Abstract
Free full text

Efficacy of three drugs for protecting against gentamicin-induced hair cell and hearing losses
Abstract
BACKGROUND AND PURPOSE
Exposure to an ototoxic level of an aminoglycoside can result in hearing loss. In this we study investigated the otoprotective efficacy of dexamethasone (DXM), melatonin (MLT) and tacrolimus (TCR) in gentamicin (GM)-treated animals and cultures.
EXPERIMENTAL APPROACH
Wistar rats were divided into controls (treated with saline); exposed to GM only (GM); and three GM-exposed groups treated with either DXM, MLT or TCR. Auditory function and cochlear surface preparations were studied. In vitro studies of oxidative stress, pro-inflammatory cytokine mRNA levels, the MAPK pathway and caspase-3 activation were performed in organ of Corti explants from 3-day-old rats.
KEY RESULTS
DXM, MLT and TCR decreased levels of reactive oxygen species in GM-exposed explants. The mRNA levels of TNF-α, IL-1β and TNF-receptor type 1 were significantly reduced in GM + DXM and GM + MLT groups. Phospho-p38 MAPK levels decreased in GM + MLT and GM + TCR groups, while JNK phosphorylation was reduced in GM + DXM and GM + MLT groups. Caspase-3 activation decreased in GM + DXM, GM + MLT and GM + TCR groups. These results were consistent with in vivo results. Local treatment of GM-exposed rat cochleae with either DXM, MLT or TCR preserved auditory function and prevented auditory hair cell loss.
CONCLUSIONS AND IMPLICATIONS
In organ of Corti explants, GM increased oxidative stress and initiated an inflammatory response that led to the activation of MAPKs and apoptosis of hair cells. The three compounds tested demonstrated otoprotective properties that could be beneficial in the treatment of ototoxicity-induced hearing loss.
Introduction
Aminoglycoside antibiotics are used clinically for treating tuberculosis as well as other illnesses produced by aerobic Gram-negative bacteria (Bertolaso et al., 2003; Nordang and Anniko, 2005; Xie et al., 2011). Although the use of these agents has declined in recent years, aminoglycosides are still widely used in developing countries, mostly due to their cost, effectiveness and availability. Unfortunately, this practice has resulted in a high incidence of aminoglycoside-associated hearing loss in these countries. There is evidence that aminoglycosides can enter sensory hair cells via their mechanotransduction channels and once inside accumulate within the lysosomes. Scanning electron microscopic observations range from disorganization and fusion of stereocilia at low doses of an aminoglycoside to complete hair cell loss at higher doses (Alharazneh et al., 2011; Cunningham and Tan, 2011). The antibacterial effect of aminoglycosides is achieved through the inhibition of protein synthesis at the ribosome level and the pore-forming effect that this class of antibiotics has on cell membranes. Positively charged aminoglycoside molecules are attracted to the negatively charged outer plasma membrane of affected cells including those that make up the cochlear sensory receptor. Using an energy-dependent process, aminoglycoside molecules enter cells, where they initiate the formation of free radicals using a pathway that has been implicated in the pathology of several inner ear disorders. The generation of reactive oxygen species (ROS) involves the formation of an aminoglycoside iron complex, which then catalyses the production of ROS from unsaturated fatty acids. This is noteworthy because several reports have concluded that the generation of ROS is linked to ototoxicity of the aminoglycosides due to the ability of these free radicals to promote both apoptotic and necrotic cell death (Ylikoski et al., 2002; Rybak and Ramkumar, 2007; Cunningham and Tan, 2011). One signalling enzyme activated by aminoglycosides via ROS is JNK, an enzyme of the MAPK family signalling pathway, which has been shown to contribute to the apoptosis of damaged cells (Davis, 2000). One of the downstream targets of JNK is the transcription factor activating protein-1 (AP-1), which is of special interest considering that gentamicin (GM) treatment of organ of Corti explants is known to result in increased AP-1 activity in outer hair cells (OHCs; Pirvola et al., 2000; Ylikoski et al., 2002; Wang et al., 2003).
The aim of the present study was to determine the protective efficacy of three compounds (i.e. dexamethasone, DXM; tacrolimus, TCR; melatonin, MLT) against GM-initiated damage to the cochlea using both in vivo and in vitro experiments. These three compounds are currently used clinically. DXM has long been employed by physicians to limit the effect of cochlear injury on hearing thresholds, while TCR is currently used for inhibiting transplant rejection, and MLT is a common dietary supplement for the treatment of insomnia. These compounds have different properties, but all have the potential to prevent hair cell (HC) death by acting at different points in a cell death pathway in which GM treatment of organ of Corti explants leads to pro-inflammatory cytokines and ROS production with subsequent activation of MAPK signalling. MLT is a potent antioxidant and free radical scavenging hormone, while DXM is an anti-inflammatory and anti-allergy drug that is known to inhibit AP-1 (González et al., 2000). TCR, used for its immunosuppressive effect, is a MAPK/JNK pathway inhibitor and therefore should limit the formation of AP-1 (Kaminska, 2005).
Methods
In vitro studies
Organ of Corti explants
Three-day-old (P-3) rats of the Wistar strain of laboratory rats (Harlan Interfauna Iberica, Barcelona, Spain and Charles River Laboratories, Wilmington, MA, USA) were anaesthetized with ice for 30 min. All animal experiments were conducted in accordance with the guidelines established by the European Union on Animal Care (CEE Council 86/609). Housing conditions and experimental procedures were approved and monitored by the Institutional Ethics Committee of the University of Valencia, Spain. In addition, animal experiments performed at the University of Miami Ear Institute with P-3 rats were in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publications no. 80-23, revised 1996) and in accordance with the University of Miami, Internal Animal Care and Use Committee, protocol # 11–086.
Organ of Corti explants were dissected en bloc and subsequently placed in serum-free media consisting of Dulbecco's modified Eagle's medium supplemented with glucose (final concentration at 6 g·L−1), 1% of N-1 supplement (Sigma Aldrich, St. Louis, MO, USA) and penicillin G (30 U·mL−1).
RNA extraction and real-time reverse transcription (RT)-PCR
Twenty-four organ of Corti explants for each real-time RT-PCR experiment were cultured for 24 h, under the following conditions: saline (S); GM (100 µM); GM (100 µM) + DXM (50 µM); DXM (50 µM); GM (100 µM) + MLT (50 µM); MLT (50 µM); GM (100 µM) + TCR (50 µM); TCR (50 µM). Three independent experiments were carried out (n = 3 explants/condition). RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. RNA purity and concentration were determined by the absorbance at 260 nm and 280 nm using Nano Drop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using iScript kit (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR was performed in duplicate by using iQ SYBR Green Supermix (Bio-Rad) on iCycler Real-Time CFX96 Detection System (Bio-Rad). The mRNA level was normalized by housekeeping gene β-actin. The primers were designed based on the cDNA sequences obtained from Ensembl Genome Browser (http://www.ensembl.org). The primers set used were: TNF-α 5′-AACTCGAGTGACAAGCCCGTAG-3′ and 3′-GTACCACCAGTTGGTTGTCTTTGA-5′ (ENSRNOT00000001110); TNF receptor superfamily member 1A (TNFRSFIA) 5′-GAACACCGTGTGTAACTGCC-3 and 3′-ATTCCTTCACCCTCCACCTC-5′ (ENSRNOT00000048529); IL-1β 5′-ACCCAAGCACCTTCTTTTCC-3′ and 3′-AGACAGCACGAGGCATTTTT-5′ (ENSRNOT00000006308); IL1R1 5′-TGACCCAGGATCCACGATAC-3′ and 3′-AGTCAGGAACTGGGTATAC-5′ (ENSRNOT00000019673); inducible nitric oxide synthase (iNOS or NOS2) 5′-CTGGGACTGCACAGA ATGTTC C-3′ and 3′-TTTGCCTCTTTGAAGGAGCC-5′ (ENSRNOT00000016133); β-actin 5′-CGTTGACATCCGTAAAGACC-3′ and 3′-AGCCACCAATCCACACAGAG-5′ (Sigma Aldrich). Real-time PCR was carried out to 40 cycles at 95°C, 61°C and 72°C, 50 s each. Melting curves were also performed to ensure primer specificity and evaluate for any contamination. Relative changes in mRNA levels of genes were assessed using the 2(−ΔΔCT) method and normalized to the housekeeping gene β-actin and then to the expression levels of control organ of Corti explants. The formula used to calculate % inhibition was [(x–y)/x]*100, where x is levels of mRNA in GM group and y is levels of mRNA in treatment groups.
ERK 1/2, p-ERK 1/2; p38 MAPK, p-p38 MAPK; JNK, p-JNK-elisa s
Eighty organ of Corti explants for each elisa experiment were cultured for 24 h, under the following conditions: S; GM (100 µM); GM (100 µM) + DXM (50 µM); DXM (50 µM); GM (100 µM) + MLT (50 µM); MLT (50 µM); GM (100 µM) + TCR (50 µM); TCR (50 µM) and then incubated at 37°C in 5% CO2 with 95% relative humidity. Three independent experiments were carried out (n = 4 explants/condition). As recommended by the manufacturer, PD98059, an inhibitor of MAPK kinases and anisomycin (both from Sigma Aldrich), an activator of JNK/SAPK and p38 were used as controls for the elisa assays of the organ of Corti explants. Each specimen was placed in an individual well of a 96-well culture plate, and ERK 1/2, phosphorylated-ERK 1/2; p38 MAPK, phosphorylated-p38; JNK, phosphorylated JNK proteins were detected using the RayBio® Cell-based ERK1/2 elisa sampler kit (Ray Biotech, Inc, Norcross, GA, USA) as described in the manufacturer's protocol and quantified at 450 nm wavelength light using a VersaMax™ Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The supernatant was removed, the tissues were washed three times with PBS and the proteins were quantified by Bradford's method (Bio-Rad). In order to normalize the data obtained for each organ of Corti explant, the results are presented as the ratio of elisa absorbance at 450 nm/Bradford absorbance at 595 nm.
Assessment of antioxidant enzyme levels
Ninety-six organ of Corti were cultured for 24 h in either S; GM (100 µM); GM (100 µM) + DXM (50 µM); DXM (50 µM); GM (100 µM) + MLT (50 µM); MLT (50 µM); GM (100 µM) + TCR (50 µM); TCR (50 µM). Specimens were placed in PBS containing phosphatase and proteinase inhibitors cocktail (Thermo Fisher Scientific, Pittsburgh, PA, USA) on ice and the cells were lysed with the aid of a Misonix XL-2000 series sonicator (Misonix, Farmingdale, NY, USA). The samples were centrifuged at 14 000×g, 15 min at 4°C and the supernatants were collected. The activity of superoxide dismutase (SOD) was measured by monitoring the rate of inhibition of nitroblue tetrazolium dye (NBT) reduction (Kono, 1978). Briefly, 10 µL of supernatant was mixed with 0.1 mM EDTA solution containing 50 mM sodium carbonate pH 10, 0.6% Triton X-100 and 20 mM hydroxylamine hydrochloride pH 6. The reaction started by adding 90 µM NBT (all reactives from Sigma Aldrich) and the rate of NBT reduction was recorded every 20 s for 2 min at 560 nm in a SpectraMax 190™ Microplate Reader (Molecular Devices). One enzyme unit was expressed as inverse of the amount of protein (mg) required to inhibit the reduction rate by 50% in 1 min.
Catalase activity was assayed by the method of Luck (1971); 10 µL of supernatant was mixed with 50 mM sodium phosphate buffer pH 7 and the reaction started by adding 30 mM H2O2 and the changes in absorbance were recorded every 20 s for 2 min at 240 nm in a SpectraMax 190™ Microplate Reader. Enzyme activity was calculated using the molar extinction coefficient of H2O2 (0.071 M−1·cm−1). One unit of catalase activity was defined as µM H2O2 decomposed·min−1·mg−1 protein.
The levels of NO in the supernatants were measured by the method of Griess (1879; Sigma Aldrich). The absorbance was measured at 490 nm and the results were expressed in µM·mg−1 protein.
Total proteins were measured in the supernatants by Bradford's method and were used to normalize the data obtained from each experiment.
ROS detection
Twenty organ of Corti explants (n = 4 explants per group) were cultured for 24 h. The groups were S; GM (500 µM); GM (500 µM) + DXM (50 µM); GM (500 µM) + MLT (50 µM); GM (500 µM) + TCR (50 µM). Then CellROX Deep Red (5 µM, Invitrogen) was added to each well and incubated at 37°C for 30 min. The samples were then washed three times with PBS fixed in 4% paraformaldehyde, 1% methanol in 0.1 M PBS for 20 min. The explants were washed three times in PBS and subsequently incubated in 5% normal goat serum (Sigma Aldrich), 1% Triton X-100 (Fluka) in PBS for 30 min at 25°C, then were incubated with fluorescein isothiocyanate (FITC)-labelled phalloidin for 45 min at 25°C. After being washed, the organ of Corti explants were incubated in 600 nM 4′,6-diamidino-2-phenylindole (DAPI) solution (Sigma Aldrich) for 5 min at 25°C following three additional washes with de-ionized H2O and transferred to a glass slide with mounting medium, cover slipped and viewed under a confocal Zeiss Axiovert 700 microscope. ImageJ 1.45h (http://imagej.nih.gov/ij) software was used for processing and analysing the images. Red signal intensity was measured and background was subtracted. The size of the region of interest was the same for all images and the brightness was in the range of 0 to 255 arbitrary units.
Cleaved caspase-3 immunofluorescent labelling
Thirty organ of Corti explants (n = 6 explants/condition) were cultured in the same conditions described above. The groups were S; GM; GM + DXM; GM + MLT; GM + TCR. The tissues were then fixed in 4% paraformaldehyde, 1% methanol in 0.1 M PBS for 48 h at 4°C. The explants were washed three times in PBS and subsequently incubated in 5% normal goat serum, 1% Triton X-100 in PBS for 30 min at 25°C. The organ of Corti explants were then incubated with anti-cleaved caspase-3 (Asp175) rabbit polyclonal antibody (Cell Signaling Technology, MA, USA) at a 1:200 dilution overnight at 4°C. After being washed with PBS, the tissues were incubated with secondary antibody tetramethyl rhodamine isothiocyanate (TRITC)-labelled goat anti-rabbit IgG (Invitrogen) at a 1:200 dilution for 90 min at 25°C. The tissues were then washed in PBS three times and incubated with FITC-labelled phalloidin for 45 min at 25°C. After being washed, the organ of Corti explants were incubated in 600 nM DAPI (Sigma) solution for 5 min at 25°C following three additional washes with de-ionized H2O and transferred to a glass slide with mounting medium, cover slipped and viewed under a confocal Zeiss Axiovert 700 microscope. ImageJ 1.45h (http://imagej.nih.gov/ij) software was used for processing and analysing the images. Red signal intensity was measured as described previously.
In vivo studies
Experimental subjects
All animal care and experimental procedures were conducted in accordance with the guidelines established by the European Union on Animal Care (CEE Council 86/609). Housing conditions and experimental procedures were approved and monitored by the Institutional Ethics Committee of the University of Valencia, Spain. Twenty-five male (n = 5 rats for each group) Wistar rats weighing 220–250 g from Harlan Interfauna Iberica (Barcelona, Spain) were used. The rats were fed a standard diet ad libitum and were kept on a 12 h light–dark cycle. The ambient noise level in the housing facility was 35–45 dB sound pressure level (SPL).
Damage protocol
During surgery, the animal's body temperature was maintained at 36.5–37.5°C with the aid of a heating pad. Animals were anaesthetized with isoflurane (4% for induction and then 2%, Aerrane, Baxter, and Norfolk, UK), buprenorfine (0.05 mg·kg−1, Buprex, Schering-Plough, Madrid, Spain) was the analgesic. The depth of anaesthesia was assessed by paw pinch reflex, in which the toe of the rat was pinched between the fingers to see a withdrawal response by the animal. Also a corneal reflex was used during the surgical process in order to (a) not to touch non-sterile areas (such us the paws) and (b) this was closer to the surgical area. All surgical procedures where carried out under a stage 3, plane 2 level of anaesthesia. Under aseptic conditions, temporal bones were exposed via a post auricular approach. Tympanic bullae were exposed and holes were made with an 18G needle. In the left ears, the tip of the catheters were placed in the round window membrane niches of each cochlea, these catheters were attached to mini-osmotic pumps (MP, Alzet, model 2001, Cupertino, CA, USA) that were implanted s.c. under the skin of the rat's back between the scapulae. In the right ears 2 mm2 pieces of gelfoam were placed in the round window membrane niches. The animal treatment groups were S (MP: 200 µL of saline; gelfoam: 40 µL of saline); GM (MP: 10 mg of GM in 200 µL of saline; gelfoam: 10 mg of GM in 40 µL of saline); GM + DXM; GM + MLT; and GM + TCR (MP: 10 mg of GM and DXM, MLT or TCR respectively, with each at 500 µM final concentration in 200 µL of saline; gelfoam: 10 mg of GM and DXM, MLT or TCR, respectively, with each at 500 µM final concentration in 40 µL of saline) (GM, DXM and MLT from Sigma Aldrich; TCR from LC Laboratories, Woburn, MA, USA). The MP catheter was attached to the bone of the bulla, which was sealed with tissue adhesive and the skin was sutured. After 7 days, the MPs were removed and the remaining mixture of GM and respective otoprotective compound was subjected to HPLC analysis under the following conditions: 74:26 methanol-H2O, 0.007% trifluoracetic acid, pump L-6200, detector L-7455, pre-column and column Lichrospher C18 (Merck Hitachi, Damstadt, Germany). At 7 days, all three compounds tested were found to be stable in the presence of GM (data not shown).
Auditory function studies
An otoscopic examination was performed on the rats' tympanic membranes on each testing day to rule out possible middle ear pathologies. Observation of any middle ear pathology would exclude an animal from the study. Measurements of auditory function were carried out under brief anaesthesia with isoflurane before GM challenge and on post-exposure days 0, 10, 15 and 20.
Determination of distortion product otoacoustic emissions (DPOAEs, DPgrams)
The DPOAEs were recorded and analysed with the aid of a Navigator-Pro (Bio-logic, Natus, IL, USA) cochlear emission analyser. The DPOAEs were recorded at fixed stimulus levels (L1 = L2 = 70 dB SPL), with an f2/f1 ratio of 1.22 and three points per octave for f2 ranging from 1453 Hz to 10 kHz.
Auditory brainstem response recordings (ABRs)
Three stainless steel needle electrodes were placed as follows: (1) subdermal over the vertex; and (2) over the right and (3) left mastoids of each animal. The ABR stimulus was a 100 ms click presented at a rate of 21.4/s. ABR thresholds were recorded by attenuating the click stimulus 5 dB stepwise until no reproducible response could be recorded. The responses to 1000 acoustic stimuli were then averaged. The signal from the electrodes was amplified with the aid of Navigator-Pro fitted with pass filters with a range of 150 Hz to 3 kHz.
Morphological study
Histological preparation and cytocochleogram: the rats were deeply anaesthetized with an i.p. injection of pentobarbital (100 mg·kg−1, Dolethal, Vetoquinol, France). The cochleae were removed, fixed with 10% formalin in 0.1 M phosphate buffer at pH 7.4, decalcified in 1 M formaldehyde with 2.7 M formic acid and then the organ of Corti surface preparations were dissected and stained with FITC-labelled phalloidin (Sigma Aldrich). Inner and outer hair cells (IHCs and OHCs respectively) were examined with the aid of a Nikon Eclipse E600 microscope (Nikon Instruments Europe B.V., Amstelveen, the Netherlands) and counted in 0.15 mm segments of organ of Corti sensory epithelia and were averaged from 2–3 consecutive segments from the apex to the base of each cochlea to obtain the cytocochleograms.
Statistical analysis
One-way anova test was used for ABR threshold and gene expression experiments and for the assessment of the results of antioxidant enzyme activity. For the rest of the experiments a two-way anova test was employed. Both anova statistical analyses were followed by a Bonferroni post-test for multiple comparisons. The data are expressed as mean ± SD and a P-value < 0.05 was considered significant. All calculations were performed on a computer equipped with Graph Pad v 4.00 software for Windows® (GraphPad Software, Inc. La Jolla, CA, USA).
Results
In vitro studies
Gene expression
As it is shown in Figure 1A, TNF-α was up-regulated in the GM-only group of organ of Corti explants, while treatment of GM-challenged explants with DXM, MLT and TCR reduced the mRNA levels of this cytokine. TNF-α was down-regulated in DXM-only organ of Corti explants compared with S control group. Similarly, the levels of mRNA for TNFRSF1A were reduced in the treatment groups GM + DXM and GM + TCR when compared with GM-only group of organ of Corti explants (Figure 1B). Exposure to GM induced an increase in IL-1β mRNA levels that were reduced in GM + DXM treatment group, and also in the GM + MLT and GM + TCR groups (Figure 1C). Interestingly, mRNA levels of IL-1β were enhanced in the organ of Corti explants exposed to MLT only when compared to the S group explant value. IL1R1 mRNA levels were not changed by the treatments, but the levels of this cytokine receptor were increased in the DXM group compared to the values of the S treatment group (Figure 1D). Exposure of the organ of Corti to GM alone did not affect the mRNA levels of the inducible form of NOS (Figure 1E).
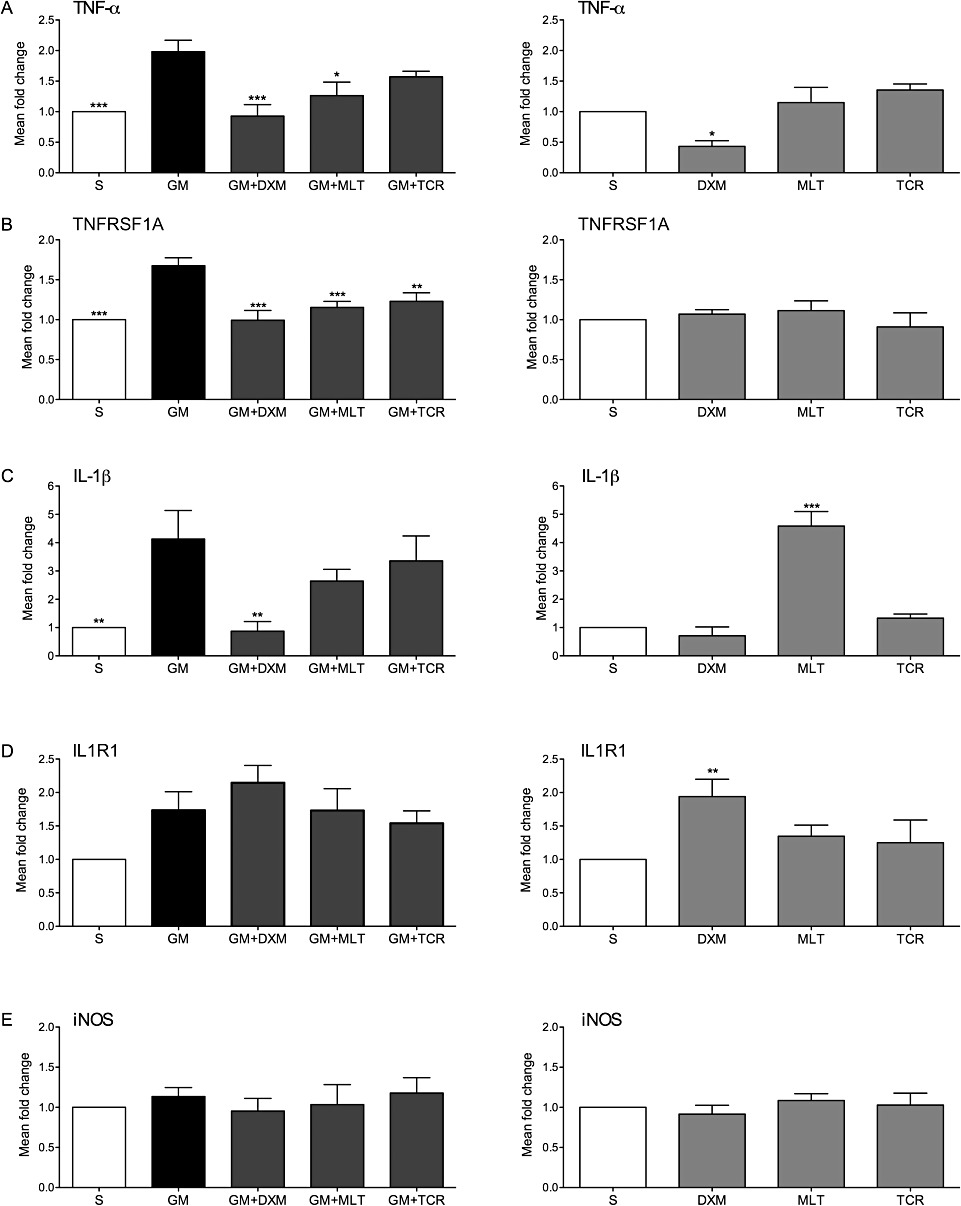
Gene expression analysis of two pro-inflammatory cytokines and their receptors (A–D) and of iNOS (E) in organ of Corti exposed to GM. Histograms on the left present mRNA levels of the genes studied in organ of Corti explants exposed to GM + treatment with either DXM, MLT or TCR compared to GM group explant expression levels. Histograms on the right column present mRNA levels of the genes studied in explants treated with either DXM, MLT or TCR-only in the absence of GM and compared to S group explant expression levels. mRNA levels corresponding to (A) TNF-α, (B) TNFRSF1A, (C) IL-1β, (D) IL1R1, (E) iNOS. Expression levels are presented as mean values ± SD; one-way anova followed by Bonferroni test; n = 9 explants per group in duplicates; *P < 0.05, **P < 0.01, ***P < 0.001.
ERK 1/2, p-ERK 1/2; p38 MAPK, p-p38 MAPK; JNK, p-JNK-elisa s
Treatment of the organ of Corti explants with GM did not induce a significant increase in the phosphorylation of ERK1/2 compared to the S-treated control explant values (Figure 2A). However, treatment of GM-exposed organ of Corti explants with DXM, MLT or TCR induced an increase in phospho-ERK1/2 levels. A decrease in ERK1/2-values was observed in the MLT and TCR-only treated groups when compared with the S group, while in the DXM alone treated explants the levels of phospho-ERK1/2 increased.
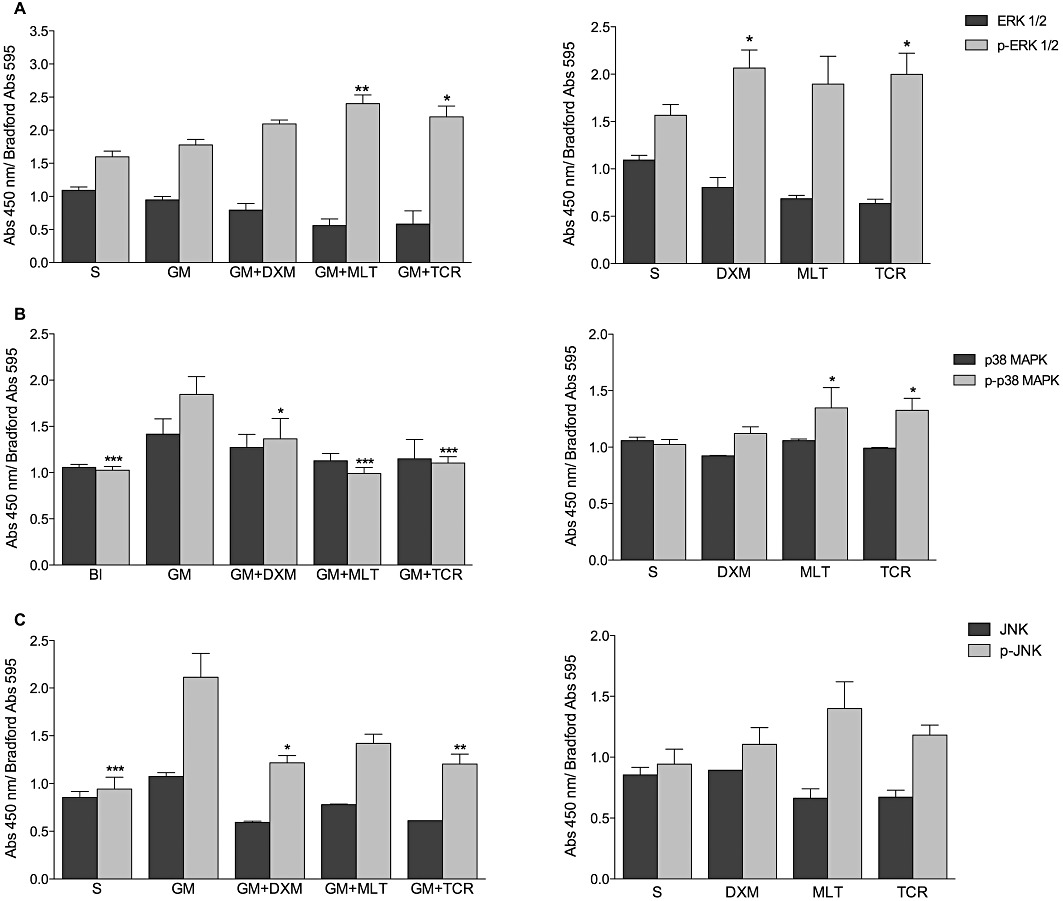
GM exposure of organ of Corti explants results in increases in the levels of phospho(p)-p38 MAPK and phospho(p)-JNK but did not affect the levels of phospho(p)-ERK1/2. (A–C) elisa assay results for the activation of three MAPK subfamilies with results presented as mean values ± SD. (A) ERK 1/2 and p-ERK1/2, (B) p38 MAPK and p-p38 MAPK and (C) JNK and p-JNK in organ of Corti explants exposed to GM (GM) or saline (S) and treated with DXM, MLT or TCR. In order to normalize the data obtained for each organ of Corti explant, the results are presented as the ratio of elisa absorbance at 450 nm/Bradford absorbance at 595 nm. Two-way anova, in which the phosphorylated and non-phosphorylated form for each enzyme were compared between groups was followed by Bonferroni test; n = 12 explants per group in duplicates; *P < 0.05, **P < 0.01, ***P < 0.001.
Phospho-p38 MAPK levels (Figure 2B) increased in the GM-only treated group of explants compared with the S group. The p-38 levels and its phosphorylated form were decreased in the GM + DXM, GM + MLT or GM + TCR groups of explants compared to the GM-alone group.
Phospho(p)-JNK levels increased in organ of Corti explants exposed to GM only when compared with the S group. A decrease in JNK and p-JNK levels was observed in GM + DXM; GM + MLT; and GM + TCR-treated explants (Figure 2C) when compared with the GM-alone explant group. The levels of the p-JNK were higher in the MLT-only treated group.
Assessment of antioxidant enzymes
SOD activity decreased in organ of Corti explants exposed to GM, while an increase in SOD activity that reached S group levels occurred in GM + DXM, GM + MLT and GM + TCR-treated groups of explants (Figure 3A). In organ of Corti explants not exposed to GM, the levels of the SOD activity were higher in DXM-only and MLT-only treated groups of explants compared to the levels for this enzyme present in the S explants group, while the TCR-only group of explants showed a decrease in SOD activity.
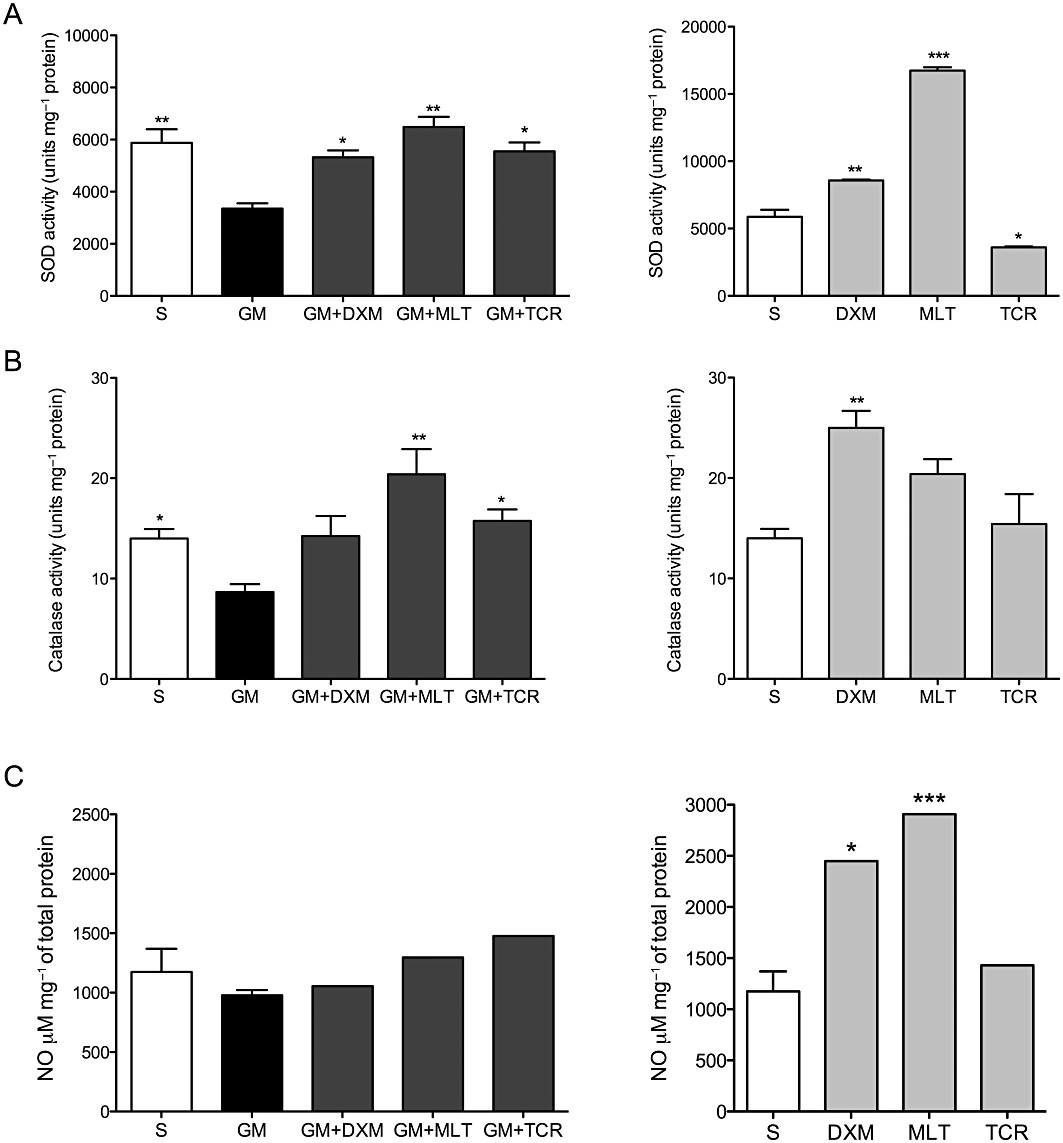
GM exposure of organ of Corti explants causes a decrease in SOD and catalase activity. Treatment of GM-exposed explants with either DXM, MLT or TCR protects the organ of Corti explants from GM-initiated free radical-induced oxidative stress. Histograms on the left present enzyme activity levels in organ of Corti explant exposed to GM and treated individually with each of the three protective compounds in this study. Histograms on the right present enzyme activity levels in organ of Corti explants treated with DXM, MLT or TCR-only in the absence of GM and compared to the saline treated (S) explant group levels. Antioxidant enzyme activity levels corresponding to (A) SOD and (B) catalase. (C) iNOS activity levels were addressed indirectly by measuring the levels of nitrite (NO2–), which is a primary, stable and non-volatile breakdown product of NO. In order to normalize the data obtained for each experiment, the results are presented in the case of SOD and catalase as the ratio of units of each enzyme activity/mg of total protein. In the case of iNOS, activity levels are presented as the ratio of concentration of NO2-mg-1 of total protein. One-way anova followed by Bonferroni test; n = 6 explants per group in duplicates; *P < 0.05, **P < 0.01, ***P < 0.001.
Treatment of organ of Corti explants with GM only reduced catalase activity compared with S explant group levels. An increase of catalase activity occurred in GM + DXM, GM + MLT and GM + TCR-treated explant groups compared with catalase levels in the GM-only group of explants. Catalase activity was enhanced in DXM-only and MLT-only treated explant groups compared to its activity in the S explants (Figure 3B).
No difference was observed in levels of NO for all treatment groups tested: i.e. S, GM-only, GM + DXM, GM + MLT, GM + TCR treatment groups (Figure 3C). Consistent with these NO results, exposure of organ of Corti to GM did not affect the mRNA levels of the inducible form of NOS (Figure 1E). DXM and MLT-only treated explants showed higher levels of NO compared to S explant group values for NO.
ROS detection
Organ of Corti explants exposed to GM only experienced an increase in ROS compared with the S group of explants in the base turn (Figure 4B and E), ROS was not observed in the apex of the specimens (data not shown). The mean of red signal intensity units (miu), corresponding to CellROX Deep Red reagent for GM-only group was higher than that of the S group in the auditory HCs and Deiter and pillar cells, referred to as supporting cells (SCs-) of the base (Figure 4P). A decrease in red signal intensity was observed in the GM + DXM, GM + MLT and GM + TCR groups (Figure 4H, K, N and P). The mean of red signal intensity was higher in GM-exposed group of explants than in the S, GM + DXM, GM + MLT and GM + TCR groups (Figure 4C, F, I, L, O and Q).
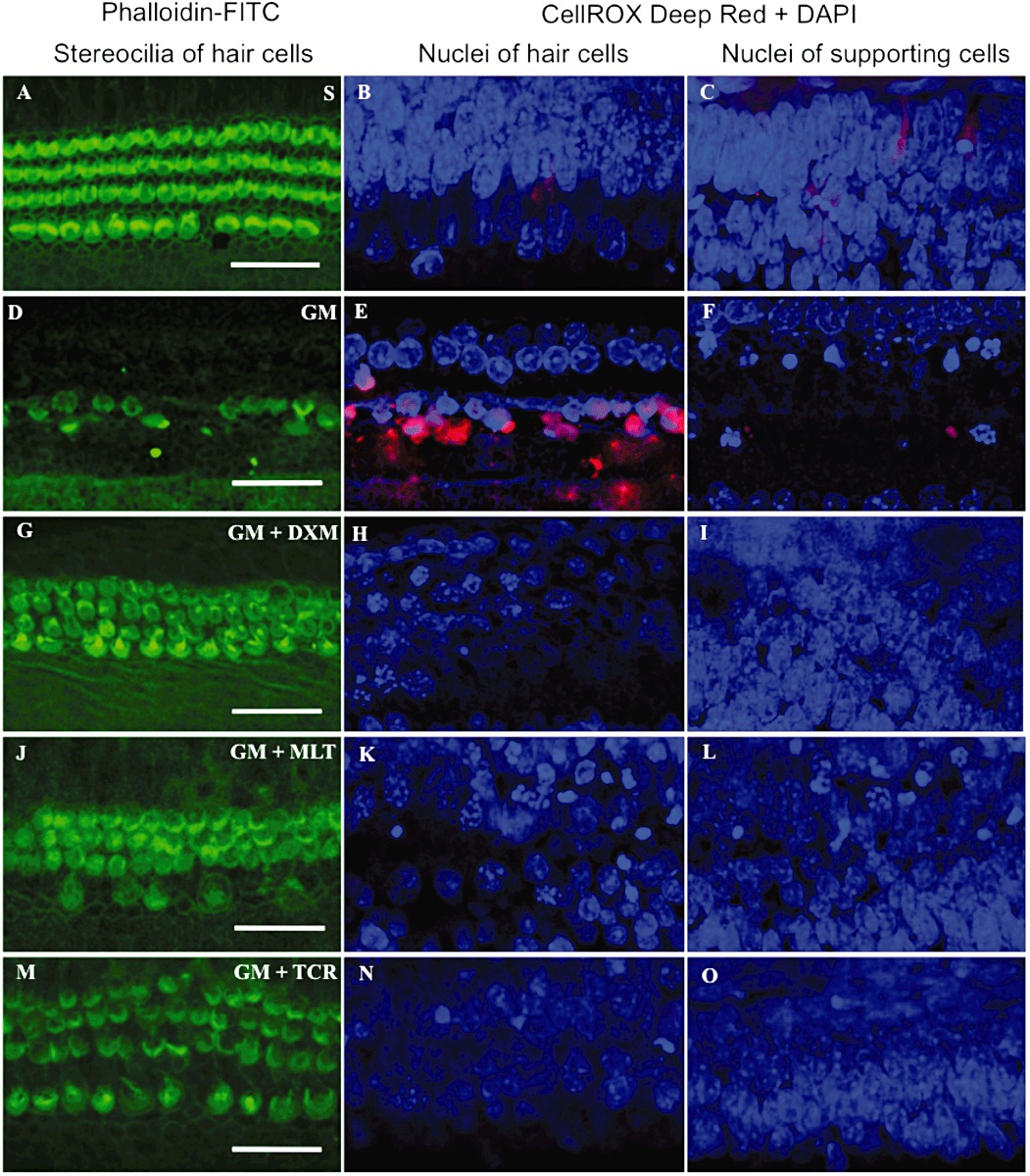
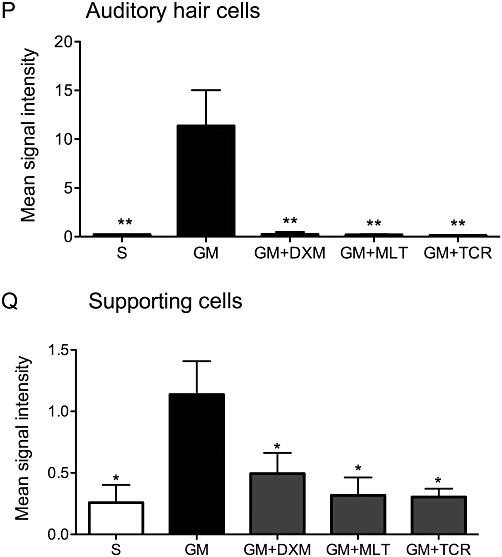
Organ of Corti explants exposed to GM (D–F) experienced an increase in ROS compared with the S-treated group of explants (A–C), and those exposed to GM + treated with DXM (G–I), MLT (J–L) or TCR (M–O) in the basal turn area of the explants. Treatment of GM-exposed explants with DXM, MLT and TCR resulted in a reduction in the loss of hair cells compared to GM-only explants (compare G, J and M to D). Bars in all pictures = 25 µm. The photomicrographs in A–O are representative of the results obtained from six explants per treatment group. (P and Q) shows differences in the mean signal intensity in the red channel for auditory hair cells and supporting cells, respectively, for the saline (S) control and the four treatment groups (mean values ± SD). One-way anova followed by Bonferroni test; n = 6 explants per group; *P < 0.05, **P < 0.01.
Cleaved caspase-3 immunostaining
As shown in Figure 5P and Q, there was a marked difference in the intensity of immunofluorescent signal for cleaved (i.e. activated) caspase-3 between GM-exposed organ of Corti explants and the S, GM + DXM, GM + MLT and GM + TCR groups, in auditory hair cells and supporting cells in the basal turn, while no activation of this enzyme was detected in the apex area (data not shown). Minimal activation of this apoptosis-associated enzyme occurred in the hair cells and supporting cells of organ of Corti explants treated with saline (S, Figure 5B and C). In sharp contrast, there was a marked increase in activated caspase-3 in the HCs and SCs of the GM-only exposed organ of Corti cultures (GM, Figure 5E and F); they also showed shrunken nuclei due to chromatin condensation and apoptotic bodies representing breakdown of affected cell nuclei. The auditory HCs and SCs in the GM + DXM, GM + MLT and GM + TCR treated organ of Corti explants showed lower levels of activated caspase-3 compared to the immunostaining levels observed in the GM-only explants (compare Figure 5H, I; K, L and N, O, respectively, with Figure 5E and F). A high level of HC loss was observed in the organ of Corti explants treated with GM-only (Figure 5D). Treatment of GM-exposed explants with DXM, MLT and TCR resulted in a reduction in the loss of HCs compared to that in the GM-only explants (compare Figure 5G, J and M to 5D).
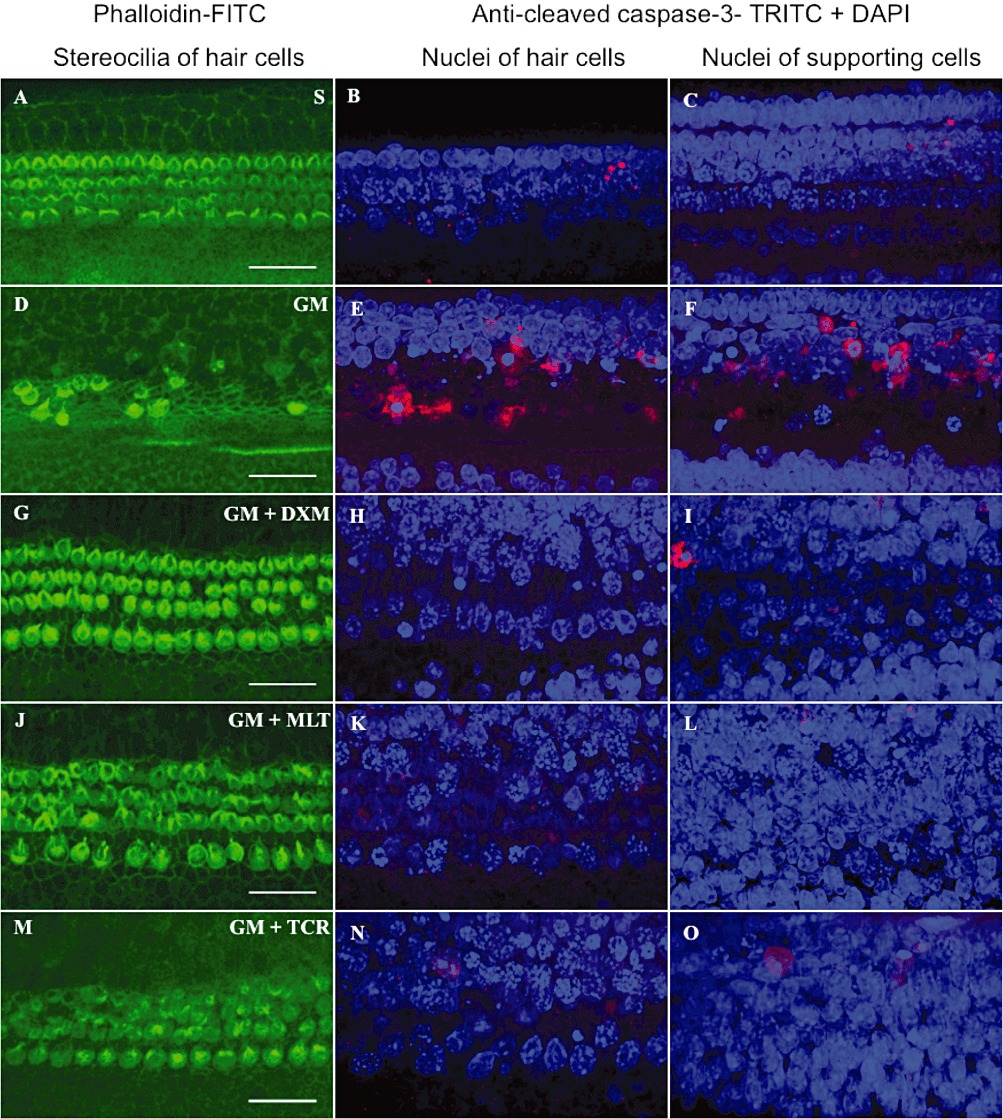
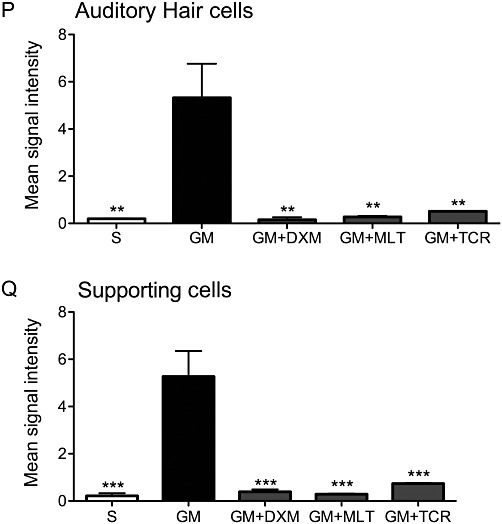
GM exposure of organ of Corti explants initiated the activation of caspase-3 in both auditory hair cells and supporting cells. This activation of pro-caspase-3 into an active form was prevented by the treatment of the GM-challenged explants with either DXM, MLT or TCR (G–O). Explant treatment groups: (A–C) saline (S) control; (D–F) GM, gentamicin-only treated; (G–I) GM + DXM, gentamicin and dexamethasone treated; (J–L) GM + MLT, gentamicin and melatonin treated; and (M–O) GM + TCR, gentamicin and tacrolimus treated. Phalloidin-FITC stain, green (A, D, G, J, M); anti-activated caspase-3, TRITC, red combined with DAPI, blue (B–C, E–F, H–I, K–L, N–O), bars in all pictures = 25 µm. The photomicrographs presented in A–O are representative of the results obtained from the six explants per treatment group. Treatment of GM-exposed explants with DXM, MLT and TCR resulted in a reduction in the loss of hair cells compared to GM-only explants (compare G, J and M to D). (P and Q) shows differences in the mean signal intensity in the red channel for auditory hair cells and supporting cells, respectively, for the S control explants and for the four experimental groups of this study. One-way anova followed by Bonferroni test; n = 6 explants per group; *P < 0.05, **P < 0.01, ***P < 0.001.
In vivo studies
DPgram
DPgrams corresponding to the different groups are shown in Figure 6. For both of the local delivery systems (Figure 6A and B), the cochleae treated with saline had DPOAE values that were similar to the base line values that were obtained before the onset of treatment. A decrease in DPOAE values for the ears treated with GM was observed on post-exposure days 10, 15 and 21 (Figure 6C and D). The difference in DPgrams is significant at the higher frequencies, that is, F2, 6000 to 10500 Hz, suggesting that the process of surgery did not affect the auditory system and that it was the GM-induced cochlear injury that was responsible for the changes in thresholds. There were no significant differences between basal levels and DPOAE values for GM + DXM; GM + MLT and GM + TCR groups of treatment in the gelfoam delivery system; however, a decrease (ns; P > 0.05) in DPOAE values was observed for the treatment groups in which GM was delivered locally to the round window membrane niche by the MPs (Figure 6F, H and J).
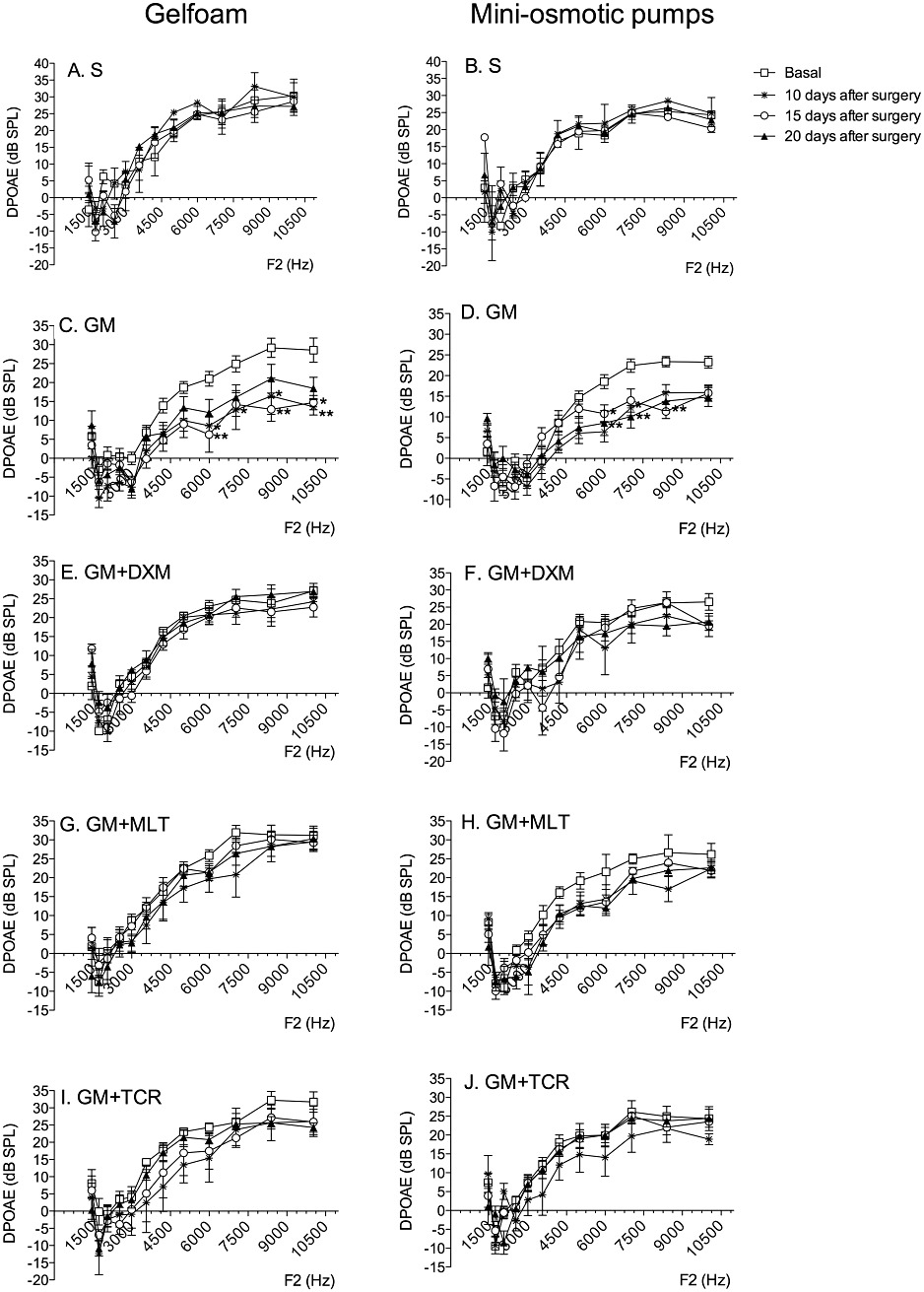
GM exposure causes a loss in the intensity of the DPOAEs (thought to reflect a loss of outer hair cell function) while local treatment with either DXM, MLT or TCR protected against this GM-induced loss of DPOAE intensity. DPOAEs for gelfoam (left column) and MP (right column) of animals treated with saline (S) (A, B); GM (C, D); GM + DXM (E, F); GM + MLT (G, H); and GM + TCR (I, J). In each graph, the symbols correspond to untreated, 10 days post-treatment, 15 days post-treatment and 20 days post-treatment values. Error bars show SD; two-way anova, Bonferroni's test; n = 5 animals per group; *P < 0.05, **P < 0.01.
ABRs
The ABR threshold values in response to a click stimulus presented in Figure 7 show that S-treated ears only showed a significant elevation in threshold in the gelfoam treatment group at 10 days post-treatment and then a return to a pretreatment threshold at 15 days post-treatment (Figure 7A and B), while cochleae treatment with GM by either gelfoam or via a MP showed significant increases (P < 0.05) in ABR threshold with respect to their baseline values on post-treatment days 10–20 (Figure 7C and D). In the GM + DXM (Figure 7E and F); GM + MLT (Figure 7G and H) and GM + TCR (Figure 7I and J) treated groups, the ABR threshold values were similar to those values recorded for the S-treated ears with only a transient elevation in thresholds in the MP GM + DXM group at 10 days and in the MP GM + TCR group at 15 days post-treatment.
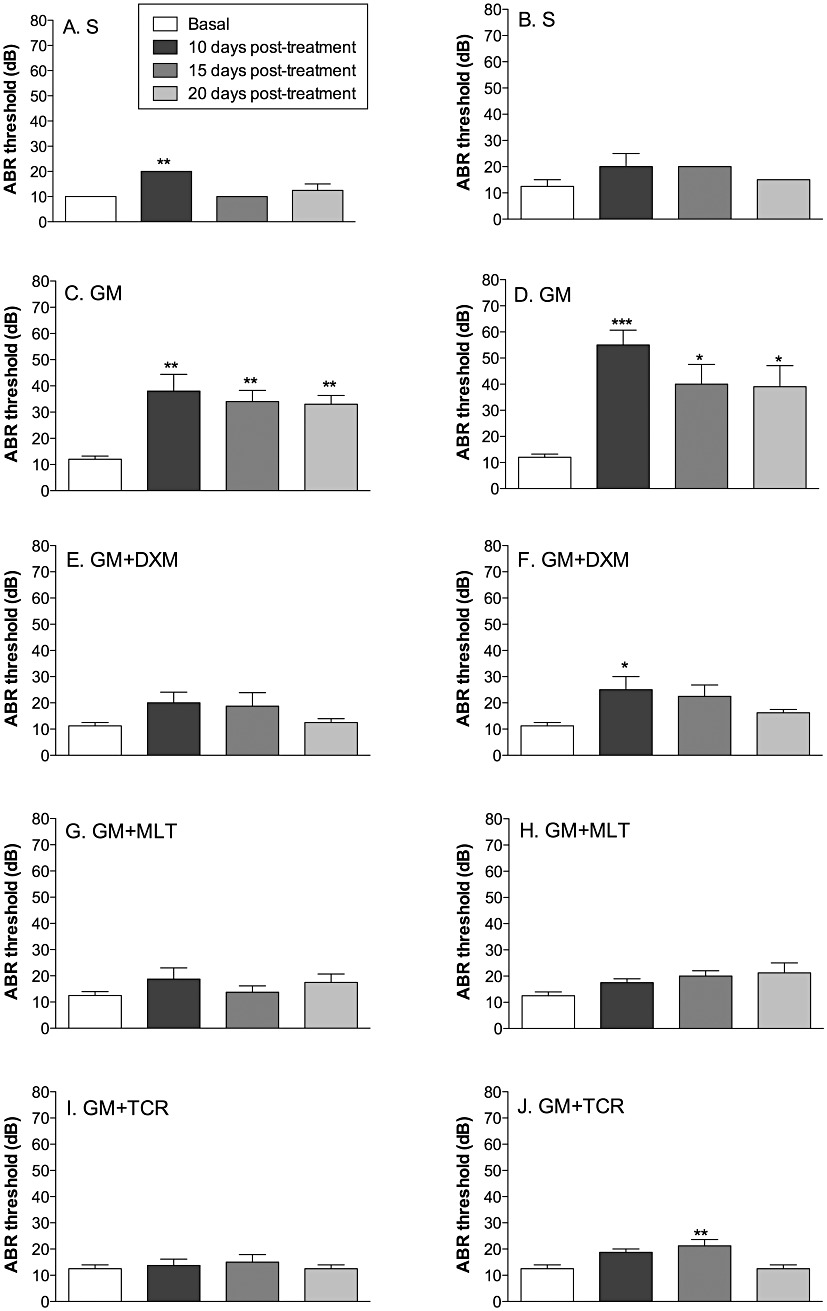
GM exposure causes an increase in auditory evoked brain stem (ABR) thresholds to a click stimulus while local treatment of cochleae with either DXM, MLT or TCR protects against this GM-induced elevation in hearing thresholds. ABR thresholds of animals treated with saline (S) (A, B); GM (C, D); GM + DXM (E, F); GM + MLT (G, H); and GM + TCR (I, J). In the right column are the graphs for the ears implanted with the MP, in the left column are the graphs for the ears implanted with gelfoam. Error bars show SD; one-way anova, Bonferroni's test; n = 5 animals per group; *P < 0.05, **P < 0.01, ***P < 0.001.
Cytocochleogram
Analysis of the cochleograms constructed from the hair cell counts taken from the organ of Corti FITC-phalloidin-stained surface preparations from the five groups of animals (Figure 8) indicate a loss of OHCs in the mid- to high-frequency regions (i.e. 55 to 100% from the apex) of the cochleae treated with GM while the cochleae treated with saline lost very few auditory sensory cells. Treatment of cochleae with GM did not affect IHCs viability (data not shown). In the mid- to high-frequency regions, the treatment of GM-exposed cochleae with either DXM, MLT or TCR prevented most of the GM exposure-induced OHC losses.
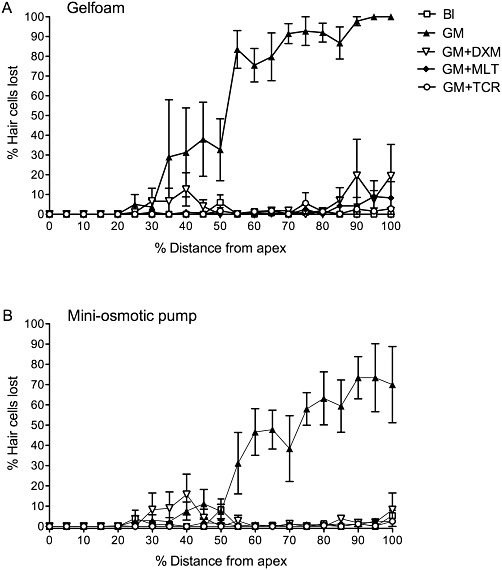
GM exposure causes a loss of OHCs from the cochlea while treatment of cochleae with either DXM, MLT or TCR protects these sensory cells against GM-induced losses of OHCs. Cytocochleogram for organ of Corti surface preparations from adult rat cochleae that had their round window membrane niches implanted with (A) gelfoam or (B) MP, which delivered each of the five different treatment solutions: baseline (Bl) (gelfoam: P < 0.001 for 45–100% distance from apex to base; MP: P < 0.001 for 60–100%); GM (group values of OHC losses to which the four other groups were compared); GM + DXM (gelfoam: P < 0.001 for 45–100%; MP: P < 0.001 for 60–100%); GM + MLT (gelfoam: P < 0.01 for 45–50% and P < 0.001 for 55–100%; MP: P < 0.001 or 55–100%); GM + TCR (gelfoam: P < 0.001 for 45–100%, MP: P < 0.001 or 55–100%). Error bars show SD; two-way anova, Bonferroni's test; n = 5 cochleae per treatment group.
Discussion
Oxidative stress
Several reports have concluded that the generation of ROS is linked to the ototoxicity of aminoglycosides, probably due to the ability of ROS to promote apoptotic and/or necrotic cell death of hair cells (Ylikoski et al., 2002; Rybak and Ramkumar, 2007; Cunningham and Tan, 2011). Choung et al. (2009) observed in GM-challenged organ of Corti explants that the formation of highly reactive oxygen species (hROS) was most prominent within the OHCs and showed both a base to apex as well as a first to the third row of OHCs intensity gradient. Moreover, they observed that patterns of stereocilia loss correlated with the accumulation pattern of hROS within hair cells of the GM-exposed explants. Our results demonstrate that GM treatment of organ of Corti explants increases the levels of ROS and also decreases the activity levels of the enzymes SOD (an important intracellular antioxidant enzyme) and catalase, which protects against H2O2. Treatment of GM-exposed explants with DXM, MLT and TCR reduced the levels of ROS production, but only DXM and MLT promoted an increase in the levels of SOD and catalase. These results suggest that the tested compounds reduce the levels of ROS in the HCs by increasing the activity of existing enzymes, such as SOD and catalase. Reactive nitrogen species are also known to be involved in cochlear injury induced by GM (Xie et al., 2011). Heinrich et al. (2008) demonstrated that GM caused hearing loss in association with an increase in NO production by the cochlea's lateral wall tissues and organ of Corti of guinea pigs. However, a study by Nordang and Anniko (2005) showed that the NO inhibitor L-nitro-arginine methyl ester (L-NAME) can provide only moderate otoprotection against GM-induced HC and hearing losses. Based on our results, the lack of an iNOS response by GM-exposed explants or by any of the treatments groups, clearly demonstrate that iNOS does not play a major role in GM ototoxicity. Because iNOS transcription remained at a basal level, the increase in NO in DXM-only and MLT-only treated explant groups could be due to an increase in endothelial NOS and/or neuronal NOS.
MAPK signalling
One signalling enzyme activated by aminoglycoside exposure via production of ROS is JNK, which has been shown to contribute to the initiation of apoptosis of damaged cells (Davis, 2000). While Kalinec et al. (2005) reported that gentamicin ototoxicity was mediated by the inhibition of JNK pathway and that a weak and transient activation happened mainly in supporting cells, Pirvola et al. (2000) and Ylikoski et al. (2002) found that JNK signalling was activated in auditory HCs after exposure to either an ototoxic antibiotic or a damaging level of noise. In this context, Van De Water and colleagues (Eshraghi et al., 2007) demonstrated that hearing loss induced by either exposure to an ototoxic antibiotic or mechanical trauma caused during cochlear implantation can be prevented by blocking JNK signalling with local administration of the inhibitory peptide, that is, D-JNKI-1. In this study, we showed that treatment with GM produces an increase in phosphorylated JNK and that treatment with all three compounds tested (i.e. DXM, MLT and TCR) lowered phospho-JNK levels.
Phospho-p38 levels, which are known to control cellular responses to stress and pro-inflammatory cytokines, were significantly reduced in the OC treated with MLT or TCR. Lahne and Gale (2008) reported the activation of ERK1/2 in supporting cells surrounding pyknotic HC nuclei in the neomycin-exposed organ of Corti, suggesting that ERK signalling promotes HC death and indicating a direct role for supporting cells in the regulation of HC death. In our study, DXM and MLT treatment of GM-exposed explants increased the levels of phosphorylated-ERK1/2, which can phosphorylate and activate the transcription factors, for example, Elk-1, c-fos and c-myc, thereby increasing a stressed cell's ability to survive (Hipskind et al., 1991). However, since in this study, the levels of ERK1/2 and the activated form of this kinase were measured in whole OC, it is not possible to determine if the ERK activation occurred in the supporting cells, as reported by Lahne and Gale (2008).
Pro-inflammatory cytokines
So et al. (2007) reported that ROS are formed in response to trauma, which in the present study, was caused by exposure to GM. GM exposure produces high levels of ROS and an inflammatory response where the phosphorylation of p38 MAPK and JNK play important roles in the stress-related loss of auditory HCs (Op de Beck et al., 2011). Our results show that exposure of explants to GM results in increased levels of TNF-α, IL-1β and TNFRSF1A expression. We also showed that DXM treatment of GM-exposed explants down-regulated the expression of both of these pro-inflammatory cytokines and TNFRSF1A. DXM treatment of the GM-exposed explants did not reduce the level of IL1R1 transcripts, while DXM-only treated explants had increased mRNA levels for this cytokine receptor. MacGillivray et al. (2000) reported that IL-1β can induce the activation of ERK through its receptor, that is, IL1R1. This observation could explain the correlation we observed between IL1R1 mRNA up-regulation and higher levels of phospho-ERK1/2 in the DXM-only treated group. Treatment of GM-exposed explants with MLT resulted in significant down-regulation of TNF-α and TNFRSF1A expression, while MLT had no significant effect on IL-1β and IL1R1 expression in GM-exposed explants. In contrast, MLT-only treatment of explants initiated an increase in the expression of IL-1β mRNA, which correlates with an increase in the levels of the phosphorylated form of ERK1/2, p-38 MAPK and JNK, although none were significantly different from their activated forms in the S-treated explants.
MLT treatment
Treatment of rats with MLT protected against GM-induced ototoxicity as shown by the auditory function results and by the histological assays of HCs present in the cytocochleograms. These findings are in accord with Ye et al. (2009), who observed that MLT attenuated HC loss caused by exposure of guinea pigs to an ototoxic level of gentamicin. MLT has previously been demonstrated to have both anti-inflammatory and neuroprotective properties by reducing IL-1β and TNF-α levels and by inhibiting the phosphorylation of p38, JNK and ERK1/2 in a spinal cord injury model (SCI; Esposito et al., 2009). The differences in phosphorylation levels obtained for ERK1/2 in the present study may be explained by the fact that our model is not limited to neurones, since the organ of Corti explant is a complex structure composed of several different kinds of HCs and SCs. In contrast, Joo and Yoo (2009) reported MLT induced apoptosis in an androgen-sensitive human prostate adenocarcinoma cell line [i.e. lymph node carcinoma of the prostate (LNCaP)] via JNK and p38, but not ERK signalling. The present study indicates an anti-apoptotic effect of MLT, since treatment of GM-exposed explants with MLT inhibited the activation of caspase-3 and HC death. In this context and according to our findings, Kim et al. (2009) reported the protective effect of MLT treatment in GM-exposed utricular explants was as a result of an inhibitory effect on both ROS production and caspase-3 activation.
DXM treatment
DXM is known to inhibit AP-1 activity (González et al., 2000). Local administration of DXM into the inner ear has been shown to be otoprotective when applied before the administration of an aminoglycoside with a loop diuretic; however, only mild protection occurred when DXM was applied after exposure to an aminoglycoside (Himeno et al., 2002). Dinh et al. (2008) reported the otoprotective properties of DXM against TNF-α in organ of Corti explants demonstrating an up-regulation of both Bcl-2 and Bcl-xl and inhibition of Bax expression that resulted in a lowering of the Bax/Bcl-2 expression ratio favouring HC survival. Our adult rat results indicate that local DXM treatment prevented both loss of auditory function and loss of their HCs in GM-exposed rats.
TCR treatment
TCR is a potent immunosuppressive drug primarily used for the reduction of allograft rejection and has been reported to be neuroprotective following CNS injury. Our results indicate that local delivery of TCR via the round window membrane of the GM-exposed rat cochlea prevented both HC loss and an increase in hearing threshold levels (i.e. hearing loss) that occurred in the GM-only treated animals. Guzmán-Lenis et al. (2008) demonstrated that TCR had a significant protective effect on spinal cord tissue following acute excitotoxic damage that was characterized by a release of pro-inflammatory cytokines, such as TNF-α and IL-1β, which are mainly produced and released by microglia. In this context, Saganováet al. (2009) mentioned that immunosuppressant drugs, including TCR, have direct inhibitory effects on microglia by inhibiting microglial secretion of neurotoxic substances such as pro-inflammatory cytokines and NO. Our in vitro results showed a significant inhibition of gene expression for TNFRSF1A occurred in the GM-exposed TCR-treated explants with no significant effect on the expression of TNF-α or IL-1β and its receptor, IL1R1. Therefore, the in vitro results obtainedfor TCR treatment of GM-exposed explants provides weak support for a possible immunosuppressive action by TCR to explain its in vivo otoprotection.
Clinical implications
All three of the compounds tested have been used clinically and all have been shown to be effective at protecting against GM ototoxicity both in vitro and in vivo. However, only local application, via the round window membrane, of one of these compounds, DXM, has been extensively used to treat hearing (Haynes et al., 2007). Therefore, this would be the drug of first choice in an attempt to limit the damage that can result from systemic therapy with an aminoglycoside antibiotic.
Conclusions
The development of local long-term delivery techniques to the cochlea will be a breakthrough in terms of reducing the levels of drugs required for effective treatment, decreasing or eliminating side effects and avoiding alteration of drugs by liver metabolism, thereby assuring that the desired concentration of a drug is achieved only in the target area, that is, the perilymph within the scala tympani. The results of our study show that local treatment of the cochlea with DXM, MLT or TCR can conserve auditory function and prevent HC loss.
Acknowledgments
The authors thank Professor Rios Cañavate from the Pharmacology Department, University of Valencia, Spain, for use of the HPLC equipment and Dr Shibing Chen from University of Miami Ear Institute, University of Miami, Miller School of Medicine, Miami, FL, USA, for teaching EB the organ of Corti explant procedure and culture techniques. EB was the recipient of a postdoctoral fellowship from Bancaixa and Hospital Clinico Universitario Foundation Research and a fellowship for exchange students from Generalitat Valenciana (BE-14/08). This work was partially supported by the Generalitat Valenciana (AP-009/09) and a grant from MED-EL Hearing Instruments, Innsbruck, Austria to VDW.
Glossary
| ABRs | auditory brainstem responses |
| AP-1 | activating protein-1 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| dB | decibel |
| DPOAEs | distortion product otoacoustic emissions |
| DXM | dexamethasone |
| ERKs | FITC, fluorescein isothiocyanate |
| GM | gentamicin |
| HCs | hair cells |
| hROS | highly reactive oxygen species |
| IHCs | inner hair cells |
| IL1R1 | IL receptor type 1 |
| iNOS | inducible nitrogen oxide syntase |
| L-NAME | L-nitro-arginine methyl ester |
| LNCaP | lymph node carcinoma of the prostate |
| MLT | melatonin |
| MP | miniosmotic pumps |
| NBT | nitroblue tetrazolium |
| ns | not significant |
| OHCs | outer hair cells |
| PD | PD98059, 2-(2-amino-3-methoxyphenyl) chromen-4-one |
| ROS | reactive oxygen species |
| RT-PCR | reverse transcription PCR |
| S | saline |
| SCI | spinal cord injury |
| SOD | superoxide dismutase |
| SPL | sound pressure level |
| TCR | tacrolimus |
| TNFRSF1A | TNF receptor type 1 |
| TRITC | tetramethyl rhodamine isothiocyanate |
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, et al. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS ONE. 2011;6:e22347. [Europe PMC free article] [Abstract] [Google Scholar]
- Bertolaso L, Bindini D, Previati M, Falgione D, Lanzoni I, Parmeggiani A, et al. Gentamicin-induced cytotoxicity involves protein kinase C activation, glutathione extrusion and malondialdehyde production in an immortalized cell line from the organ of Corti. Audiol Neurootol. 2003;8:38–48. [Abstract] [Google Scholar]
- Choung YH, Taura A, Pak K, Choi SJ, Masuda M, Ryan AF. Generation of highly-reactive oxygen species (hROS) is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience. 2009;161:214–226. [Europe PMC free article] [Abstract] [Google Scholar]
- Cunningham L, Tan J. Cell death in the inner ear. In: Reed JC, Green DR, editors. Apoptosis: Physiology and Pathology. Cambridge, UK: Cambridge University Press; 2011. pp. 182–193. [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. [Abstract] [Google Scholar]
- Dinh CT, Haake S, Chen S, Hoang K, Nong E, Eshraghi AA, et al. Dexamethasone protects organ of Corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008;157:405–413. [Abstract] [Google Scholar]
- Eshraghi AA, Wang J, Adil E, He J, Zine A, Bublik M, et al. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity. Hear Res. 2007;226:168–171. [Abstract] [Google Scholar]
- Esposito E, Genovese T, Caminito R, Bramante P, Meli R, Cuzzocrea S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J Pineal Res. 2009;46:79–86. [Abstract] [Google Scholar]
- González MV, Jiménez B, Berciano MT, González-Sancho JM, Caelles C, Lafarga M, et al. Glucocorticoids antagonize AP-1 by inhibiting the activation/phosphorylation of JNK without affecting its subcellular distribution. J Cell Biol. 2000;150:1199–1207. [Europe PMC free article] [Abstract] [Google Scholar]
- Griess P. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt ‘Ueber einige azoverbindungen.’ Chem Ber. 1879;12:426–428. [Google Scholar]
- Guzmán-Lenis MS, Vallejo C, Navarro X, Casas C. Analysis of FK506-mediated protection in an organotypic model of spinal cord damage: heat shock protein 70 levels are modulated in microglial cells. Neuroscience. 2008;155:104–113. [Abstract] [Google Scholar]
- Haynes DS, O'Malley M, Cohen S, Watford K, Labadie RF. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2007;117:3–15. [Abstract] [Google Scholar]
- Heinrich P, Helling K, Sifferath M, Brieger J, Li H, Schmidtmann I, et al. Gentamicin increases nitric oxide production and induces hearing loss in guinea pigs. Laryngoscope. 2008;118:1438–1442. [Abstract] [Google Scholar]
- Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, et al. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167:61–70. [Abstract] [Google Scholar]
- Hipskind RA, Roa VN, Muller CGF, Raddy ESP, Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991;354:531–534. [Abstract] [Google Scholar]
- Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. [Abstract] [Google Scholar]
- Kalinec GM, Fernandez-Zapico ME, Urrutia R, Esteban-Cruciani N, Chen S, Kalinec F. Pivotal role of Harakiri in the induction and prevention of gentamicin-induced hearing loss. PNAS. 2005;102:16019–16024. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy – from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. [Abstract] [Google Scholar]
- Kim JB, Jung JY, Ahn JC, Rhee CK, Hwang HJ. Antioxidant and anti-apoptotic effect of melatonin on the vestibular hair cells of rat utricles. Clin Exp Otorhinolaryngol. 2009;2:6–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–195. [Abstract] [Google Scholar]
- Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci. 2008;28:4918–4928. [Abstract] [Google Scholar]
- Luck H. Catalase. In: Bergmeyer HO, editor. Methods of Enzymatic Analysis. New York, London: Academic Press; 1971. pp. 855–927. [Google Scholar]
- MacGillivray MK, Cruz TF, McCulloch CAG. The recruitment of the interleukin-1 (IL-1) receptor-associated kinase (IRAK) into focal adhesion complexes is required for IL-1b-induced ERK activation. J Biol Chem. 2000;275:23509–23515. [Abstract] [Google Scholar]
- Nordang L, Anniko M. Nitro-L-arginine methylester: a potential protector against gentamicin ototoxicity. Acta Otolaryngol. 2005;125:1033–1038. [Abstract] [Google Scholar]
- Op de Beck K, Schacht J, Van Camp G. Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear Res. 2011;281:18–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, et al. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. [Abstract] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. [Abstract] [Google Scholar]
- Saganová K, Orendácová J, Sulla I, Filipcık P, Cızkova D, Vanicky I. Effects of long-term FK506 administration on functional and histopathological outcome after spinal cord injury in adult rat. Cell Mol Neurobiol. 2009;29:1045–1051. [Abstract] [Google Scholar]
- So H, Kim H, Lee J, Park C, Kim Y, Kim E, et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kB. J Assoc Res Otolaryngol. 2007;8:338–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-Terminal Kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. [Abstract] [Google Scholar]
- Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011 DOI: 10.1016/j.heares.2011.05.008 [Epub ahead of print] [Europe PMC free article] [Abstract] [Google Scholar]
- Ye LF, Tao ZZ, Hua QQ, Xiao BK, Zhou XH, Li J, et al. Protective effect of melatonin against gentamicin ototoxicity. J Laryngol Otol. 2009;123:598–602. [Abstract] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. [Abstract] [Google Scholar]
Articles from British Journal of Pharmacology are provided here courtesy of The British Pharmacological Society
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1476-5381.2012.01890.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3402812?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1476-5381.2012.01890.x
Article citations
Exploring the bioactive ingredients of three traditional Chinese medicine formulas against age-related hearing loss through network pharmacology and experimental validation.
Naunyn Schmiedebergs Arch Pharmacol, 02 Oct 2024
Cited by: 0 articles | PMID: 39356317
Mitochondrial-derived peptides, HNG and SHLP3, protect cochlear hair cells against gentamicin.
Cell Death Discov, 10(1):445, 21 Oct 2024
Cited by: 0 articles | PMID: 39433756 | PMCID: PMC11493991
A Diet with Amikacin Changes the Bacteriobiome and the Physiological State of Galleria mellonella and Causes Its Resistance to Bacillus thuringiensis.
Insects, 14(11):889, 17 Nov 2023
Cited by: 0 articles | PMID: 37999088 | PMCID: PMC10672437
Effect of melatonin on otoprotection in rodents: a systematic review with meta-analysis.
Braz J Otorhinolaryngol, 89(5):101288, 04 Jul 2023
Cited by: 0 articles | PMID: 37451174 | PMCID: PMC10518499
Review Free full text in Europe PMC
Considering the protective effect of exendin-4 against oxidative stress in spiral ganglion neurons.
Iran J Basic Med Sci, 26(12):1423-1430, 01 Jan 2023
Cited by: 0 articles | PMID: 37970444 | PMCID: PMC10634057
Go to all (37) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss.
Acta Otolaryngol, 129(4):385-389, 01 Apr 2009
Cited by: 19 articles | PMID: 19051071
Otoprotective properties of mannitol against gentamicin induced hair cell loss.
Otol Neurotol, 35(5):e187-94, 01 Jun 2014
Cited by: 13 articles | PMID: 24662629
Conservation of hearing and protection of auditory hair cells against trauma-induced losses by local dexamethasone therapy: molecular and genetic mechanisms.
Cochlear Implants Int, 11 Suppl 1:42-55, 01 Jun 2010
Cited by: 17 articles | PMID: 21756583
Mechanisms of hearing loss from trauma and inflammation: otoprotective therapies from the laboratory to the clinic.
Acta Otolaryngol, 130(3):308-311, 01 Mar 2010
Cited by: 28 articles | PMID: 19579145
Review




