Abstract
Background
Although generally mild, the 2009-2010 influenza A/H1N1 pandemic caused two major surges in hospital admissions in the UK. The characteristics of patients admitted during successive waves are described.Methods
Data were systematically obtained on 1520 patients admitted to 75 UK hospitals between May 2009 and January 2010. Multivariable analyses identified factors predictive of severe outcome.Results
Patients aged 5-54 years were over-represented compared with winter seasonal admissions for acute respiratory infection, as were non-white ethnic groups (first wave only). In the second wave patients were less likely to be school age than in the first wave, but their condition was more likely to be severe on presentation to hospital and they were more likely to have delayed admission. Overall, 45% had comorbid conditions, 16.5% required high dependency (level 2) or critical (level 3) care and 5.3% died. As in 1918-1919, the likelihood of severe outcome by age followed a W-shaped distribution. Pre-admission antiviral drug use decreased from 13.3% to 10% between the first and second waves (p=0.048), while antibiotic prescribing increased from 13.6% to 21.6% (p<0.001). Independent predictors of severe outcome were age 55-64 years, chronic lung disease (non-asthma, non-chronic obstructive pulmonary disease), neurological disease, recorded obesity, delayed admission (≥5 days after illness onset), pneumonia, C-reactive protein ≥100 mg/litre, and the need for supplemental oxygen or intravenous fluid replacement on admission.Conclusions
There were demographic, ethnic and clinical differences between patients admitted with pandemic H1N1 infection and those hospitalised during seasonal influenza activity. Despite national policies favouring use of antiviral drugs, few patients received these before admission and many were given antibiotics.Free full text
Original article
Predictors of clinical outcome in a national hospitalised cohort across both waves of the influenza A/H1N1 pandemic 2009–2010 in the UK
Abstract
Background
Although generally mild, the 2009–2010 influenza A/H1N1 pandemic caused two major surges in hospital admissions in the UK. The characteristics of patients admitted during successive waves are described.
Methods
Data were systematically obtained on 1520 patients admitted to 75 UK hospitals between May 2009 and January 2010. Multivariable analyses identified factors predictive of severe outcome.
Results
Patients aged 5–54 years were over-represented compared with winter seasonal admissions for acute respiratory infection, as were non-white ethnic groups (first wave only). In the second wave patients were less likely to be school age than in the first wave, but their condition was more likely to be severe on presentation to hospital and they were more likely to have delayed admission. Overall, 45% had comorbid conditions, 16.5% required high dependency (level 2) or critical (level 3) care and 5.3% died. As in 1918–1919, the likelihood of severe outcome by age followed a W-shaped distribution. Pre-admission antiviral drug use decreased from 13.3% to 10% between the first and second waves (p=0.048), while antibiotic prescribing increased from 13.6% to 21.6% (p<0.001). Independent predictors of severe outcome were age 55–64 years, chronic lung disease (non-asthma, non-chronic obstructive pulmonary disease), neurological disease, recorded obesity, delayed admission (≥5 days after illness onset), pneumonia, C-reactive protein ≥100 mg/litre, and the need for supplemental oxygen or intravenous fluid replacement on admission.
Conclusions
There were demographic, ethnic and clinical differences between patients admitted with pandemic H1N1 infection and those hospitalised during seasonal influenza activity. Despite national policies favouring use of antiviral drugs, few patients received these before admission and many were given antibiotics.
Introduction
On 11 June 2009, WHO announced an influenza pandemic after a novel strain of influenza A virus emerged and spread worldwide.1 2 In the UK the Influenza Clinical Information Network (FLU-CIN) was established in May 2009 to undertake clinical surveillance of hospitalised cases.3 Having already documented the first wave of the pandemic (May–September 2009),3 this paper presents an analysis across the first and second pandemic waves.
Methods
As previously described,3 trained FLU-CIN staff extracted demographic and clinical data from hospital case notes and electronic records. Patients with pandemic influenza A/H1N1 2009 infection (‘pandemic H1N1’) confirmed by real-time reverse transcribed PCR were included; no other selection criteria were applied. FLU-CIN was an ‘emergency’ initiative with a purposive sampling frame based on 13 sentinel hospitals situated in five clinical ‘hubs’ in Nottingham, Leicester, London, Sheffield and Liverpool, with contributions from a further 45 non-sentinel hospitals in England and 17 in Scotland, Wales and Northern Ireland. This included five children's hospitals and five tertiary respiratory referral centres (three with facilities for Extra Corporeal Membrane Oxygenation).i Participating hospitals were requested to notify all cases of confirmed pandemic H1N1 infection.
Descriptive analyses considered demographic data, pre-existing comorbidities recorded in case notes, pregnancy, physician-defined obesity, clinical parameters and clinical management details. Paediatric data were described as abnormal when values lay outside two standard deviations of normal ranges for respiratory rate, heart rate and blood pressure, adjusting for age, sex and temperature (heart rate only).3 4 We examined total and weighted comorbidity burden (the latter using Charlson's comorbidity index).5 6
Using logistic regression (Wald tests) we investigated differences by pandemic wave and identified risk factors for severe outcomes. The split between first and second waves was defined using national surveillance data (first wave: to 31 August 2009; second wave: from 1 September 2009).7 ‘Severe outcome’ was defined as admission to level 2 (high dependency unit) or level 3 (intensive care unit) facilities,ii and/or death. Age was treated as a categorical variable for univariate analyses. The lowest age band (<1 year) was used as a reference for the comparison of the two waves as the purpose was to compare distributions between waves. However, for the analyses of severe outcome, the age band of 16–24 years (least risk category) was used as the reference because we could not assume a linear relationship between age and severe outcome. Continuous variables such as serum C-reactive protein (CRP) levels were coded categorically to facilitate clinical interpretation.8 9 A multivariable regression analysis was conducted for statistically significant variables (p≤0.05) identified during univariate analyses. Two separate models were constructed to examine potential predictors of severe outcome: model 1 included patient characteristics (demographic characteristics and pre-existing comorbidities) while model 2 included clinical characteristics (symptoms, findings of clinical examination and investigations). Both models were then restricted to include only the variables that were significantly associated with an increased risk, and receiver operating characteristics (ROC) curves were plotted to explore the prediction of severe outcome. In essence, the predictive ability of the final model for severe outcome was calculated by assigning each patient an unweighted score of ‘1’ for every risk factor present, and calculating sensitivity and specificity for each cut-off value. All analyses were conducted using Stata, V.11.
Results
Overview
Data were obtained on 1520 patients with confirmed pandemic H1N1 infection. Illness onset occurred from 25 April 2009 to 26 January 2010 (online supplementary figure 1). The median length of hospital stay was 3 days (IQR 2–6). One in six (16.5%) patients needed admission to high dependency (4.1%) or intensive care (12.4%) units (respectively level 2 and level 3 care) and the in-hospital case death rate was 5.3% (children 0–15 years: 3.8%; adults 16–64 years: 5.6%; older people >65 years: 10.7%; first wave: 5.0%; second wave: 5.4%).
Patient characteristics
Table 1 summarises socio-demographic characteristics; the median age was 26 years (IQR 9–44). There were higher proportions of patients in age bands 0–4 (17%) and 16–34 (32%) compared with the general population. However, compared with pre-pandemic hospital admissions for acute respiratory infection (ARI) during the immediately preceding influenza active winter period (November 2008–March 2009), there was an inverse age distribution with fewer patients in age bands 0–4 and ≥65 and substantially higher proportions in age bands from 5 to 54. Among women, 20.8% were pregnant compared with an estimated national prevalence of pregnancy 5.6% in the female population aged 15–44 years (table 2). There was an over-representation of non-white ethnic groups in the FLU-CIN cohort compared with the UK general population and ARI admissions during the previous winter. More than half of all admitted patients (55.1%) did not have any recorded pre-existing comorbidity at the time of admission (table 2).
Table 1
Demographic characteristics of 1520 UK patients hospitalised with pandemic H1N1 infection during the 2009–2010 pandemic compared with source population and pre-pandemic hospital data on acute respiratory infection admissions
| n (%) | UK population comparison, % | Pre-pandemic hospital data, acute respiratory infections,* % | |
| Sex† | |||
 Men Men | 720 (47.4) | 48.7 | 50.4 |
 Women Women | 800 (52.6) | 51.3 | 49.6 |
| Age (years)‡ | |||
 <1 <1 | 121 (8.0) | 1.3 | 14.7 |
 1–4 1–4 | 138 (9.1) | 4.8 | 12.6 |
 5–15 5–15 | 221 (14.5) | 12.6 | 4.8 |
 16–24 16–24 | 245 (16.1) | 12.1 | 2.5 |
 25–34 25–34 | 242 (15.9) | 12.9 | 3.3 |
 35–44 35–44 | 195 (12.8) | 14.6 | 4.6 |
 45–54 45–54 | 168 (11.0) | 13.5 | 5.4 |
 55–64 55–64 | 115 (7.6) | 11.8 | 7.9 |
 65–74 65–74 | 55 (3.6) | 8.5 | 11.0 |
 >75 >75 | 20 (1.3) | 7.8 | 33.2 |
| Ethnicity§ | |||
 White White | 630 (41.5) | 92.1 | 83.3 |
 Mixed Mixed | 11 (0.7) | 1.2 | 1.9 |
 Asian/Asian British Asian/Asian British | 249 (16.4) | 4.0 | 8.7 |
 Black/black British Black/black British | 129 (8.5) | 2.0 | 3.4 |
 Chinese and other Chinese and other | 121 (8.0) | 0.8 | 2.7 |
Missing data for 380 (25%).
Table 2
Pre-admission comorbidity in 1520 patients hospitalised with pandemic H1N1 infection during the 2009–2010 pandemic compared with national prevalence data
| Underlying medical conditions | ||||
| Children (<16 years), n=480 (31.6%), n (%) | Adults, n=1040 (68.4%), n (%) | All admissions, (n=1520), n (%) | Background, prevalence in the general population,* % | |
| No. of comorbidities† | ||||
 0 0 | 346 (72.1) | 492 (47.3) | 838 (55.1) | – |
 1 1 | 115 (23.9) | 394 (37.9) | 509 (33.5) | – |
 2 or more 2 or more | 19 (4.0) | 154 (14.8) | 173 (11.4) | – |
| Comorbidity | ||||
 Cardiovascular disease Cardiovascular disease | 20 (4.2) | 168 (16.2) | 188 (12.4) | 3.5 |
 Pulmonary disease Pulmonary disease | ||||
  COPD COPD | 0 (0.0) | 83 (8.0) | 83 (5.5) | 1.5 |
  Asthma Asthma | 71 (14.8) | 314 (30.2) | 385 (25.3) | 5.9 |
  Other pulmonary disease Other pulmonary disease | 16 (3.3) | 20 (2.0) | 36 (2.4) | – |
 Diabetes Diabetes | 6 (1.3) | 96 (9.2) | 102 (6.7) | 4.1 |
 Other metabolic disease Other metabolic disease | 8 (1.7) | 4 (0.4) | 12 (0.8) | – |
 Neurological disease Neurological disease | 36 (7.5) | 51 (5.0) | 87 (5.7) | – |
 Cerebrovascular disease Cerebrovascular disease | 0 (0.0) | 5 (0.5) | 5 (0.5) | 1.7 |
 Obesity recorded on admission† Obesity recorded on admission† | 3 (0.6) | 46 (4.4) | 49 (3.2) | 8.1‡ |
 Pregnancy Pregnancy | 1 | 82 (20.3)§ | 83 (20.8)§ | 5.6¶ |
Preadmission care
The mean interval between symptom onset and admission to hospital was 2 days (median 2 days; IQR 1–4). ‘Delayed admission’ was defined as an interval of ≥5 days between symptom onset and presentation at hospital.iii After excluding missing data (n=450), 227 of 1070 patients (21.2%) had a delayed admission. Prior to admission, 417 of 1520 patients (27.4%) consulted a general practitioner (GP) with influenza-like symptoms. Patients who experienced delayed admission were not significantly different to ‘early’ admissions in relation to age (median 27.0 years vs 25.0 years; p=0.361) or number of comorbidities (median 0 vs 1; p=0.023). However, radiological pneumonia (unadjusted OR 1.83; 95% CI 1.27 to 2.63) and severe outcome (unadjusted OR 1.67; 95% CI 1.15 to 2.43) were associated with delayed admission. Pre-admission GP consultation was significantly associated with delayed admission (unadjusted OR 2.09; 95% CI 1.41 to 3.11). Pre-admission antiviral drugs and antibiotics had been given to 172 (11.3%) and 280 (18.4%) of the cohort, respectively. There was no difference in pre-admission antiviral use between early and delayed admissions (96 of 843 (11.4%) vs 25 of 227 (11.0%), respectively) but a threefold increase in the likelihood of receiving pre-admission antibiotics in patients with delayed admission (76 of 227 (33.4%) vs 118 of 843 (14.0%); unadjusted OR 3.09; 95% CI 2.21 to 4.33). Of the 87 patients (38.3%) with delayed admission who had also seen a GP, 8 (9.2%) were prescribed antiviral drugs in contrast to 50 (57.5%) prescribed antibiotics.
There were 987 cases admitted prior to 23 October 2009 who would not have had the opportunity to be vaccinated or to have seroconverted (even if vaccinated) prior to illness onset. In the remaining 533 patients, 2009 seasonal and pandemic vaccination was recorded in only 21 and 12 instances, respectively.
Clinical presentation and results of early investigations
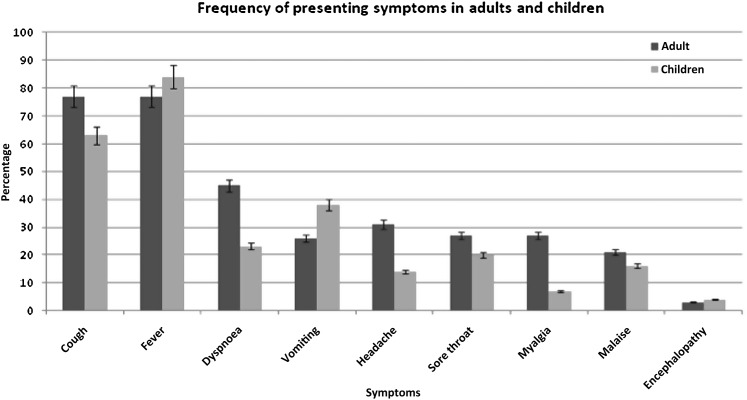
The most common presenting symptoms upon admission in adults and children are summarised in figure 1. Online supplementary table 1 summarises vital signs on admission and early investigations for the FLU-CIN cohort.
Pneumonia
There were 239 (15.7%) pneumonia cases on the basis of radiological reports. A manual review of unreported chest x-ray findings recorded in the case notes (by an unblinded respiratory physician) found 15 additional cases that could be classified as pneumonia based on documentation of acute pulmonary infiltrates and bilateral changes, giving a total of 254 (16.7%) radiological pneumonia cases in the FLU-CIN cohort. The median age of patients with pneumonia was 42 years (IQR 29–54) compared with 23 years (IQR 7–41) for non-pneumonia patients (p<0.001). Of pneumonia cases, 27% had been prescribed pre-admission antibiotics whereas only 13% had been prescribed pre-admission antiviral drugs. From 2087 specimens including 941 nose/throat swabs, 503 blood cultures, 234 urine, 70 stool and 195 sputum specimens taken, the following bacteria were identified: Staphylococcus aureus (n=7), Streptococcus pneumoniae (n=5), Escherichia coli (n=5), Haemophilus influenzae (n=5), Pseudomonas aeruginosa (n=2), Klebsiella spp. (n=1), coagulase-negative staphylococci (n=1) and mixed bacterial flora (n=11).
Differences by pandemic wave
These data are summarised in table 3. Significantly lower proportions of school-age children and young adults (age 5–24 years) were admitted during the second wave, and the proportion of people from non-white ethnic groups also declined significantly (becoming similar to that seen during seasonal influenza activity). But higher proportions of patients presented with dyspnoea, altered consciousness and CRP levels ≥31 mg/litre. Hospital stays <2 days' duration were 33% less likely in the second wave (unadjusted OR 0.67; 95% CI 0.52 to 0.88), but the likelihoods of delayed admission (unadjusted OR 1.93; 95% CI 1.42 to 2.63), and needing level 2/3 care (unadjusted OR 1.76; 95% CI 1.31 to 2.37) were both higher, although there were no apparent differences in mortality. The frequency of prescribing of pre-hospital antiviral drugs decreased from 13.3% to 10.0% between the first and second waves, respectively (p=0.048), whereas the use of pre-hospital antibiotics increased from 13.6% to 21.6% (p<0.001).
Table 3
Comparative analysis of comorbidity, demography, clinical characteristics and selected investigations in first wave versus second wave for patients hospitalised with pandemic H1N1 infection during the 2009–2010 pandemic (n=1520)
| Patient characteristic | First wave, n=601 (39.5%), n (%) | Second wave, n=919 (60.5%), n (%) | Unadjusted OR* (95% CI) | Age-adjusted OR (95% CI) |
| Age (years) | ||||
 <1 <1 | 39 (6.5) | 82 (8.9) | 1.00 (reference) | – |
 1–4 1–4 | 60 (10.0) | 78 (8.5) | 0.62 (0.37 to 1.03) | |
 5–15 5–15 | 116 (19.3) | 105 (11.4) | 0.43 (0.27 to 0.68) | |
 16–24 16–24 | 106 (17.6) | 139 (15.1) | 0.62 (0.39 to 0.99) | |
 25–34 25–34 | 87 (14.5) | 155 (16.9) | 0.85 (0.53 to 1.35) | |
 35–44 35–44 | 72 (12.0) | 123 (13.4) | 0.81 (0.50 to 1.31) | |
 45–54 45–54 | 59 (9.8) | 109 (11.9) | 0.88 (0.54 to 1.44) | |
 55–64 55–64 | 33 (5.5) | 82 (8.9) | 1.18 (0.68 to 2.06) | |
 ≥65 ≥65 | 29 (4.8) | 46 (5.0) | 0.75 (0.41 to 1.38) | |
| p Value | p trend=0.010 | |||
| Ethnicity | ||||
 White White | 180 (30.0) | 450 (49.0) | 1.00 | 1.00 |
 Other Other | 337 (56.1) | 173 (18.8) | 0.21 (0.16 to 0.26) | 0.21 (0.16 to 0.27) |
 Missing Missing | 84 (14.0) | 296 (32.2) | – | – |
| p Value | <0.001 | <0.001 | ||
| Neurological disorders | 45 (7.5) | 42 (4.6) | 0.59 (0.38 to 0.91) | 0.60 (0.39 to 0.92) |
| p Value | 0.018 | 0.020 | ||
| Radiological pneumonia | 79 (13.1) | 175 (19.0) | 1.55 (1.16 to 2.07) | 1.41 (1.04 to 1.90) |
| p Value | 0.003 | 0.025 | ||
| C-reactive protein (mg/litre) | ||||
 ≤30 ≤30 | 171 (28.5) | 222 (24.2) | 1.00 | 1.00 |
 31–99 31–99 | 91 (15.1) | 171 (18.6) | 1.45 (1.05 to 2.00) | 1.39 (0.99 to 1.93) |
 100 100 | 40 (6.7) | 122 (13.3) | 2.35 (1.56 to 3.54) | 2.22 (1.46 to 3.39) |
 Missing Missing | 299 (49.8) | 404 (44.0) | – | – |
| p Value | p trend <0.001 | P trend <0.001 | ||
| Altered consciousness | 21 (3.5) | 53 (5.8) | 1.69 (1.01 to 2.83) | 1.72 (1.03 to 2.89) |
| p Value | 0.046 | 0.040 | ||
| Dyspnoea | 191 (31.8) | 384 (41.8) | 1.54 (1.24 to 1.91) | 1.46 (1.17 to 1.82) |
| p Value | <0.001 | 0.001 | ||
| Pre-admission antibiotics | 82 (13.6) | 198 (21.6) | 1.74 (1.31 to 2.30) | 1.69 (1.27 to 2.24) |
| p Value | <0.001 | <0.001 | ||
| Pre-admission antiviral drugs | 80 (13.3) | 92 (10.0) | 0.72 (0.53 to 0.99) | 0.73 (0.53 to 1.00) |
| p Value | 0.048 | 0.049 | ||
| Delayed admission | ||||
 No No | 420 (69.9) | 423 (46.0) | 1.00 | 1.00 |
 Yes Yes | 77 (12.8) | 150 (16.3) | 1.93 (1.42 to 2.63) | 1.91 (1.41 to 2.60) |
 Missing Missing | 104 (17.3) | 346 (37.6) | – | – |
| p Value | <0.001 | <0.001 | ||
| Required supplemental oxygen on admission | 154 (25.6) | 281 (30.6) | 1.28 (1.02 to 1.61) | 1.21 (0.96 to 1.53) |
| p Value | 0.037 | 0.104 | ||
| Intravenous fluid replacement on admission | 179 (29.8) | 211 (23.0) | 0.70 (0.56 to 0.89) | 0.70 (0.55 to 0.88) |
| p Value | 0.003 | 0.002 | ||
| Oxygen saturation <94% on air | ||||
 No No | 61 (10.2) | 315 (34.3) | 1.00 | 1.00 |
 Yes Yes | 178 (29.6) | 439 (47.8) | 0.48 (0.35 to 0.66) | 0.49 (0.35 to 0.68) |
 Missing Missing | 362 (60.2) | 165 (18.0) | – | – |
| p Value | <0.001 | <0.001 | ||
| Length of hospital stay | ||||
 <2 days <2 days | 137 (22.8) | 152 (16.5) | 1.00 | 1.00 |
 ≥2 days ≥2 days | 395 (65.7) | 650 (70.7) | 1.48 (1.14 to 1.93) | 1.41 (1.08 to 1.84) |
 Missing Missing | 69 (11.5) | 117 (12.7) | – | – |
| p Value | 0.003 | 0.012 | ||
| Adverse outcomes (death or level 2 or 3 admission) | 80 (13.3) | 188 (20.5) | 1.67 (1.26 to 2.23) | 1.64 (1.23 to 2.18) |
| p Value | <0.001 | 0.001 | ||
| Level 2 or 3 admission | 72 (12.0) | 178 (19.4) | 1.76 (1.31 to 2.37) | 1.73 (1.29 to 2.33) |
| p Value | <0.001 | <0.001 | ||
No statistically significant differences were found by pandemic wave in the following patient characteristics: sex; comorbidities such as asthma, chronic obstructive pulmonary disease (COPD) and chronic pulmonary conditions other than asthma or COPD; hepatic disease, cardiovascular disease, diabetes, hypertension, immunocompromised status, or total comorbidity burden; recorded obesity, smoking status, pregnancy; inpatient treatment with antivirals or antibiotics; and mortality.
Factors associated with severe outcome
Univariate analysis
The risk of severe outcome varied by age band and was generally highest in the youngest children (age <1 year) and those aged 45 years and over (table 4). However, a further sensitivity analysis using quinquennial age bands showed an increased risk in children under 1 year, those aged 31–40 and older adults (age >50 years), following a W-shaped distribution (figure 2). An increased risk of severe outcome was associated with specific comorbidities (pre-existing chronic lung disease (excluding asthma and chronic obstructive pulmonary disease (COPD)), neurological disorders and cardiovascular disease); but there was no relation to total comorbidity burden. Recorded obesity was also associated with increased risk. In contrast, pre-existing asthma was associated with significantly decreased risk of severe outcome. No significant association was observed between pregnancy and severe outcome (unadjusted OR 0.92; 95% CI 0.47 to 1.83).iv
Table 4
Analysis of comorbidity, demography, clinical characteristics, care pathway and selected investigations as risk factors for severe outcomes (level 2 or 3 admission and/or death) in patients hospitalised with pandemic H1N1 infection during the 2009–2010 pandemic (n=1520)
| Patient characteristic | Cases (severe outcomes), n=268 (17.6%), n (%) | Controls, n=1252 (82.4%), n (%) | Unadjusted OR (95% CI) | p Value |
| Age (years) | ||||
 <1 <1 | 25 (9.3) | 96 (7.7) | 1.87 (1.04 to 3.34) | 0.036 |
 1–4 1–4 | 23 (8.6) | 115 (9.2) | 1.43 (0.80 to 2.58) | 0.231 |
 5–15 5–15 | 29 (10.8) | 192 (15.3) | 1.08 (0.63 to 1.87) | 0.776 |
 16–24 16–24 | 30 (11.2) | 215 (17.2) | 1.00 (reference) | – |
 25–34 25–34 | 44 (16.4) | 198 (15.8) | 1.59 (0.96 to 2.63) | 0.070 |
 35–44 35–44 | 37 (13.8) | 158 (12.6) | 1.68 (0.99 to 2.83) | 0.053 |
 45–54 45–54 | 33 (12.3) | 135 (10.8) | 1.75 (1.02 to 3.00) | 0.042 |
 55–64 55–64 | 30 (11.2) | 85 (6.8) | 2.53 (1.44 to 4.45) | 0.001 |
 ≥65 ≥65 | 17 (6.3) | 58 (4.6) | 2.10 (1.08 to 4.07) | 0.028 |
| Asthma | 43 (16.0) | 342 (27.3) | 0.51 (0.36 to 0.72) | <0.001 |
| Chronic pulmonary conditions excluding asthma or COPD | 12 (4.5) | 24 (1.9) | 2.40 (1.18 to 4.86) | 0.015 |
| Neurological disorders | 31 (11.6) | 56 (4.5) | 2.79 (1.76 to 4.43) | <0.001 |
| Cardiovascular disease | 50 (18.7) | 138 (11.0) | 1.85 (1.30 to 2.64) | 0.001 |
| Radiological Pneumonia | 94 (35.1) | 160 (12.8) | 3.69 (2.73 to 4.98) | <0.001 |
| C-reactive protein (mg/litre) | ||||
 ≤30 ≤30 | 38 (14.2) | 355 (28.4) | 1.00 | |
 31–99 31–99 | 41 (15.3) | 221 (17.7) | 1.73 (1.08 to 2.78) | 0.022 |
 ≥100 ≥100 | 73 (27.2) | 89 (7.1) | 7.66 (4.86 to 12.09) | <0.001 |
 Missing Missing | 116 (43.3) | 587 (46.9) | – | |
| Recorded obese | 17 (6.3) | 32 (2.6) | 2.58 (1.41 to 4.72) | 0.002 |
| Altered consciousness | 40 (14.9) | 34 (2.7) | 6.28 (3.89 to 10.14) | <0.001 |
| Dyspnoea | 141 (52.7) | 434 (34.7) | 2.09 (1.60 to 2.73) | <0.001 |
| Pre-admission antibiotics | 74 (27.6) | 206 (16.5) | 1.94 (1.43 to 2.63) | <0.001 |
| Required supplemental oxygen on admission | 163 (60.8) | 272 (21.7) | 5.59 (4.23 to 7.40) | <0.001 |
| Intravenous fluid replacement on admission | 93 (34.7) | 297 (23.7) | 1.71 (1.29 to 2.27) | <0.001 |
| Delayed admission | ||||
 No No | 114 (42.5) | 729 (58.2) | 1.00 | |
 Yes Yes | 47 (17.5) | 180 (14.4) | 1.67 (1.15 to 2.43) | 0.008 |
 Missing Missing | 107 (39.9) | 343 (27.4) | – | |
| Length of hospital stay | ||||
 <2 days <2 days | 5 (1.9) | 284 (22.7) | 1.00 | |
 ≥2 days ≥2 days | 170 (63.4) | 875 (69.9) | 11.04 (4.49 to 27.12) | <0.001 |
 Missing Missing | 93 (34.7) | 93 (7.4) | – | |
| In-hospital antibiotic therapy | 235 (87.7) | 1020 (81.5) | 1.62 (1.10 to 2.40) | 0.016 |
No statistically significant association was observed between severe outcomes (level 2 or 3 admission or death) and the following patient characteristics: sex, ethnicity; comorbidities such as chronic obstructive pulmonary disease (COPD), hepatic disease, diabetes, hypertension, immunocompromised status or total comorbidity burden; smoking status, pregnancy, pre-admission antiviral use, oxygen saturation <94% on air; and inpatient treatment with antivirals.
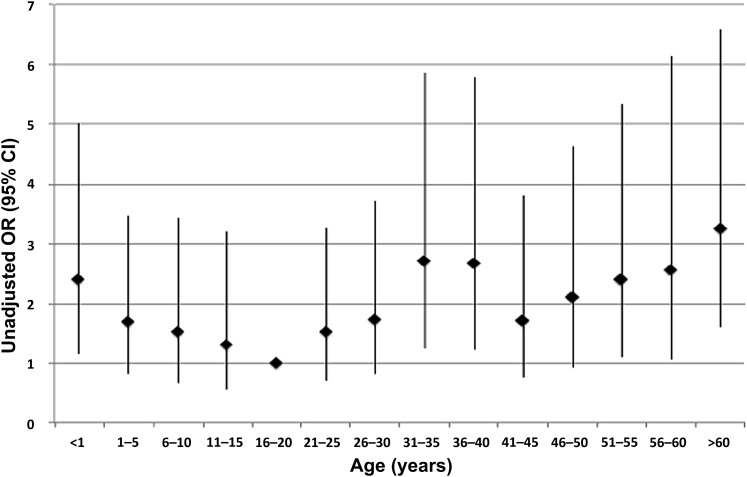
Unadjusted OR of severe outcome by age (OR and 95% CI) for patients hospitalised with pandemic H1N1 infection during the 2009–2010 pandemic. Severe outcome defined as admission to high dependency (level 2) or intensive (level 3) care facilities, and/or death.
Dyspnoea, altered consciousness, levels of CRP ≥100 mg/litre, need for supplemental oxygen or intravenous fluid replacement on admission were all associated with an increased risk of severe outcome, as was the use of pre-admission antibiotics. Radiologically confirmed pneumonia was strongly associated with severe outcome.
Demographic and comorbid independent predictors of severe outcome
Model 1 considered patient characteristics (socio-demographic and comorbidities) that could predict severe outcome: age, asthma, cardiovascular disease, chronic lung disease (non-asthma, non-COPD) and neurological disorders (table 5). We observed statistically significant increased risks associated with the age band 55–64 years (adjusted OR 2.08; 95% CI 1.16 to 3.74), pre-existing lung disease (excluding asthma and COPD) (adjusted OR 2.40; 95% CI 1.17 to 4.93), neurological disorders (adjusted OR 2.59; 95% CI 1.62 to 4.15) and recorded obesity (adjusted OR 2.22; 95% CI 1.18 to 4.18). Pre-existing asthma was associated with reduced likelihood of severe outcome (adjusted OR 0.49; 95% CI 0.34 to 0.70). In view of the complex, nonlinear relationship between age and severe outcome, model 1 was also analysed excluding age, with similar findings (online supplementary table 2).
Table 5
Multivariable analysis: Patient characteristics independently predictive of severe outcomes in pandemic influenza during the 2009–2010 pandemic (n=1520)
| Patient characteristic | Adjusted* OR (95% CI) | p Value |
| Socio-demographic characteristics | ||
 Age (years) Age (years) | ||
  <1 <1 | 1.60 (0.88 to 2.90) | 0.123 |
  1–4 1–4 | 1.20 (0.66 to 2.19) | 0.545 |
  5–15 5–15 | 0.91 (0.52 to 1.59) | 0.740 |
  16–24 16–24 | 1.00 (reference) – | |
  25–34 25–34 | 1.60 (0.96 to 2.66) | 0.071 |
  35–44 35–44 | 1.47 (0.86 to 2.52) | 0.164 |
  45–54 45–54 | 1.46 (0.84 to 2.56) | 0.183 |
  55–64 55–64 | 2.08 (1.16 to 3.74) | 0.014 |
  ≥65 ≥65 | 1.45 (0.71 to 2.93) | 0.308 |
| Underlying comorbidities | ||
 Asthma Asthma | 0.49 (0.34 to 0.70) | <0.001 |
 Cardiovascular disease Cardiovascular disease | 1.43 (0.96 to 2.13) | 0.075 |
 Other lung disease excluding COPD and asthma Other lung disease excluding COPD and asthma | 2.40 (1.17 to 4.93) | 0.018 |
 Neurological disorders Neurological disorders | 2.59 (1.62 to 4.15) | <0.001 |
 Recorded obesity Recorded obesity | 2.22 (1.18 to 4.18) | 0.013 |
COPD, chronic obstructive pulmonary disease.
Clinical parameters independently predictive of severe outcome
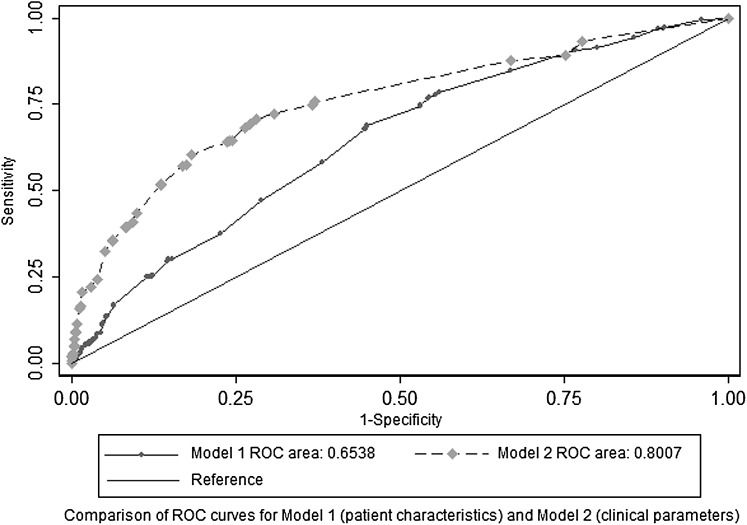
Model 2 included delayed admission because this had a significant effect on severe outcomes in the univariate analysis (table 6). Radiologically confirmed pneumonia (adjusted OR 1.83; 95% CI 1.27 to 2.64), delayed admission (adjusted OR 1.67; 95% CI 1.09 to 2.56), altered consciousness on presentation (adjusted OR 6.53; 95% CI 3.73 to 11.41) and CRP levels ≥100 mg/litre (adjusted OR 3.78; 95% CI 2.41 to 5.94) were independently associated with severe outcomes as was a need for supplemental oxygen or intravenous fluid replacement on admission (adjusted OR 4.34; 95% CI 3.09 to 6.08; and adjusted OR 1.86; 95% CI 1.30 to 2.66, respectively). Figure 3 shows the ROC curves and area under curve values for the two resulting models with only the independent predictors of increased risk. In the case of model 1 a new variable representing the age group 55–64 years was created to construct the ROC curve, to allow for the nonlinear increase in risk observed with age.
Table 6
Multivariable analysis: clinical parameters independently predictive of severe outcomes in pandemic influenza during the 2009–2010 pandemic (n=1520)
| Clinical parameter | Adjusted* OR (95% CI) | p Value |
| Delayed admission | 1.67 (1.09 to 2.56) | 0.019 |
| Radiologically confirmed pneumonia | 1.83 (1.27 to 2.64) | 0.001 |
| Presenting symptoms | ||
 Dyspnoea Dyspnoea | 1.22 (0.88 to 1.67) | 0.231 |
 Altered consciousness Altered consciousness | 6.53 (3.73 to 11.41) | <0.001 |
 Required supplemental oxygen on admission Required supplemental oxygen on admission | 4.34 (3.09 to 6.08) | <0.001 |
 Intravenous fluid replacement on admission Intravenous fluid replacement on admission | 1.86 (1.30 to 2.66) | 0.001 |
| Clinical parameters | ||
 C-reactive protein (mg/dl) (≥100 vs <100) C-reactive protein (mg/dl) (≥100 vs <100) | 3.78 (2.41 to 5.94) | <0.001 |
Discussion
Although limited to severe influenza cases requiring hospitalisation, these data provide important information on predictors of severe outcomes and the impact of clinical management. They also suggest potential for improvement in the community management of pandemic influenza that might influence disease progression. Hospitals were asked to notify all cases with confirmed pandemic H1N1 and cases were followed up without selection. Nevertheless, the inclusion of several children's hospitals and tertiary respiratory referral centres may alter the representativeness of our findings and case ascertainment may not have been complete in all centres.
Our analysis identified that only 11% of the FLU-CIN cohort had been prescribed pre-admission antiviral drugs despite a national policy for making them widely available.10 The significant decline in use of antiviral drugs between first and second waves (despite the opening of the National Pandemic Flu Service in late July 2009)10 and the concomitant increase in the frequency of antibiotic use suggest that GPs may have reverted to the use of antibiotics rather than antivirals during the second wave. However, very few (<3%) bacterial infections were confirmed in the cohort. These data also imply that affordable, specific and sensitive near-patient tests for influenza could, in future, offer a significant advance to influenza management strategies by informing the appropriate use of antiviral drugs. One in five admissions were delayed and such patients were more likely to suffer a severe outcome, as reported elsewhere.11 Patients with delayed admission were not different in terms of age or comorbidities. However, without access to primary care data, we cannot determine whether such patients could have been identified for earlier admission or treatment.
The age bands 0–4 and 16–34 years were markedly over-represented in the FLU-CIN cohort compared with the UK general population. However, in comparison to winter seasonal admissions for ARI, the age bands from 5 to 54 years were over-represented. There was also an over-representation of non-white ethnic groups compared with the source population and in relation to ARI admissions during seasonal influenza activity (first wave only), but no differences in progression to severe outcome were noted. The association between severe outcome and age was nonlinear, with infants <1 year and the over 50s showing an increased risk. Moreover, figure 2 describes a W-shaped distribution, as seen in the severe pandemic of 1918,12 and in Canada and the Netherlands in 2009.13 14 This phenomenon may be related to antigenic similarity between 1918-like and 2009 viruses,15 and immune complex-mediated disease in some middle-aged adults.16
Over half of patients had no pre-existing comorbidity and about 41% of in-hospital deaths occurred in those who were previously healthy. People with pre-existing neurological disease and chronic pulmonary disease (other than asthma or COPD), and those with physician-defined obesity were at increased risk of severe outcomes as observed worldwide.17–23 Although 65% of patients recorded as obese had one or more underlying comorbidities, after adjustment, this remained an independent risk factor, possibly explained by respiratory compromise or a pro-inflammatory state.24 25
The most common comorbidity was asthma, which was associated with a decreased risk of severe outcome and is consistent with other reports.17 18 22 Possible explanations include a lower threshold for admission26 or earlier admission (patients with asthma were significantly less likely to have a delayed admission compared with those without asthma; unadjusted OR 0.68, 95% CI 0.48 to 0.96). Lastly, it is possible that corticosteroid administration as part of asthma management protected patients with asthma from severe outcomes. These hypotheses are discussed in more detail in a subsequent manuscript.
All of the following were independent predictors of severe outcome: altered consciousness, need for supplemental oxygen or intravenous fluid replacement at admission, CRP ≥100 mg/litre and influenza-related pneumonia, as noted elsewhere.17 18 23 27
Comparing pandemic waves, we found a 33% decreased likelihood of a length of stay <2 days, higher proportions of dyspnoea, altered consciousness and raised CRP; and a higher likelihood of delayed admission and of needing level 2 or 3 care, but lower levels of pre-admission antiviral use in the second wave. Together, these data may reflect greater confidence among GPs and receiving physicians to manage milder cases at home, and a lower perceived benefit from antiviral drugs for milder cases. In addition, in-hospital mortality appeared unchanged between waves and we found no statistically significant differences in the factors influencing severe outcomes by wave. However, other work suggests that case death rate increased from 0.015% to 0.025% between the first and second waves in the UK.28
Data from the ROC analysis suggest that patient characteristics in model 1 (age 55–64 years, lung disease other than asthma or COPD, neurological disorders, recorded obesity) were not good predictors of severe outcomes (ROC area under the curve 0.65). This was probably because the combination of these variables is too rare to serve as a generic prediction rule. However, the clinical parameters in model 2 proved better predictors of severe outcomes with a ROC area under the curve value of 0.80 (delayed admission, pneumonia, altered consciousness, the need for supplemental oxygen or intravenous fluid on admission, CRP levels ≥100 mg/litre). It is, of course, arguable whether any model based on hospital observations has any use unless earlier intervention would be possible. However, patients with markers of potential severity on admission could be earmarked for early review by specialised respiratory physicians and intensivists. The next step would be to conduct a formal, prospective, head-to-head comparison of our algorithm with other triage tools.29
Conclusions
During the H1N1 pandemic in the UK in 2009–2010, among hospitalised patients, independent predictors of severe outcomes were increasing age, pre-existing chronic lung disease (excluding asthma or COPD), neurological disease and recorded obesity, but asthma was associated with decreased risk. Additional independent predictors of severe outcome were delayed admission, dyspnoea, radiographical pneumonia, altered consciousness, need for supplemental oxygen or intravenous fluid replacement on admission and CRP levels ≥100 mg/litre. The age-related risk of severe outcome followed a W-shaped distribution similar to that described for mortality in the 1918–1919 pandemic. An increase in the proportion of non-white patients compared with ARI admissions during seasonal influenza activity, seen in the first pandemic wave, was not maintained during the second wave. In-patient mortality rates were unchanged between waves but the threshold for admission probably increased during the second wave.
Supplementary Material
Acknowledgments
We gratefully acknowledge the teams who helped identify cases and collated clinical data: Alison Booth, Margaret Charlesworth, Sarah Rodenhurst, Angela Ballard and Alison Holmes at Imperial College Healthcare NHS Trust, London, UK; Sally Batham, Phayre Parkinson, Tracy Kumar and Aiden Dunphy at the University Hospitals of Leicester NHS Trust, Leicester, UK; Anne Tunbridge, Patty Hempsall, Joyce Linskill, Aimee Turner, Sharon Grindle, Dawn Shevlin and Eric Moulds at Sheffield University Hospitals NHS Trust, Sheffield, UK; Elvina White, Elaine Scott, Jennifer Cater, Erica Sergi and Helen Hill at Alder Hey Children's Hospital NHS Foundation Trust, Liverpool, UK; Deborah Fleetwood, Lorna Roche, Sarah Dyas, and Maria Boswell at the Royal Liverpool and Broadgreen University Hospital's Trust, Liverpool, UK; Gillian Vernon, Gillian Houghton, Heather Longworth and Angela Kerrigan at Liverpool Women's Hospital, Liverpool, UK; Sonia Greenwood, Gemma Thompson, Emily Jarvis and Charlotte Minter at the Nottingham University Hospitals NHS Trust, Nottingham, UK; Kristina Lum Kin, Jacqueline Daglish, Sam Hayton, and Gemma Slinn at Birmingham Children's Hospital, Birmingham, UK; Michelle Lacey, Kevin Rooney, Karen Duffy, Anne Gordon, Eleanor Anderson, Hilary Davison, William Carman, Mark Cotton, Arlene Reynolds, Heather Murdoch, Karen Voy, Rosie Hague and Ali McAllister for their contribution to FLU-CIN in Scotland. Brian Smyth and Cathriona Kearns from the National Public Health Agency, Northern Ireland for identifying cases and facilitating data collection; Teresa Cunningham at the Southern Trust and Leslie Boydell at the Belfast Trust for facilitating data collection. Alemayehu Amberbir, Safaa Al-Badri, Baraa Mahgoob and Nachi Arunachalam at the University of Nottingham for data entry and obtaining background population data; also Graham Watson for database development and support; and Tom Bewick at the Nottingham University Hospitals NHS Trust, Nottingham, UK for manually reviewing the records to identify additional pneumonia cases. We also thank Professor Sir Gordon Duff, Co-Chair of the Scientific Advisory Group for Emergencies, and Professor Janet Darbyshire, who Co-Chaired the Influenza Clinical Information Network Strategy Group, for their support and constructive remarks; Dr Shona Kelly of the University of Nottingham (now at University of South Australia) for help in developing the initial data collection tool; and Dr Patrick O'Brien of University College London Hospitals NHS Foundation Trust for assistance in further developing the data set. We thank those Chief Executive Officers, clinicians, virologists and managers, too numerous to mention, who were active in notifying cases to FLU-CIN. SJB and PJMO wish to acknowledge the support of the UK NIHR Biomedical Research Centre scheme.
Footnotes
Contributors: All authors were involved with designing the study, interpreting and analysing the data, contributing to the article and approving the final version. JEE trained FLU-CIN data collectors, coordinated data collection, collated the data and oversaw data entry with JSN-V-T. PRM analysed the data. MGS adjusted the paediatric data for age and temperature. PRM, MGS, WSL and JSN-V-T wrote the article with the assistance of all co-authors and JSN-V-T acts as guarantor. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of their respective employers.
Funding: FLU-CIN was supported by the Department of Health, England (principal funder), and the Scottish government Chief Medical Officer and Public Health Directorate in Scotland. Chairmanship of the FLU-CIN Strategy Group that includes JSN-V-T, WSL, MGS, PJMO, RCR, BLT, SJB, JMcM, JEE and KGN as members was provided by the Department of Health, England. The report was approved for publication by the Department of Health, England. All authors had full access to all data in the study. The FLU-CIN Strategy Group had final responsibility for the interpretation of findings and decision to submit for publication.
Correction notice: This article has been corrected since it was published online first. The following sentence has been updated to read ‘despite the opening of the National Pandemic Flu Service in late July 2009’.
Competing interests: JS N-V-T has received funding to attend influenza related meetings, lecture and consultancy fees and research funding from several influenza antiviral drug and vaccine manufacturers. All forms of personal remuneration ceased in September 2010, but influenza-related research funding from GlaxoSmithKline, F Hoffmann-La Roche and Astra-Zeneca remains current. He is a former employee of SmithKline Beecham plc (now GlaxoSmithKline), Roche Products Ltd, and Aventis-Pasteur MSD (now Sanofi-Pasteur MSD), all prior to 2005, with no outstanding pecuniary interests by way of shareholdings, share options or accrued pension rights. PRM holds an unrestricted educational grant from F Hoffman-La Roche Ltd for research in the area of pandemic influenza. RCR has received funding for vaccine-related research from Novartis and travel funding from GlaxoSmithKline. EMG and CA are employees of the Department of Health, England; EMG has received one-off support for travel and accommodation (without fees) from Solvay (now Abbott) to give a lecture at an educational meeting on influenza. BB provides clinical advice to the Department of Health, England under non-personal consultancy terms. WSL has received unrestricted funding from Pfizer (previously Wyeth) for research in the area of pneumonia. MGS is an advisor to the Department of Health, England. SJB has received consultancy fees from GlaxoSmithKline and Baxter. JEE has received consultancy fees from GlaxoSmithKline, has performed paid work for the Department of Health, England and holds an unrestricted educational grant from Astra-Zeneca for influenza-related research. KGN has received H5 avian influenza vaccines from Novartis and H1N1 pandemic influenza vaccines from GlaxoSmithKline and Baxter to facilitate MRC and NIHR-funded trials. He has received consultancy fees from Novartis and GlaxoSmithKline and lecture fees from Baxter. A colleague of KGN at the University Hospitals of Leicester NHS Trust was Principal Investigator and recipient of research funding from Roche on antiviral resistance and from Novartis on pandemic H1N1 vaccines. All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author).
Ethics approval: Before commencement, FLU-CIN procedures were reviewed by the Ethics and Confidentiality Committee of the National Information Governance Board for Health and Social Care in England and approved for collection, storage and use of personal data for surveillance purposes.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The collection of data for the FLU-CIN database has been publicly funded and, as a public good, will be made available for new research purposes on a case-by-case basis. In general, only anonymised data will be supplied to researchers, except where the law permits the processing of identifiable data. Ownership and oversight of data access and use resides with the Pandemic Influenza Preparedness Team at the Department of Health, England. Any requests for access to FLU-CIN data should be made to Department of Health via the corresponding author, Prof JS Nguyen-Van-Tam.
iChildren's hospitals and tertiary respiratory referral centres were not mutually exclusive; one of three extracorporeal membrane oxygenation centres was a children's hospital.
iiLevel 0: patients whose care needs can be met through normal ward care; level 1: patients at risk of deteriorating or recently relocated from higher levels of care whose needs can be met on an acute ward with additional advice and support from the critical care team; level 2: patients requiring more detailed observation or intervention, including support for a single failing organ system and those ‘stepping down’ from higher levels of care—high dependency unit; level 3: patients requiring advanced respiratory support alone or basic respiratory support together with support of at least two organ systems. This includes all complex patients requiring support for multi-organ failure—intensive care unit.
iiiThe term ‘delayed admission’ defines the interval between symptom onset and admission as ≥5 days, but does not carry any imputation that the delay in all cases was undesirable or clinically suboptimal.
ivComparator restricted to non-pregnant women aged 16–44 years in the FLU-CIN cohort.
References
Full text links
Read article at publisher's site: https://doi.org/10.1136/thoraxjnl-2011-200266
Read article for free, from open access legal sources, via Unpaywall:
https://thorax.bmj.com/content/thoraxjnl/67/8/709.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1136/thoraxjnl-2011-200266
Article citations
Clinical predictors of severe forms of influenza A(H1N1)pdm09 in adults and children during the 2009 epidemic in Brazil.
PLoS One, 19(2):e0291843, 26 Feb 2024
Cited by: 0 articles | PMID: 38408061 | PMCID: PMC10896526
Obesity dysregulates the pulmonary antiviral immune response.
Nat Commun, 14(1):6607, 19 Oct 2023
Cited by: 2 articles | PMID: 37857661 | PMCID: PMC10587167
COPD, but Not Asthma, Is Associated with Worse Outcomes in COVID-19: Real-Life Data from Four Main Centers in Northwest Italy.
J Pers Med, 12(7):1184, 20 Jul 2022
Cited by: 0 articles | PMID: 35887681 | PMCID: PMC9321539
The Type 2 Asthma Mediator IL-13 Inhibits Severe Acute Respiratory Syndrome Coronavirus 2 Infection of Bronchial Epithelium.
Am J Respir Cell Mol Biol, 66(4):391-401, 01 Apr 2022
Cited by: 29 articles | PMID: 34982656 | PMCID: PMC8990122
Changes in characteristics and case-severity in patients hospitalised with influenza A (H1N1) pdm09 infection between two epidemic waves-England, 2009-2010.
Influenza Other Respir Viruses, 15(5):599-607, 04 May 2021
Cited by: 1 article | PMID: 33942500 | PMCID: PMC8404053
Go to all (52) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009).
Thorax, 65(7):645-651, 01 Jul 2010
Cited by: 151 articles | PMID: 20627925 | PMCID: PMC2921287
Changes in severity of 2009 pandemic A/H1N1 influenza in England: a Bayesian evidence synthesis.
BMJ, 343:d5408, 08 Sep 2011
Cited by: 54 articles | PMID: 21903689 | PMCID: PMC3168935
Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009.
CMAJ, 182(18):1981-1987, 08 Nov 2010
Cited by: 50 articles | PMID: 21059773 | PMCID: PMC3001504
Impact of H1N1 on socially disadvantaged populations: systematic review.
PLoS One, 7(6):e39437, 25 Jun 2012
Cited by: 18 articles | PMID: 22761796 | PMCID: PMC3382581
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Chief Scientist Office
Medical Research Council (3)
MOSAIC - cohort of hospitalised H1N1 patients
Professor Peter Openshaw, Imperial College London
Grant ID: MC_G1001212
MRC-Asthma UK Centre in Allergic Mechanisms of Asthma
Professor Sebastian Johnston, Imperial College London
Grant ID: G1000758
Grant ID: G1000758B
National Institute for Health Research (NIHR) (1)
Grant ID: NF-SI-0508-10212

 1 and on behalf of the Influenza Clinical Information Network (FLU-CIN)
1 and on behalf of the Influenza Clinical Information Network (FLU-CIN)