgroup to detect a significant difference.
Neurologic Assessment
All patients with the tuberous sclerosis complex had cortical tubers; 64% had subependymal nodules. None had hydrocephalus or giant-cell astrocytomas. There were no changes in the size of the tubers, in the characteristics of the tubers on MRI, or in cerebral vasculature or perfusion.
Adverse Events
The most common adverse events included aphthous ulcers, diarrhea, and upper respiratory infections (Table 3). Nine patients were hospitalized with 10 serious adverse events. Six of these events were possibly or probably related to, or exacerbated by, the use of sirolimus: diarrhea with dehydration, community-acquired pneumonia, pyelonephritis, cellulitis of the lower leg from a cat bite, stomatitis, and hemorrhage of a renal angiomyolipoma that occurred 2 days after the initiation of sirolimus therapy. Four serious adverse events were categorized as unrelated to the use of sirolimus because they occurred when the patient was not receiving the drug: sinus bradycardia after the initiation of a beta-blocker, palpitations, abdominal pain, and a thyroid papillary carcinoma that was retrospectively found to be present on baseline imaging. Renal function did not change during the study, except in one patient, who had a short-term increase in the creatinine level of 0.6 mg per deciliter (53.0 μmol per liter), from a baseline value of 2.9 mg per deciliter (256.4 μmol per liter), while receiving sirolimus.
Table 3
| Category | No. of Patients | No. of Events | Events Probably or Possibly Related to Sirolimus | Events Unlikely to Be Related to Sirolimus | Events Unrelated to Sirolimus |
|---|---|---|---|---|---|
| no. of events (no. of serious events) | |||||
| Gastrointestinal | 19 | 34 | 31 (2) | 3 | 0 |
 Aphthous ulcer or Aphthous ulcer or  mucositis mucositis | 17 | 22 | 22 (1) | 0 | 0 |
 Diarrhea Diarrhea | 7 | 12 | 9 (1) | 3 | 0 |
| Infection | 17 | 31 | 29 (3) | 0 | 2 |
 Upper respiratory infection, sinusitis, or bronchitis Upper respiratory infection, sinusitis, or bronchitis | 11 | 18 | 18 | 0 | 0 |
 Pneumonia Pneumonia | 2 | 2 | 2 (1) | 0 | 0 |
 Pharyngitis Pharyngitis | 2 | 2 | 1 | 0 | 1 |
 Cellulitis Cellulitis | 3 | 3 | 3 (1) | 0 | 0 |
 Urinary tract infection or pyelonephritis Urinary tract infection or pyelonephritis | 4 | 4 | 3 (1) | 0 | 1 |
 Conjunctivitis Conjunctivitis | 1 | 1 | 1 | 0 | 0 |
 Salivary gland infection Salivary gland infection | 1 | 1 | 1 | 0 | 0 |
| Skin-related | 13 | 17 | 10 | 1 | 6 |
 Folliculitis Folliculitis | 4 | 4 | 3 | 0 | 1 |
 Acne Acne | 3 | 3 | 3 | 0 | 0 |
 Other Other | 8 | 10 | 4 | 1 | 5 |
| Metabolic or laboratory | 13 | 14 | 14 | 0 | 0 |
 Hyperlipidemia Hyperlipidemia | 13 | 13 | 13 | 0 | 0 |
 Hypokalemia Hypokalemia | 1 | 1 | 1 | 0 | 0 |
| Pain | 12 | 15 | 7 | 2 | 6 (1) |
 Headache Headache | 4 | 4 | 3 | 0 | 1 |
 Abdominal or flank pain Abdominal or flank pain | 4 | 4 | 2 | 1 | 1 (1) |
 Other Other | 6 | 7 | 2 | 1 | 4 |
| Pulmonary or upper respiratory | 9 | 13 | 8 | 1 | 4 |
 Dyspnea Dyspnea | 3 | 3 | 3 | 0 | 0 |
 Hypoxia Hypoxia | 2 | 2 | 2 | 0 | 0 |
  With exercise With exercise | 1 | 1 | 1 | 0 | 0 |
  At rest At rest | 1 | 1 | 1† | 0 | 0 |
 Pneumothorax Pneumothorax | 1 | 1 | 0 | 1 | |
 Cough Cough | 3 | 3 | 2 | 0 | 1 |
 Abnormality on radiography Abnormality on radiography | 2 | 2 | 1 | 0 | 1 |
 Allergic rhinitis Allergic rhinitis | 2 | 2 | 0 | 1 | 1 |
| Sexual or reproductive function | 4 | 6 | 3 | 3 | 0 |
 Irregular menses Irregular menses | 3 | 3 | 3 | 0 | 0 |
 Ovarian or breast cyst Ovarian or breast cyst | 1 | 3 | 0 | 3 | 0 |
| Musculoskeletal or soft-tissue swelling | 4 | 5 | 5 | 0 | 0 |
| Cardiac | 4 | 4 | 2 | 0 | 2 (2) |
 Hypertension Hypertension | 2 | 2 | 2 | 0 | 0 |
 Palpitations or arrhythmia Palpitations or arrhythmia | 2 | 2 | 0 | 0 | 2 (2) |
| Hemorrhage | 3 | 3 | 2 (1) | 0 | 1 |
 Renal-angiomyolipoma hemorrhage Renal-angiomyolipoma hemorrhage | 1 | 1 | 1 (1) | 0 | 0 |
 Uterine bleeding Uterine bleeding | 2 | 2 | 1 | 0 | 1† |
| Endocrine | 2 | 2 | 0 | 0 | 2 (1) |
 Hypoglycemia Hypoglycemia | 1 | 1 | 0 | 0 | 1 |
 Thyroid papillary carcinoma Thyroid papillary carcinoma | 1 | 1 | 0 | 0 | 1 (1) |
| Constitutional | 3 | 4 | 2 | 1 | 1 |
 Fatigue Fatigue | 2 | 3 | 2 | 0 | 1 |
 Anxiety Anxiety | 1 | 1 | 0 | 1 | 0 |
| Secondary skin or basal-cell cancer | 1 | 1 | 1† | 0 | 0 |
| Neurologic | 2 | 2 | 1 | 0 | 1 |
 Forgetfulness Forgetfulness | 1 | 1 | 0 | 0 | 1 |
 Migraine Migraine | 1 | 1 | 1 | 0 | 0 |
| Blood or bone marrow (leukopenia) | 1 | 1 | 1 | 0 | 0 |
| Renal or genitourinary | 1 | 2 | 1 | 0 | 1 |
 Proteinuria Proteinuria | 1 | 1 | 1 | 0 | 0 |
 Hematuria Hematuria | 1 | 1 | 0 | 0 | 1 |
Discussion
Sirolimus therapy in patients with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis was associated with a reduction in angiomyolipoma volume of nearly 50% and, in the patients with lymphangioleiomyomatosis, improvements in airflow and gas trapping (measured as residual volume). The renal and pulmonary benefits of treatment with sirolimus tended to reverse after the drug was withdrawn, though the improvements were persistent in some patients.
In patients with the tuberous sclerosis complex, renal disease is a leading cause of death or disability, second only to neurologic disease.24 Angiomyolipomas are slow-growing hamartomas that can lead to renal failure or spontaneous hemorrhage and do not spontaneously regress. Although nephron-sparing surgical and interventional radiologic techniques have largely supplanted nephrectomy for the management of problematic angiomyolipomas, there is a clear need for less-invasive therapies. The identification of tuberous sclerosis complex genes and the implication of their role in pathogenic cell signaling have suggested inhibition of the activity of the mammalian target of rapamycin (mTOR) as a potential pharmacologic approach to managing angiomyolipomas in patients with the tuberous sclerosis complex and sporadic lymphangioleiomyomatosis. Preclinical studies have suggested that angiomyolipomas might respond to sirolimus therapy.10,11 Case reports of sirolimus-induced reduction in angiomyolipoma size were published while this article was in press.25,26 The observation that angiomyolipoma size correlates with the risk of hemorrhage suggests that pharmacotherapy that maintains or reduces angiomyolipoma size may reduce the risk of bleeding.27 We speculate that the regression in angiomyolipoma size found in our patients might be related to apoptosis or cell-volume reduction. The variable recurrence of tumors, ranging from a rapid return to baseline dimensions to a sustained reduction in size, after the withdrawal of sirolimus might be consistent with both processes.
Eleven patients with lymphangioleiomyomatosis were evaluated for pulmonary outcomes. The typical rate of change in FEV1 for such patients is reported to be -75 ml per year.23 After 1 year of sirolimus therapy, the FEV1 increased by 118 ml, and the FVC increased by 390 ml. It is unlikely that these spirometric responses can be explained by the reversal of airflow obstruction alone, given that the increase in FVC was more than three times the increase in FEV1. The most likely explanation for the observed increase in FVC is the relief of gas trapping, indicated by the reduction in residual volume that occurred with the use of sirolimus. A decrease in the infiltration of smooth-muscle cells, or in lung remodeling, in association with the reduction in cyst volume may have contributed to this response. The volumetric CT data revealed a trend toward a reduction in cyst size, but the results were not significant. The lack of a significant response in total lung capacity suggests that sirolimus does not markedly affect the elastic recoil of the lung. DlCO, which reflects the integrity of the pulmonary capillary bed and is considered a sensitive indicator of lymphangioleiomyomatosis progression, did not change significantly during the period of sirolimus therapy, probably because the destruction of the pulmonary parenchyma in lymphangioleiomyomatosis is irreversible. The lack of a significant increase in the 6-minute walk distance suggests that improvement in lung function was not accompanied by an increase in exercise capacity. However, the high exercise tolerance of our patients at baseline might have confounded detection of a treatment effect. Among the pulmonary responses, FVC and residual volume remained the most improved, significantly so, 1 year after sirolimus was stopped, as compared with the baseline values.
There was no change in neuroimaging results or neurologic status during the study. Tubers, the most prevalent neurologic lesions in the study, are dysplastic rather than neoplastic, and may not be responsive to antiproliferative treatment strategies. However, sirolimus appears to have activity in the central nervous system, on the basis of reports of its effects on subependymal giant-cell astrocytomas28 and recurrent glioblastoma multiforme.29
Reported adverse effects of sirolimus include leukopenia, thrombocytopenia, hypertriglyceridemia, hypercholesterolemia, aphthous ulcers, edema, joint pain, interstitial pneumonia, delayed wound healing, and infection.30-34 Montalbano et al. reported that sirolimus had to be discontinued because of side effects in 24% of liver-transplant patients who were receiving multidrug immunosuppressive therapy and that there were sirolimus-related deaths.35
The sirolimus monotherapy used in our trial also resulted in a high rate of adverse events. Approximately 50% of patients had elevations in their serum lipid levels requiring dietary or pharmacologic intervention. Oral ulcers occurred to some degree in the majority of patients but typically resolved with topical therapy or a short-term reduction in sirolimus dose. There were six serious adverse events while patients were receiving sirolimus, as well as four during follow-up after the therapy was stopped. Sirolimus was discontinued in five patients, in four because of side effects and in one because of nonadherence to protocol. The use of sirolimus did not appear to be associated with an increased risk of convulsions, and pneumothorax did not occur after sirolimus was initiated. One patient with severe sporadic lymphangioleiomyomatosis, who withdrew from the trial to be able to receive the sirolimus off-label, later died suddenly at home. No autopsy was performed, and the cause of death was presumed to most likely have been arrhythmia or pulmonary embolism. Further research regarding the safety, efficacy, and dosing of sirolimus is indicated in this population of patients who typically have chronic, slowly progressive disease.
In conclusion, treatment with sirolimus for 1 year resulted in a decrease in the size of angiomyolipomas and an improvement in lung function in adults with the tuberous sclerosis complex or sporadic lymphangioleiomyomatosis. One year after the drug was discontinued, the angiomyolipoma size and the FEV1 approached, but did not completely return to, the baseline values, and the effects on residual volume and FVC were durable in most patients. Our study has important limitations, including the open-label design, the lack of a control group, the small number of patients, and the effort-dependent nature of pulmonary-function tests. Collectively, the data suggest that mTOR inhibition may hold promise for treating the tuberous sclerosis complex and sporadic lymphangioleiomyomatosis and that sirolimus monotherapy is associated with clinically important side effects. Additional trials will be needed to define the relative risks and benefits of the use of sirolimus in patients with these diseases.
Acknowledgments
Supported by grants from the National Cancer Institute (CA103486), the LAM Foundation, the Tuberous Sclerosis Alliance, and the Kettering Fund (to Drs. Bissler, McCormack, and Franz) and from the National Institutes of Health (M01 RR08084) to the General Clinical Research Center of the Cincinnati Children's Hospital Medical Center. Wyeth supplied the sirolimus for this trial but did not provide financial support.
Drs. Bissler and Franz report receiving grant support from Novartis; Dr. McCormack, grant support from Wyeth; and Dr. Franz, consulting fees from OncoImmune and lecture fees from Abbott Laboratories and Novartis.
References
Full text links
Read article at publisher's site: https://doi.org/10.1056/nejmoa063564
Read article for free, from open access legal sources, via Unpaywall:
https://www.nejm.org/doi/pdf/10.1056/NEJMoa063564?articleTools=true
Free to read at content.nejm.org
http://content.nejm.org/cgi/content/abstract/358/2/140
Subscription required at content.nejm.org
http://content.nejm.org/cgi/content/full/358/2/140
Subscription required at content.nejm.org
http://content.nejm.org/cgi/reprint/358/2/140.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1056/nejmoa063564
Article citations
mTOR/miR-142-3p/PRAS40 signaling cascade is critical for tuberous sclerosis complex-associated renal cystogenesis.
Cell Mol Biol Lett, 29(1):125, 27 Sep 2024
Cited by: 0 articles | PMID: 39333852 | PMCID: PMC11429883
Therapeutic Approaches to Tuberous Sclerosis Complex: From Available Therapies to Promising Drug Targets.
Biomolecules, 14(9):1190, 21 Sep 2024
Cited by: 0 articles | PMID: 39334956 | PMCID: PMC11429992
Review Free full text in Europe PMC
Successful Treatment of Postmenopausal Exacerbation of Abdominal Lymphangioleiomyomatosis With Sirolimus: A Report of a Rare Case.
Cureus, 16(9):e69549, 16 Sep 2024
Cited by: 0 articles | PMID: 39416572 | PMCID: PMC11483176
Surgical Management of Solitary Extrapulmonary Lymphangioleiomyomatosis in the Mesentery: A Case Report.
Cureus, 16(9):e69042, 09 Sep 2024
Cited by: 0 articles | PMID: 39391387 | PMCID: PMC11464949
Targeted therapies for vascular malformations.
Front Med (Lausanne), 11:1446046, 03 Sep 2024
Cited by: 0 articles | PMID: 39290395 | PMCID: PMC11405217
Review Free full text in Europe PMC
Go to all (722) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00457808
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis.
N Engl J Med, 358(2):200-203, 01 Jan 2008
Cited by: 126 articles | PMID: 18184971
Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial.
Clin Cancer Res, 17(12):4071-4081, 27 Apr 2011
Cited by: 192 articles | PMID: 21525172
Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: a two years trial.
Orphanet J Rare Dis, 13(1):43, 27 Mar 2018
Cited by: 25 articles | PMID: 29587809 | PMCID: PMC5870799
Efficacy and safety of sirolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: a systematic review.
J Urol, 192(5):1424-1430, 09 May 2014
Cited by: 20 articles | PMID: 24813310
Review
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: CA103486
Grant ID: R21 CA103486-01
Grant ID: R21 CA103486
Grant ID: R21 CA103486-02
NCRR NIH HHS (2)
Grant ID: M01 RR008084
Grant ID: M01 RR08084





