Abstract
Free full text

Pharmacogenetic approaches to the treatment of alcohol addiction
Abstract
Addictive disorders are partly heritable, chronic, relapsing conditions that account for a tremendous disease burden. Currently available addiction pharmacotherapies are only moderately successful, continue to be viewed with considerable scepticism outside the scientific community and have not become widely adopted as treatments. More effective medical treatments are needed to transform addiction treatment and address currently unmet medical needs. Emerging evidence from alcoholism research suggests that no single advance can be expected to fundamentally change treatment outcomes. Rather, studies of opioid, corticotropin-releasing factor, GABA and serotonin systems suggest that incremental advances in treatment outcomes will result from an improved understanding of the genetic heterogeneity among patients with alcohol addiction, and the development of personalized treatments.
Addictive disorders account for an extensive disease burden, and disproportionately affect people in the prime of their lives. In industrialized countries, alcohol use alone causes about 10% of total disability-adjusted life years lost1, and a recent evaluation in the United Kingdom concluded that in aggregate, the harm to self and others inflicted by alcohol exceeds that caused by heroin or cocaine2. Alcohol consumption in the population is markedly skewed, and a large proportion of alcohol-related disability is due to alcohol addiction, hereafter equated with alcoholism. This is a condition that in the United States affects more than 12% of the population at some point in their life3. Alcoholism is a chronic, relapsing disorder that shares many characteristics with other complex chronic conditions, such as diabetes or hypertension: it has a considerable component of genetic susceptibility, is under marked influence of environmental factors, and its onset and course are fundamentally shaped by behavioural choices4,5. This prompts the question of whether alcoholism can be tackled with medical treatments. Some efficacy of medications for alcoholism6 as well as opiate7 and nicotine8 addiction has been documented and supports the feasibility of addiction pharmacotherapy. However, with the exception of methadone or buprenorphin maintenance therapy for opioid addictions, the effect sizes of these treatments are small. Despite evidence-based guidelines that pharmacotherapy be considered in all patients with alcoholism, and in particular in those who are not successfully treated with behavioural interventions alone9, only a small minority of patients receive medication for their alcoholism10. Clearly, extensive unmet medical needs remain in this therapeutic area.
In this Review, we first show that there is considerable heterogeneity among people with alcohol addiction, and that this heterogeneity suggests a need for personalized treatment approaches based on, among other factors, genetic variation. We then review evidence for functional genetic variation within biological systems that mediate positive and negative reinforcement from alcohol — including the opioid and corticotropin-releasing factor (CRF; also known as CRH) systems — and summarize evidence that this variation is likely to moderate treatment effects. An overarching objective of this Review is to point the way for translating the considerable advances recently made in the basic neuroscience of alcohol addiction into therapeutic gains for patients.
‘Alcoholics’ differ from each other
A clinical diagnosis of alcoholism is currently made on the basis of diagnostic criteria that are standardized across addictive disorders by the Diagnostic and Statistical Manual of Mental Disorders, which is currently in its fourth edition (DSM IV)11. In the absence of reliable biomarkers, this approach eliminates some of the subjective judgement involved in making diagnoses, and has clinical utility. However, there is reason to believe that patients diagnosed using this approach are markedly heterogeneous. In fact, such heterogeneity was already proposed in the 1980s on the basis of clinical characteristics such as age of onset, but also on family history, which is a marker of genetic susceptibility12. Numerous other attempts at clinical subtyping of people with alcoholism have since followed. The use of genetic markers offers the possibility of more reliably and consistently capturing the heterogeneity of people with alcoholism, in ways that are closer to its biological underpinnings.
Among individuals in the general population who fulfil diagnostic criteria for alcoholism, the majority — about three-quarters — never receive treatment3. Available data indicate that those people who go on to enter treatment and those who do not are fundamentally different with regard to personality traits, alcohol use patterns and long-term outcomes13–15. Furthermore, classic longitudinal studies show that long-term outcomes and alcohol-related harm vary markedly between individuals in ways that do not seem to have a simple correlation with participation in treatment or the level of alcohol use13,14.
A clinical diagnosis of alcoholism is probably best viewed as an ‘end-stage disease’, similar to congestive heart failure. In this view, the diagnostic category of alcoholism consists of conditions that are phenotypically similar (or constitute ‘phenocopies’), but patients arrive at the disease state through fundamentally different trajectories. This is captured by a conceptualization that was first put forward for major depression16, but is also likely to apply to addiction (BOX 1). In a kindling-like process, brain exposure to cycles of intoxication and withdrawal induces progressive neuroadaptations that ultimately result in escalation of alcohol intake17,18. In the absence of significant genetic susceptibility, escalation will only result following prolonged exposure to alcohol and the environmental factors with which it interacts, such as stress. By contrast, when genetic risk factors are present, progression can be fast. These individuals can be viewed, in terminology borrowed from the depression literature16, as ‘pre-kindled’, or ‘already there’.
Emerging evidence indicates that individuals with alcohol addiction who are on trajectories that are driven by different biological mechanisms or who are in different stages of addiction can be expected to respond to different treatments. Fundamentally, treatments for alcohol addiction must intervene with biological mechanisms that provide motivation for alcohol seeking and consumption19. These mechanisms largely fall into two main categories. First, in a similar way to other drugs of abuse, alcohol can activate brain reward pathways, leading to positively reinforced alcohol seeking and use. Secondly, alcohol can acutely suppress negative emotions that result from stress or withdrawal from alcohol itself, such as anxiety and dysphoria, thus setting the scene for negatively reinforced alcohol use18,20. To highlight the distinction between these two incentives for alcohol use, the terms ‘reward drinking’ and ‘relief drinking’ have been introduced21. It is reasonable to expect that these different types of excessive alcohol use will require different treatments.
Alcoholism has a moderate to high heritability, and in part shares genetic susceptibility factors with other addictions5. Genetic and environmental factors in alcoholism can result in very different types of vulnerability, ranging from heightened impulsivity and reward from alcohol to enhanced stress responses and anxious personality traits12. Genetic variants that alter alcohol reward- or stress-related emotional processing are therefore probable modifiers of disease trajectories and of responses to treatments that target reward and stress systems.
Targeting opioid-mediated alcohol reward
Alcohol reward is in part mediated by endogenous opioids
Although the exact role of mesolimbic dopamine in addiction remains controversial, activation of this pathway is thought to confer incentive salience to addictive drugs, to ‘reward’ their pursuit or consumption, or to be otherwise related to their addictive properties20,22–24. Accordingly, studies in experimental animals25,26 and humans27,28 have demonstrated that alcohol activates the mesolimbic dopamine circuitry. Dopamine neurotransmission in the corticomesolimbic system is modulated by the mu-opioid receptor (MOR; also known as MOR1). Inhibitory tone from GABAergic interneurons onto dopamine cell bodies in the ventral tegmental area (VTA) is removed through MOR activation on GABA neurons by endogenous opioids, which ultimately results in increased dopamine release in terminal areas in the ventral striatum29,30. The exact mechanism by which alcohol interacts with this circuitry remains unknown. However, studies in experimental animals show that MOR blockade in the VTA largely prevents accumbal dopamine release induced by alcohol intake, indirectly showing that alcohol leads to release of endogenous opioids within this structure and thereby drives dopamine release31 (FIG. 1). Another, independent line of research led to development and approval of the opioid receptor antagonist naltrexone as a medication for alcoholism6 (BOX 2). A synthesis of these two research lines leads to the hypothesis that the mechanism through which naltrexone exerts its therapeutic action is by disrupting the cascade that leads to striatal dopamine release following alcohol intake.
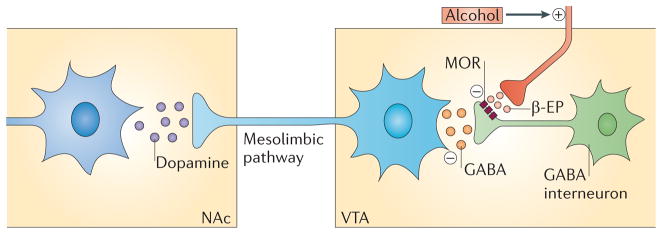
Schematic of the alcohol–opioid–dopamine cascade that is thought to be the target of naltrexone, based on integration of circuitries originally proposed in REFS 29,156. Dopaminergic ventral tegmental area (VTA) neurons that project to the nucleus accumbens (NAc) are under tonic inhibition by GABAergic interneurons within the VTA. GABA release from these neurons is in turn under negative regulation by the mu-opioid receptor (MOR). When alcohol is ingested, endogenous opioids such as β-endorphins (β-EPs) are released, resulting in inhibition of GABA release in the VTA and removal of the inhibitory tone from the dopamine cells. This cascade ultimately results in increased dopamine release in the terminal areas in the NAc.
However, although a meta-analysis6 supports the efficacy of naltrexone treatment in alcoholism, the average effect size is small, with a Cohen’s D of approximately 0.2. One possible conclusion is that endogenous opioids only play a minor part in alcohol reward and excessive alcohol use, limiting the utility of treatments that target this mechanism. In fact, despite solid evidence for its efficacy, naltrexone has not come into widespread clinical use, and scepticism about its efficacy is one of the reasons given by clinicians10.
However, an alternative interpretation of the limited overall effect size of naltrexone is that it reflects heterogeneity of response among patients. In fact, both clinical experience and meta-analyses have long indicated a heterogeneity of naltrexone responses in people with alcoholism, and have implied a possible role of genetic factors in this heterogeneity. For instance, a meta-analysis of available clinical trials suggests that a family history of alcoholism is associated with clinical improvement in response to naltrexone treatment32. Support for a role of family history in the clinical response to naltrexone has also been found in laboratory studies; family history influenced both the effect of naltrexone on subjective feelings of a ‘high’ from a standard alcohol dose33 and the level of alcohol self-administration34. Although a role of family history could reflect genetic or environment factors (or both), emerging evidence strongly suggests a major role of pharmacogenetics in the clinical response to naltrexone, as discussed below.
Functional variation at the OPRM1 locus as a pharmacogenetic determinant
The possibility of pharmacogenetic heterogeneity in the response to naltrexone is particularly important to consider, because more than a decade ago a common functional variant was discovered in the OPRM1 gene, which encodes the MOR, the target for naltrexone35,36. This non-synonymous 118A→G single nucleotide polymorphism (SNP), rs1799971, encodes an asparagine (N) → aspartate (D) substitution in position 40 of the receptor protein (N40D). The exchange occurs in the amino-terminal extracellular loop of the receptor, and results in the loss of a putative glycosylation site (BOX 2). The frequency of the less common (minor) 118G allele at this locus varies between populations of different ancestry (see below). The precise functional consequences of the N40D substitution for MOR function remain unclear, and its role as a genetic risk factor in addictive disorders is controversial36–41. However, based on a secondary analysis of three clinical trials, it was suggested that this polymorphism might moderate the therapeutic efficacy of naltrexone, and that beneficial effects of naltrexone might be largely restricted to OPRM1 118G carriers42. This finding was subsequently replicated in a secondary analysis of the large, US National Institute on Alcohol Abuse and Alcoholism (NIAAA)-sponsored COMBINE trial, in which naltrexone almost doubled the proportion of patients with a ‘good clinical outcome’ in the group of 118G carriers (from ~50% to ~90%), but had no effect on outcome in 118A homozygous patients43. Although one clinical study failed to replicate this finding44, a role of OPRM1 variation as a moderator of alcohol reward and naltrexone effects was also supported by results of elegant human laboratory studies45,46.
The evaluation of pharmacogenetic factors poses considerable challenges. Unless subjects in clinical trials are a priori recruited and randomization is stratified by genotype, undetected sources of bias may obscure true findings. Drug effects that are restricted to carriers of a minor allele are difficult to detect, because the sample size may simply be too small. Rodent models cannot easily be used to address the role of specific human genetic variants in drug responses, because variants that are functionally equivalent to those found in humans are rarely if ever found in rodents owing to the large phylogenetic distance between these species. Studies in non-human primates can be helpful in this regard, because functional equivalents of behaviourally important human variants have frequently arisen in non-human primates47. This is of evolutionary interest in its own right, but it also offers a resource for addressing questions of addiction vulnerability and pharmacogenetics in humans (BOX 3).
Accordingly, an OPRM1 SNP that is functionally equivalent to the human A118G polymorphism, namely C77G (resulting in a proline (P) → arginine (R) exchange in position 26 of the receptor protein, or P26R amino acid exchange) was identified in the rhesus macaque48. Male carriers of the rhesus OPRM1 77G allele showed increased psychomotor stimulation in response to alcohol, increased alcohol preference and increased frequency of alcohol consumption to intoxication49. Because psychomotor stimulation is a proxy marker of mesolimbic dopamine activity, these findings suggested that activation of the mesolimbic circuitry in response to alcohol primarily occurs in OPRM1 77G carriers. This prompted the hypothesis that OPRM1 77G carriers would also be preferentially sensitive to suppression of alcohol preference by naltrexone. When this was tested, naltrexone indeed only suppressed alcohol preference in carriers of the 77G variant50, a finding that has been independently corroborated51. Both the rhesus and the human data may have limitations when considered separately, but their convergence supports a role of OPRM1 variation as a moderator of naltrexone effects, in a manner that is very similar for the rhesus and human variants (FIG. 2a,b).
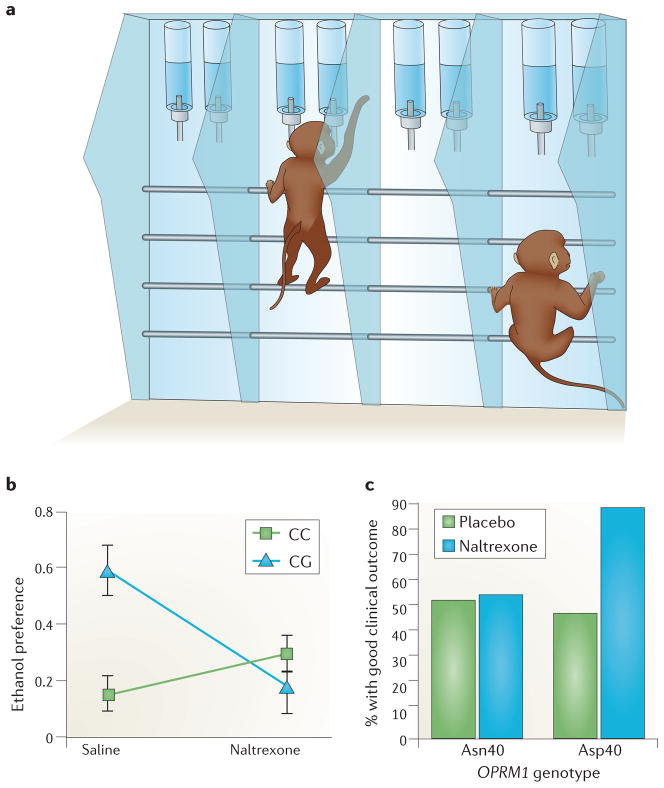
Results from studies indicating that carriers of the minor 77G (rhesus) or 118G (human) alleles of OPRM1 (which encodes the mu-opioid receptor (MOR)) are more sensitive to effects of naltrexone on alcohol preference and consumption than non-carriers. a | Set-up of an alcohol-preference test in monkeys. Each monkey is tagged by a microchip in its collar. Alcohol is made available for 1 hour daily, 5 days a week. During this time, monkeys can walk up, place their head into one of the several ‘bar’ booths, be identified through the chip being read, and choose between an aspartame-sweetened alcohol solution or a solution of aspartame alone. b | Suppression of alcohol preference by naltrexone as a function of OPRM1 genotype. In rhesus 77G carriers (CG), which have a greater baseline alcohol preference, naltrexone suppressed alcohol preference, whereas in rhesus subjects that are homozygous for the more common 77C allele (CC), naltrexone lacked effect. Data from REF. 50. c | Selective increase in ‘good clinical outcome’ after naltrexone treatment compared to placebo in individuals with alcohol addiction carrying the 118G allele (Asp40), and lack of efficacy in subjects who are homozygous for the more common 118A allele (Asn40). ‘Good clinical outcome’ is a dichotomous composite measure of clinical efficacy that includes abstinence or absence of heavy drinking and improvement with regard to negative consequences of drinking. Figure is modified, with permission, from REF. 43 © (2009) American Medical Association.
Interestingly, in monkeys that were homozygous for the major (OPRM1 77C) allele, naltrexone tended to increase alcohol preference, an effect opposite to that observed in the 77G carriers. This pattern parallels that of a human laboratory study in which naltrexone suppressed alcohol self-administration in individuals with a positive family history of alcoholism, but increased it in people without such a family history34. These observations highlight that treatments may need to be personalized not only to achieve therapeutic benefits but perhaps also to avoid worsening outcomes in other patients.
OPRM1 118G: correlation or causation?
Establishing whether the OPRM1 A118G SNP is causal for the functional phenotypes described above is challenging. Because a high degree of linkage disequilibrium is present between numerous SNPs across the OPRM1 locus, their genotypes are highly correlated, and their respective contribution to phenotypic outcomes cannot be easily disentangled in association studies. For instance, one human study found that polymorphisms other than A118G within the same haplotype block were associated with diagnoses of alcohol and drug dependence52. By contrast, a haplotype-based reanalysis of the COMBINE study found naltrexone responses to be specifically attributable to OPRM1 118G53. Furthermore, evidence was recently reported for a functional role of another OPRM1 SNP, rs563649, for pain sensitivity and MOR expression54. This SNP is located in the 5′ untranslated region of the OPRM1 gene, and is strongly associated with the expression of a novel MOR isoform, MOR1K. Although consequences of this variant for alcohol or naltrexone effects have, to our knowledge, not yet been examined, modulation of other opioid-mediated phenotypes by rs563649 suggests that such effects are possible. Because the rs563649 SNP is in strong linkage disequilibrium with other SNPs within the OPRM1 locus, an association between any of those SNPs and clinical naltrexone response could be indirect and be caused by differential expression of the MOR1K isoform. Against this background, combining the non-human primate and human alcohol and naltrexone data reviewed above helps to isolate the influence of OPRM1 77G (in rhesus macaques) and OPRM1 118G (in humans) from that of other functional polymorphisms with which the respective variants might be in linkage disequilibrium. The findings show that the OPRM1 C77G SNP in rhesus macaques and the OPRM1 A118G SNP in humans are directly linked to alcohol reward and the response to naltrexone.
These links are, however, still correlational. Subsequent studies have obtained direct evidence for a causal role of the human 118G variant in alcohol reward using a translational strategy — perhaps more appropriately termed a reverse translational strategy — in humans and genetically modified mice. First, a positron emission tomography (PET) study was carried out to determine whether alcohol-induced dopamine release in the striatum varies as a function of the OPRM1 A118G genotype in humans55. Displacement of the dopamine-D2 receptor ligand [11C]-raclopride was used to determine endogenous dopamine release. In this approach, a high level of displacement — that is, reduction in [11C]-raclopride binding potential — reflects high dopamine release. In response to an alcohol challenge in social drinkers, evidence for alcohol-induced dopamine release in the ventral striatum (which encompasses the human equivalent of the rodent nucleus accumbens (NAc)) was only detected in 118G carriers, whereas in subjects who were homozygous for the more common (major) 118A allele, the data suggested reduced dopamine release following the alcohol challenge55 (FIG. 3).
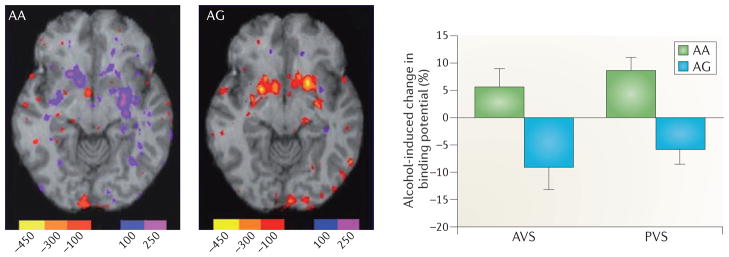
The effect of alcohol on activation of the dopaminergic brain reward circuitry in carriers of the OPRM1 118G allele, as assessed using positron emission tomography (PET) and [11C]-raclopride displacement. Alcohol given to male social drinkers under closely controlled conditions induced a robust dopamine release (detected as reduced binding potential of the radioligand) in minor 118G allele carriers (AG), whereas no measurable release was observed in subjects homozygous for the major 118A allele (AA). The units in the PET scan represent the change in binding potential (nCi ml–1). AVS, anterior ventral striatum; PVS, posterior ventral striatum. Figure is modified, with permission, from REF. 55 © (2011) Macmillan Publishers Ltd. All rights reserved.
Paralleling the human PET study, the consequences of A118G variation for alcohol-induced dopamine-release were investigated in two humanized mouse lines, in which the mouse Oprm1 gene was replaced with the human sequence. These two mouse lines carried two identical copies of the human OPRM1 sequence either with an A (OPRM1 118AA) or a G (OPRM1 118GG) in position 118, but were otherwise identical. Following administration of alcohol, brain microdialysis experiments showed a fourfold higher dopamine release in the NAc of the 118GG line compared to the 118AA line, indicating that the OPRM1 118A→G substitution is sufficient to cause elevated alcohol-induced dopamine release in this area55.
Using a different targeting strategy, the functional role of the human OPRM1 118A→G SNP was independently studied in another pair of mouse lines. In these experiments, a 112A→G mutation was introduced directly into the genetic background of C57/BL6 mice, resulting in an N→D substitution in amino acid position 38 (N38D) of the mouse MOR that is thought to be functionally equivalent to the human N40D substitution56. No alcohol data are to our knowledge yet available from the N38D model, but functional equivalence of the two mouse models is suggested by other observations. In both cases, introduction of the A→G mutation in position 118 or 112 resulted in decreased sensitivity to morphine56,57, a seemingly paradoxical phenotype that is also found in human OPRM1 118G carriers58. As already mentioned, it is currently unclear how the N40D substitution that is encoded by the human OPRM1 118G variant modifies MOR function. The mutation seems to be a loss-of-function mutation in terms of its effects on morphine sensitivity56, but a gain-of-function mutation in terms of its effects on alcohol-induced dopamine release55. The reason for this discrepancy is a most striking issue that awaits resolution. Nevertheless, in both cases, introducing the human MOR variant into mice consistently reproduces the human phenotype — that is, enhanced alcohol-induced dopamine release55 and attenuated sensitivity to morphine58. This suggests that the human OPRM1 118G allele is not only correlated with these effects, but in fact causes them.
The human PET data combine with the microdialysis findings from the humanized mouse lines to form a consistent pattern with regard to the effect of OPRM1 118G on alcohol-induced dopamine release. It seems that 118G carriers activate dopaminergic reward circuitry in response to alcohol, and that this activation is mediated through actions of endogenous opioids. Activation of this cascade offers a target for naltrexone on the basis of the idea that naltrexone can inhibit alcohol-induced dopamine release by blocking the MOR upstream of the dopamine neurons. Conversely, the data indicate that administration of alcohol is largely without influence on dopaminergic reward circuitry in 118A homozygotes, and that there is therefore nothing for naltrexone to block in these subjects.
Importantly, OPRM1 118G carrier frequencies vary across populations of different ancestry, with evidence for recent positive selection. The frequency of 118G (40D) carriers is less than 1 in 10 among African Americans, about 1 in 3 among most white populations, and about 1 in 2 among individuals of Asian descent59. Some observations of population-specific effects suggest that an individual’s genetic background can modify the effects of the OPRM1 A118G variation. Thus, similarly to subjects with a family history of alcoholism60, white OPRM1 118G carriers showed elevated adrenocorticotropic hormone (ACTH) and cortisol responses to a challenge with the injectable naltrexone analogue naloxone compared to white individuals who are homozygous for 118A. By contrast, no such difference was found among individuals of Asian descent61,62. It is unclear whether opioid antagonist effects on ACTH and cortisol responses are mechanistically related to therapeutic efficacy in alcoholism, but they have been shown to be biomarkers of clinical naltrexone response63. The differential effects of OPRM1 A118G genotype on naloxone-induced ACTH and cortisol responses in populations of different ancestry therefore suggests the possibility that variation at this locus may not be equally predictive of clinical naltrexone efficacy in all populations. However, at least one study in patients of Asian ancestry did find that OPRM1 118G carriers took longer to relapse when treated with naltrexone, whereas no such effect was seen in 118A homozygous participants64.
In summary, it seems that the small mean effect size of naltrexone in a mixed patient population is likely to represent a robust effect in the minority of patients who are 118G carriers, and that this effect is diluted by the absence of effects in the remaining patient population43. Expressed differently, a biologically defined population of individuals with alcohol addiction — namely, those individuals who are 118G carriers and therefore have what could be termed ‘endorphin-dependent alcoholism’ (approximately one-third of alcohol-addicted individuals of European ancestry) — stands to robustly benefit from naltrexone, and should receive this treatment. Even before pharmacogenetic tests become widely available in clinical practice, behavioural phenotypes that are characteristic of ‘reward drinking’, such as pronounced psychomotor stimulation by alcohol21, may help to identify patients with a high probability of being responsive to naltrexone. Furthermore, disease progression is likely to be as important to consider as genetic factors in personalized treatments, in that reward drinking is likely to have a greater role in relatively early stages of the addictive process. Patients with the right genetic make-up who, in addition, are in these early stages may therefore be particularly good candidates for naltrexone treatment. Other medications will be needed for individuals with alcohol addiction who are unlikely to respond to naltrexone.
Targeting brain stress systems
Brain stress systems and ‘relief-drinking’
The use of alcohol to alleviate social anxiety at a party illustrates the well-known ability of this drug to suppress negative emotional states, such as anxiety or dysphoria. In a clinical context, this ability sets the scene for negatively reinforced alcohol use, an incentive that is clearly distinct from that which is driven by activation of brain reward circuitry. Recent preclinical evidence has pointed to the potential for a vicious circle — or rather a spiral — in which negative reinforcement drives the progressive escalation of alcohol consumption over time. In this process, withdrawal from an episode of heavy intoxication leads to symptoms of anxiety. With repeated cycles of heavy intoxication and withdrawal, this negative emotional state sensitizes — or increases in strength — ultimately resulting in negative emotionality that persists and provides a powerful incentive for resumption of alcohol intake (relapse)65.
The dynamics of this process closely parallel the ‘opponent process theory of affective regulation’, which was originally proposed more than three decades ago66 and has subsequently been applied to drug addiction67,68. In this conceptualization, drug use initially engages a group of processes that mediate pleasurable emotional states and therefore drive positively reinforced drug seeking and taking. This triggers an activation of opponent processes in the CNS that mediate negative emotional states, such as dysphoria and anxiety, in an attempt to bring emotional homeostasis back to its normal level. With repeated drug use, the opponent processes increase in strength and duration, and ultimately remain activated. This in turn results in an emotional setpoint shift, or ‘allostasis’, such that negative emotionality is experienced in the absence of the drug and drives negatively reinforced drug seeking and use. A progressive increase in the activity of brain systems that mediate behavioural stress responses is a crucial process behind this allostatic setpoint shift. The transition to negatively reinforced drug use is what has become known as “the dark side of addiction”69.
Corticotropin-releasing factor and relief drinking
Extrahypothalamic CRF systems are crucial for the process described above. CRF is a 41 amino acid peptide that is highly expressed within neurons of the hypothalamic paraventricular nucleus (PVN). These neurons release CRF into the portal vein system, which runs along the pituitary stalk and delivers CRF to the anterior pituitary. In the pituitary, CRF acts as the releasing factor for ACTH, which in turn stimulates the release of cortisol from the adrenal glands70. CRF-positive cells are, however, also present in extrahypothalamic structures, including the central nucleus of the amygdala (CeA), the bed nucleus of stria terminalis (BNST) and the brainstem71. These extrahypothalamic CRF cells also release CRF in response to stress and mediate a broad range of behavioural (rather than endocrine) stress responses, primarily through actions at CRF receptor 1 (CRF1; also known as CRFR1 and CRHR1), a seven transmembrane domain Gs-coupled receptor of the secretin receptor family72,73. CRF1 is an attractive therapeutic target because its endogenous ligand CRF is not typically released in extrahypothalamic areas under basal, unstressed conditions. Instead, extrahypothalamic CRF systems become activated in response to sustained, high-intensity, uncontrollable stress74,75, illustrating the principle that neuropeptides are commonly released at high neuronal firing frequencies and act as ‘alarm systems’76. This suggests that CRF1 antagonists may have little if any in vivo activity unless central stress systems are activated, and would therefore have an advantageous safety and tolerability profile. Animal experiments support this notion74,77.
Alcohol withdrawal is a highly stressful state. Rats do not typically self-administer alcohol in amounts that result in physical dependence and in withdrawal symptoms upon cessation of alcohol intake unless they have been genetically selected for high alcohol preference. However, physical alcohol dependence can be induced in rats, for example, by allowing them to consume a liquid alcohol diet or breathe alcohol vapour. Studies in rats made dependent on alcohol in this manner have shown that CRF is released in the CeA during acute alcohol withdrawal and mediates anxiety-like behaviour78–80. Behavioural withdrawal signs in rats peak after about 12 hours, and are gone within 48 to 72 hours into withdrawal. However, if the alcohol-dependent state is maintained for a month or more (before withdrawal), and in particular if the exposure to alcohol is in the form of repeated intoxication and withdrawal cycles, more persistent behavioural consequences of withdrawal are observed. Under these conditions, behavioural stress sensitivity remains upregulated long after acute withdrawal signs have subsided, and this emotional dysregulation is accompanied by escalated voluntary intake of alcohol18,65. At this stage, the increased behavioural stress sensitivity reflects a shift in responsiveness rather than an increase in baseline anxiety. For example, during protracted abstinence from alcohol, rats with a history of dependence showed no increase in baseline anxiety-like behaviour in the elevated plus-maze, a pharmacologically validated animal model of anxiety. However, when these animals were challenged with a stressor before testing, anxiety-like behaviour was markedly accentuated compared to animals without a history of alcohol dependence. This anxiety-like response to stress was blocked by intracerebroventricular administration of the non-selective CRF receptor antagonist D-Phe CRF12–41 (REF. 81). Similar findings of increased behavioural sensitivity to stress in rats with a prolonged history of alcohol dependence have been obtained using several other models82–86. A parallel of these findings in humans is suggested by the observation that brain responses to aversive visual stimuli, as measured by the blood oxygen level-dependent (BOLD) response, were elevated in patients with alcohol addiction during protracted abstinence. Here, the greatest differences were found in a network of cortical structures, presumably reflecting the different nature of the stressor, species differences, or both87. Based on these and other observations, it has been proposed that repeated cycles of intoxication and withdrawal drive a progression to sensitized stress responses and escalation of alcohol intake, and that upregulated expression of Crfr1 (also known as Crhr1), the gene that encodes the CRF1 receptor, has a major role in this process18,82,88.
The CRF system plays a part in relapse. Relapse is a key element of addictive disorders and can be modelled in laboratory animals89. When alcohol self-administration is established in rats and then extinguished over the course of several weeks, relapse to alcohol seeking is reliably triggered by footshock stress, even if there was no prior physical dependence and the animal did not show any withdrawal signs90. Blockade of CRF1 in the brain blocks this stress-induced relapse to alcohol seeking77,91–93, but the exact neurocircuitry that mediates this activity is not clear. Many anti-stress actions of CRF1 antagonists have been mapped to the amygdala, and this structure, along with the BNST, is also implicated in relapse to drug-seeking94. In addition, however, blockade of stress-induced relapse is in part mediated by CRF1 blockade in the median raphe nucleus (MRN)95. Projections from the MRN are largely restricted to midline subcortical structures and do not include the amygdala or the BNST96. This suggests that CRF activity may contribute to stress-induced relapse at multiple sites along the complex neurocircuitry that drives this behaviour94.
In addition to stress, relapse can be triggered by exposure to alcohol-associated cues or alcohol itself (‘priming’)89. CRF1 antagonism does not block cue- or priming-induced relapse to alcohol seeking. Conversely, naltrexone blocks relapse induced by both alcohol-associated cues and alcohol priming, but leaves stress-induced relapse unaffected92,97. This suggests that it may be possible to target these two mechanisms in an additive manner for clinical treatment. CRF1 blockade also blocks relapse to alcohol seeking induced by a pharmacological stressor, the α2-adrenergic antagonist yohimbine98. Furthermore, non-selective CRF receptor antagonists or CRF1-selective antagonists decrease the escalated levels of alcohol self-administration that are observed in rats following a prolonged period (a month or longer) of alcohol dependence, and this decrease is mediated through actions in the amygdala77,99,100. The same effect of CRF1 antagonism is seen when escalation of alcohol self-administration is induced by yohimbine98. Finally, the CRF system may become engaged in earlier stages of the addictive process than previously thought if alcohol consumption occurs in a binge-like pattern101,102.
In summary, work in animal models shows that increased activity of the CRF system is associated with both escalated voluntary alcohol intake and increased sensitivity to stress-induced relapse. It can be speculated that different populations of CRF neurons differentially contribute to these behaviours, with the amygdala driving escalated consumption and the BNST and MRN being involved in relapse. However, the precise contribution of these brain structures to the respective effects remains to be determined. Nevertheless, the findings with CRF1 antagonists together suggest that CRF1 is an attractive treatment target for alcohol addiction.
Genetic variation within the CRF system and alcohol-related behaviours in animal studies
We have so far described findings that establish a role for central CRF signalling in escalated alcohol intake and stress-induced relapse once this system has been sensitized through a prolonged history of exposure to cycles of alcohol intoxication and withdrawal. These findings also predict that an individual with innate or stress-induced upregulation of central CRF activity would be at higher risk for escalated alcohol use and stress-induced relapse than an individual who is genetically protected against such an upregulation. By the same token, it would be expected that individuals with upregulated CRF function would be more responsive to CRF1 antagonism as a therapy for alcohol addiction. Data are beginning to emerge in support of these predictions.
The first indication that genetic variation within the CRF system might moderate alcohol intake and stress-induced relapse came from experiments with genetically selected Marchigian-Sardinian alcohol-preferring (msP) rats93. These rats have an innate behavioural sensitivity to stress and high voluntary alcohol intake in the absence of prior exposure to alcohol. They are thus partial pheno-copies of rats in which the same traits emerge and persist following a prolonged period of alcohol dependence, as described above. A differential gene expression screen revealed that expression of Crfr1 was increased in several brain regions, including the amygdala, of alcohol-naive msP rats compared to alcohol-naive animals of the parental Wistar strain93. The increased expression was accompanied by increased receptor density and signalling, and may be due to a Crfr1 promoter variant that is unique to the msP line. Rats with a history of dependence show similarly increased Crfr1 expression in the amygdala82 (FIG. 4). When msP rats are given free access to alcohol, Crfr1 expression in the amygdala is downregulated to the level of non-dependent, non-selected animals, suggesting the possibility that msP rats drink alcohol to normalize their CRF function103. In addition, both in rats with a history of alcohol dependence and in msP rats, stress-induced relapse is blocked by systemic administration of the prototypical CRF1 antagonist antalarmin in doses that are insufficient to block this behaviour in non-selected animals without a history of dependence93 (FIG. 5).
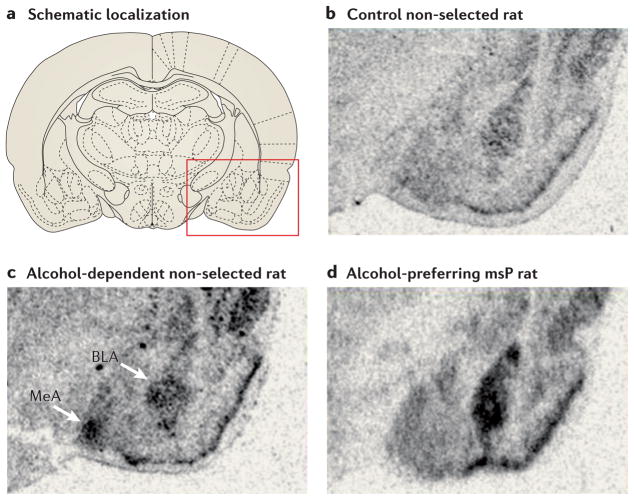
a | Schematic localization on a coronal section of the rat brain. The red box indicates the area that approximately corresponds to the subsequent in situ expression panels. b | Low expression of Crfr1 in a control rat (not genetically selected for high alcohol preference and without a history of alcohol exposure). c | Markedly upregulated Crfr1 expression (darkened areas) in the medial nucleus of the amygdala (MeA) and basolateral nucleus of the amygdala (BLA) of an unselected rat that was exposed for 7 weeks to intoxicating blood alcohol levels, 3 weeks after exposure to alcohol was terminated. d | Similarly upregulated Crfr1 expression in the BLA of a Marchigian-Sardinian alcohol-preferring (msP) rat, observed in the absence of any alcohol exposure. These findings show that increased expression of Crfr1 in the BLA can result from a ‘kindling’ process induced by exposure to cycles of alcohol intake and withdrawal, but it can also be an innate trait that is present in the absence of any alcohol exposure, such as in the msP rat line, which has been genetically selected for high alcohol preference. Part a reproduced, with permission, from REF. 157 © (2005) Elsevier. Parts b and d reproduced, with permission, from REF. 93 © (2006) National Academy of Sciences. Part c reproduced, with permission, from REF. 82 © (2008) Elsevier.
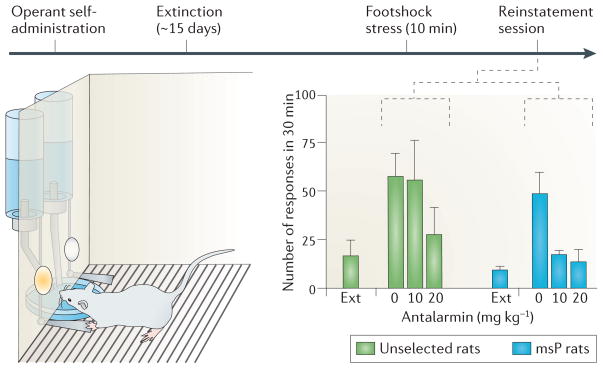
In the stress-induced relapse model, animals are first trained to establish operant self-administration of alcohol. Once stable self-administration rates are achieved, this behaviour is extinguished by removing alcohol as reinforcer, after which lever-pressing rates decline to low levels over the course of about 2 weeks (Ext). Exposure to a stressor — a 10 minute footshock — reinstates response rates on the previously alcohol-reinforced lever, even though alcohol continues to be absent. Antalarmin, a corticotropin-releasing factor receptor 1 (CRF1) antagonist, blocks stress-induced relapse-like behaviour in Marchigian-Sardinian alcohol-preferring (msP) rats at doses that are ineffective in rats that are not selected for high alcohol preference. This shows that the CRF1 receptor is crucial for stress-induced relapse, and that the activity of the CRF system is higher in msP rats compared to non-preferring rats. Figure is reproduced, with permission, from REF. 93 © (2006) National Academy of Sciences.
These data are consistent with subsequent findings in non-human primates. A screen of genetic variation within the CRF (also known as CRH) gene in rhesus macaques identified a CRF –284C→T SNP. This variant renders hypothalamic CRF expression insensitive to end-product feedback inhibition by cortisol acting at glucocorticoid response elements in the CRF promoter. This means that under stress conditions, when cortisol levels are high, CRF levels remain elevated in 284T carriers and continue to drive a hypercortisolemic state. In contrast to CRF expression in the PVN, CRF expression in the amygdala and the BNST is thought to be under positive regulation by cortisol104, and so the unrestrained activity of the hypothalamus–pituitary–adrenal (HPA) axis in 284T carriers would be expected to result in further upregulation of CRF activity in these structures. As a functional consequence of the continuing high levels of CRF, –284T carriers are predicted to have increased alcohol intake (compared to subjects that are homozygous for the more common allele) following sensitization of the stress systems by sustained and uncontrollable stress, but to show normal levels of alcohol consumption otherwise. In agreement with this prediction, alcohol consumption in late adolescence and young adulthood in –284T carriers reared under normal conditions did not differ from that of –284C homozygotes with the same rearing history. By contrast, –284T carriers that had been reared under conditions of high adversity had markedly elevated HPA axis responses to stress and increased voluntary alcohol consumption compared to –284C carriers also reared under high adversity105. Thus, an early life exposure to a sustained stressor seems to set the gain for acute stress responses later in life as a function of CRF –284C/T genotype, and this in turn is linked to the level of voluntary alcohol intake.
Human genetic variation within the CRF system and alcohol-related phenotypes
An association between genetic variation at the human CRFR1 gene locus and alcohol-related phenotypes was first reported in the Mannheim Study of Risk Children (MARC), a cohort enriched for individuals who had been exposed to adversity early in life106. At the time of the last assessment, the subjects in this cohort were still in adolescence. After determining haplotype structure based on 14 markers within the CRFR1 gene, the authors selected haplotype tagging SNPs (htSNPs) — rs1876831 and rs242938 — that tag two separate blocks of CRFR1 and examined their association with drinking phenotypes. Both htSNPs were independently associated with binge drinking and lifetime prevalence of intoxication, indicating that variation affecting either haplotype block could influence these behaviours. No evidence for an interaction of the two markers was found106. In the same study, an association of rs1876831 with high alcohol consumption was found in an independent sample of adult individuals with alcohol addiction. Most importantly, a follow-up analysis from the MARC cohort showed that an interaction between adverse life events and rs1876831 influences alcohol-related phenotypes, with the minor (less common) allele being protective107.
The latter finding was subsequently replicated and extended in a large independent sample108. Together with several other genes, CRFR1 is located in a large haplotype block on chromosome 17 that may have resulted from a local chromosomal inversion. The minor allele of rs1876831 is within the H2 haplotype at this locus. An Australian–American study examined the possible interaction between genotype at this locus and the effects on alcohol-related behaviour of childhood sexual abuse — a type of adversity known to constitute a risk factor for alcohol use disorders109. More than 1,100 participants in the Australian Nicotine Addiction Genetics project were assessed for alcohol dependence, lifetime alcohol consumption and exposure to childhood sexual abuse. A history of childhood sexual abuse was associated with significantly higher lifetime alcohol consumption and increased risk for alcohol dependence108. Furthermore, childhood sexual abuse was found to interact with the rs1876831 genotype both for measures of alcohol consumption and for a diagnosis of alcohol dependence. Specifically, the presence of the H2 haplotype, which is tagged by the minor allele of rs1876831, was protective. In these subjects, childhood sexual abuse exposure was not associated with increase in risk for any of the outcome measures108.
An attractive interpretation of these data is that H2 carrier status is protective because it prevents a functional upregulation of CRF system activity that would be caused by exposure to sustained, uncontrollable stress such as childhood sexual abuse, prolonged heavy alcohol use, or both. In studies that have examined populations of European ancestry, about one-third of subjects are carriers of the minor rs1876831 allele that tags the H2 haplotype. A challenge posed by these findings is that none of the markers within the H2 haplotype examined so far seems to be positioned to change the function of CRF1. For instance, rs1876831 is intronic. In fact, because the extended linkage disequilibrium block at this locus encompasses additional genes, it cannot currently be excluded that genes other than CRFR1 account for or contribute to the observed effects. Nevertheless, the hypothesis that patients with alcohol addiction who are not H2 carriers engage the central CRF system under conditions of stress or heavy alcohol use — and would therefore be predicted to respond to CRF1 antagonist therapy — is an attractive one.
The animal and human genetic data reviewed above suggest the possibility that pharmacogenetic variation will be found in the response to treatments that target the CRF system. This hypothesis has not yet been addressed in clinical trials, but if it is confirmed, it will be crucial to take CRFR1 genotype into account when selecting patients for CRF1 antagonist treatment. Along the way, this will also be important during clinical development of CRF1 antagonists, because demonstrating their efficacy will be difficult if an effect in a genetically defined subpopulation of subjects is diluted by a lack of effect in other study participants110.
The prospects of CRF1 antagonists as therapeutics for alcohol addiction
CRF1 antagonists were originally developed for the treatment of depression and anxiety. For a long time, the discovery of safe, orally available and brain-penetrant CRF1 antagonists proved challenging. The first such molecule — R121919 — given to humans in an open label, uncontrolled trial in patients with depression seemed promising111, but the trial was terminated owing to evidence of treatment-emergent hepatotoxicity. Compounds with better properties have since been developed, but trials that tested these compounds for use in the treatment of anxiety and depression have been disappointing112,113. Failure to take into account the genotype of patients may have contributed to these negative results. As indicated above, the central CRF system is quiescent under non-stressed conditions, and pathological activation of this system may be a feature in some, but not in other cases of depression and anxiety. By contrast, as reviewed above, given sufficient duration of alcohol exposure, the brain CRF system does seem to be consistently activated in animal models. This may make alcohol addiction the most promising indication for CRF1 antagonists.
Functional loci at genes other than CRFR1 have also been implicated in the modulation of stress responses and resilience, and could therefore interact with, or act in parallel with, the CRF system to modulate alcohol-induced plasticity of brain stress systems. Putative stress-modulatory functional polymorphisms have been found in the genes that encode FK506 binding protein 5 (FKBP5)114, neuropeptide Y (NPY)115–117, the serotonin transporter (SLC6A4)118 and catechol-O-methyltransferase (COMT)119. It is outside the scope of this Review to describe in detail the role of genetic factors in stress resilience in general. It is, however, noteworthy that although several lines of evidence exist for a stress-modulatory role of these functional loci, findings in traditional case–control association studies have been controversial — this is perhaps best illustrated by the contradictory data regarding the possible interaction between SLC6A4 variation and stress to produce depression120. As pointed out recently121, this may reflect limitations to approaching hypotheses of gene × environment interactions using association studies alone, and highlights the complementary value of experimental approaches that utilize intermediate phenotypes and animal modelling. This is echoed by the progress made in the case of OPRM1 and CRFR1 using the approaches reviewed above.
CRF1 antagonists may become clinically available for the treatment of alcoholism before pharmacogenetic tests become widely available in clinical practice. In a parallel to what has already been stated for the opioid antagonist naltrexone, careful clinical assessment may go a long way towards identifying patients with phenotypes conveniently grouped under the ‘relief drinking’ label, who might be particularly good candidates for CRF1 antagonist treatment. Much of the literature reviewed by us also suggests that an important role for relief drinking will be particularly likely in later stages of the addictive process. By extension, it can be expected that patients in those stages of the addictive process will be the most likely to benefit from CRF1 antagonist treatment.
Other neurotransmitter systems
With the decreasing cost of genotyping, it will become progressively easier to conduct unbiased genome-wide searches for pharmacogenetic predictors of alcoholism treatment responses. It is, however, important to recognize that this will require stringent statistical thresholds, and this necessitates the collection of very large samples of participants that are consistently recruited, treated and evaluated. In addition, the cost of the clinical studies will remain a challenge. Meanwhile, studies that are designed to target particular genes based on strong biological hypotheses are statistically advisable and potentially fruitful. Two additional neurotransmitter genes that have been implicated in alcohol addiction based on function are 5HT3A (also known as HTR3A), which encodes the ionotropic 5-HT3 receptor for serotonin, and GABRA2, which encodes the α2-subunit of the GABAA receptor. GABRA2 has been implicated on the basis of both its function and previous alcoholism linkage studies. The findings reviewed below are largely exploratory at this point, but are presented to illustrate the general approach and opportunities for translational and experimental medicine for alcohol addiction.
GABAergic transmission and the GABRA2 gene
Among its wide range of CNS effects, alcohol potently influences GABAergic transmission in multiple ways, including modulation of presynaptic GABA release as well as post-synaptic chloride flux. These actions are thought to contribute to some of the subjective effects of alcohol, such as behavioural disinhibition at lower doses and sedation and ataxia at higher doses26. It is therefore perhaps not surprising that the first robust finding of a nervous system-related genetic susceptibility factor in alcoholism was GABRA2, the gene that encodes the α2-subunit of the ionotropic GABAA receptor. Several markers within a haplotype of this gene have in multiple studies been associated with attenuated P300 event-related potentials, an established marker of familial risk for alcoholism. Associations have also been found directly with a diagnosis of alcoholism and with various drinking variables122–127.
Experiments in animal models suggest that the effects of alcohol on GABAergic transmission are in part mediated by neuroactive steroids128. An elegant laboratory study set out to examine whether this translates to the human situation129. This study used finasteride, a 5α-steroid reductase inhibitor that blocks the synthesis of several neuroactive steroids. Pretreatment with high-dose finasteride potently interacted with the participants’ genotype at markers within the GABRA2 gene to moderate subjective alcohol responses such as stimulation, sedation and desire to obtain more alcohol. Specifically, subjects were genotyped for rs279858, a SNP marker informative of the GABRA2 haplotype that had been identified as a susceptibility factor in association studies122–127. Subjects who were homozygous for the major A allele at this locus reported markedly higher psycho-motor stimulant-like effects on the ascending limb of the blood alcohol concentration curve compared to AG or GG subjects, and this was largely blocked by finasteride. By contrast, finasteride had no effect on the psychomotor response to alcohol in AG or GG subjects129. The moderating effects of this GABRA2 haplotype on subjective alcohol effects have been replicated in an independent sample130. Furthermore, a recent imaging genetics study131 showed that the same GABRA2 haplotype moderates insula activity during outcome anticipation in a monetary incentive delay task, an established imaging-based measure of brain reward system activation132,133. Thus, genetic variation in GABRA2 seems to influence alcohol reward.
The molecular mechanism for the interaction between GABRA2 genotype and alcohol effects is not clear, because none of the markers in the susceptibility haplotype of GABRA2 examined so far seems to be functional. For instance, although rs279858 is located within exon 4 of GABRA2, it is synonymous. If functional polymorphisms in significant linkage disequilibrium with rs279858 can be identified, however, an appealing hypothesis emerges. Psychomotor stimulant effects are highly correlated with activation of mesolimbic dopamine transmission, and dopamine neurons originating in the VTA are under tonic GABAergic inhibition. It can therefore be speculated that GABRA2 variation — or variations that alter the function of one of the other GABAA subunit genes found nearby in the gene cluster in which GABRA2 is located — moderates the ability of alcohol to disinhibit these dopamine cells through effects on GABAergic transmission, ultimately resulting in altered alcohol reward and psychomotor effects.
In summary, the work reviewed above provides some additional support for a role of neurosteroids in alcohol responses, a role that has been proposed on the basis of animal studies128. However, these data suggest that if drugs targeting the neurosteroid response to alcohol are developed for therapeutic use in alcoholism, subjects will need to be selected for treatment on the basis of their GABRA2 genotype. In fact, this may also apply more broadly to therapeutics that target dopamine-mediated alcohol reward, as dopamine-mediated alcohol reward is influenced by GABA transmission at VTA synapses.
Serotonergic transmission, serotonin transporter gene variation and ondansetron
The development of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (Prozac; Eli Lilly), has made the role of serotonergic transmission in psychiatric disorders the subject of great interest, not only among scientists but also among the general public. Because SSRIs have been effective in a surprisingly wide range of conditions, they were evaluated as a potential treatment for alcoholism in multiple trials, but overall were not found to be effective134. Another serotonergic medication, however, yielded promising results. Ondansetron, an antagonist of the ionotropic 5-HT3 receptor, was reported to reduce heavy drinking in individuals with early-onset alcoholism, a clinical subtype characterized by the onset of the disorder before the age of 25, and often during the teenage years. Secondary analyses indicated that reductions in craving and improvement of mood disturbances might contribute to the reduction in heavy drinking135–137. The therapeutic actions of 5-HT3-receptor antagonists in people with alcoholism might be due to the presence of 5-HT3 receptors on dopamine terminals in the NAc and their ability to modulate dopamine release138.
The serotonin transporter, which is encoded by SLC6A4, is a key element of serotonergic transmission. A variable-length polymorphism in the promoter region of this gene known as 5-HTTLPR (serotonin-transporter-linked polymorphic region) results in differential transcriptional activity139 and has been extensively studied for association with a wide range of behavioural and clinical phenotypes that are beyond the scope of this Review121. A recent randomized controlled treatment study showed that individuals who are homozygous for the higher expression (LL) allele of the 5-HTTLPR had a better treatment response to the 5-HT3-receptor antagonist ondansetron, measured both as the mean number of drinks per drinking day and as the percentage of days of total abstinence. Combining the analysis of 5-HTTLPR genotype with a SNP located in the 3′ untranslated region of the serotonin transporter transcript, rs1042173, further strengthened this pharmacogenetic effect140. Additional support for the reduction of drinking by ondansetron among alcohol-dependent individuals with the 5-HTTLPR LL genotype comes from a study that was carried out in non-treatment-seeking volunteers, and used alcohol self-administration under laboratory conditions as a measure of outcome141. Although the exact mechanism mediating these effects remains to be determined, 5-HTTLPR is clearly functional in that it regulates transcriptional activity of the serotonin transporter, whereas rs1042173 might be related to micro-RNA-mediated regulation of transcript stability142. Both are therefore well positioned to moderate the effects of a therapeutic acting on serotonergic transmission.
Thus, if ondansetron or other 5-HT3-receptor antagonists are developed for the treatment of alcohol addiction, their efficacy should be tested in patients who have been selected on the basis of their genotype at the SLC6A4 locus.
Conclusions
Addressing the extensive unmet medical needs related to alcohol addiction will require that novel pharmacotherapies be developed. Numerous mechanisms that could potentially be targeted have been discovered by basic addiction neuroscience, but clinical translation remains a challenge19. Developing therapeutics that target these mechanisms will require a considerable investment, at a time when the willingness of the pharmaceutical industry to invest in drug development for behavioural disorders has diminished143. When searching for ‘blockbuster drugs’ has become the dominant strategy, tailored treatments that target subpopulations of patients with addictive disorders are particularly endangered.
We believe that these challenges should prompt some rethinking in industry, academic institutions and government of the approach to the development of medications for addictive disorders. Small, mechanistic, experimental medicine studies that use intermediate phenotypes as surrogate markers of clinical efficacy have the potential to help guide development efforts, and to make these efforts more cost effective. These experimental medicine studies can use insights from preclinical research to guide their selection of subjects and outcome measures, increasing the probability of detecting a drug effect in limited-size studies. For instance, when developing CRF1 antagonists, it might be beneficial to recruit genetically susceptible, anxious individuals with alcohol addiction, and to measure stress-induced alcohol craving. This type of approach can be adapted to a range of diverse mechanisms, in what has been called a ‘Rosetta Stone’ approach144.
We also think that there is reason to rethink the clinical outcomes that are pursued. The search for novel treatments has largely been focused on finding medications that would be effective as measured by their ability to lead to complete abstinence. This is, for instance, the position currently held by the US Food and Drug Administration when evaluating novel addiction therapeutics for approval. However, the science clearly shows that complete abstinence, although desirable, is not the only worthwhile outcome. Even in the absence of complete abstinence, reductions in heavy drinking can have substantial clinical benefits145.
We have largely structured our presentation of the available empirical data by neurobiological system. This is convenient for the purpose of a scientific review, but clinical realities are clearly more complex. The pathophysiology of addiction may engage shifting combinations of mechanisms, not only in different individuals but also in different stages of the disease process. In some stages, reward- and relief-drinking-related mechanisms may combine, calling for combination treatment. At other times, one type of mechanism may dominate. In this sense, optimally personalized treatment will always remain a moving target. This prompts the need for developing clinical assessments — ideally based on the use of biomarkers — that will allow treatment to be tailored on an ongoing basis.
In conclusion, treatments that on average seem to produce only small improvements in ‘alcoholics’ may result in considerable clinical benefits in subpopulations of patients that are better defined with regard to their biology. We predict that genetic variation will emerge as one of the most important categories of biological factors that will need to be considered in this context, but that awareness of disease progression will also be crucial for improving treatments. Rather than ‘finding a cure’, we look forward to the addition of multiple, appropriately targeted novel treatments that will incrementally improve outcomes and help to reduce the devastating consequences of alcohol addiction.
Acknowledgments
The authors want to acknowledge many co-workers in their respective laboratories who over the years have contributed to work reviewed here; C.P.O. particularly wishes to acknowledge contributions by D. Oslin. The laboratories of M.H. and D.G. are supported by the intramural programme of the US National Institute on Alcohol Abuse and Alcoholism. W.H.B. is supported by US National Institutes of Health (NIH) grants P20-DA-025995, R01-DA-025201 and P60-DA 05186. C.P.O. is supported by NIH grants P60-DA-005186-23, 5-P50-DA-012756-11, R01-DA-024553 and R01-AA017164-2.
Glossary
| Disability-adjusted life years | Also known as DALY. A measure of disease burden, expressed as the number of years lost owing to ill-health, disability or early death |
| Phenocopy | An environmentally determined observable trait (phenotype) that mimics one that is genetic in nature. Frequently, the use of intermediate phenotypes can help to distinguish between phenocopies |
| Kindling | Originally, the act of setting something on fire. In neurology, a process by which repeated electrical or chemical stimulation, initially of insufficient intensity to initiate a seizure, ultimately leads to a lowering of the seizure threshold and spontaneous seizures |
| Withdrawal | Sudden and complete cessation of drug taking. The term is also used to denote the syndrome that results when drug is withdrawn after dependence, including tolerance to drug effects, has developed |
| Cohen’s D | A measure of standardized effect size, most commonly used in treatment studies, and defined as the difference between group means divided by the pooled variance. By convention, 0.2, 0.4 and 0.8 or greater are considered to be small, medium and large effect sizes, respectively |
| Pharmacogenetics | The study of inherited variation in the pharmacokinetic or pharmacodynamic effects of drugs. In addictive disorders, the term is used both for the genetic modulation of psychotropic effects produced by the addictive substance and the modulation of therapeutic effects produced by medications used for treatment |
| Non-synonymous | A non-synonymous polymorphism is a coding DNA variation that results in altered amino acid sequence |
| Single nucleotide polymorphism (SNP) | A one-letter exchange of the genetic code, the most common class of genetic polymorphism between individuals |
| Allele | A specific sequence variant encountered at a given position within the genome |
| Polymorphism | A common genetic variation (typically considered to be with a frequency >1.0%) within a species |
| Linkage disequilibrium | The degree with which a certain combination of alleles at different chromosomal locations is encountered together in a population, in excess of what would be expected by chance alone |
| Haplotype block | A block or stretch of DNA that encompasses polymorphisms that are in linkage disequilibrium |
| Haplotype | A combination of alleles at different loci on the same chromosome |
| Isoform | In relation to proteins, isoforms are different forms of a protein that arise from the same gene |
| Reverse translational strategy | Applying findings from humans to model organisms. For example, human genetic variants are inserted into a model organism, allowing their functional role to be studied under better controlled conditions |
| Haplotype tagging | The concept that most of the alleles and haplotypes (allele combinations) in a particular chromosomal region can be captured by genotyping a small number of markers |
| Chromosomal inversion | A chromosome rearrangement in which a segment of a chromosome is reversed from end to end. An inversion occurs when a single chromosome undergoes breakage and rearrangement within itself |
| Intronic | Located in a stretch of DNA between exons; although regulatory elements can reside within introns, genetic variation within introns is often without functional consequences |
| Intermediate phenotypes | A genetically influenced trait that is less complex and more proximal to the genetic information than the actual behavioural trait of interest, and is informative of the more distal complex trait while being possible to measure with less variance |
| Exon | A stretch of DNA that will be represented in the mature, spliced messenger RNA (mRNA) |
| Synonymous | A coding sequence variant that, owing to the redundancy of the genetic code, does not result in an amino acid substitution |
Footnotes
Competing interests statement
C.P.O. declares competing financial interests; see Web version for details. The remaining authors declare no competing financial interests.
FURTHER INFORMATION
Markus Heilig’s homepage: http://www.niaaa.nih.gov/ResearchInformation/IntramuralResearch/AboutDICBR/LCTS/Pages/default.aspx
david Goldman’s homepage: http://www.niaaa.nih.gov/ResearchInformation/IntramuralResearch/AboutDICBR/LNG/Pages/default.aspx
Wade Berrettini’s homepage: http://www.med.upenn.edu/ins/faculty/berrett.htm
Charles P. O’Brien’s homepage: http://www.med.upenn.edu/ins/faculty/obrien.htm
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nrn3110
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3408029?pdf=render
Citations & impact
Impact metrics
Article citations
The Roles of Endogenous D2R Dopamine and μ-opioid Receptors of the Brain in Alcohol use Disorder.
Curr Med Chem, 31(39):6393-6406, 01 Jan 2024
Cited by: 0 articles | PMID: 37921171
Review
<i>A paradoxical switch</i>: the implications of excitatory GABAergic signaling in neurological disorders.
Front Psychiatry, 14:1296527, 10 Jan 2024
Cited by: 1 article | PMID: 38268565
Review
Candidate gene-environment interactions in substance abuse: A systematic review.
PLoS One, 18(10):e0287446, 31 Oct 2023
Cited by: 1 article | PMID: 37906564 | PMCID: PMC10617739
Review Free full text in Europe PMC
Social Media as Pharmacovigilance: The Potential for Patient Reports to Inform Clinical Research on Glucagon-Like Peptide 1 (GLP-1) Receptor Agonists for Substance Use Disorders.
J Stud Alcohol Drugs, 85(1):5-11, 30 Oct 2023
Cited by: 2 articles | PMID: 37917019
A Review of the Characteristics of Clinical Trials and Potential Medications for Alcohol Dependence: Data Analysis from ClinicalTrials.gov.
Medicina (Kaunas), 59(6):1101, 07 Jun 2023
Cited by: 0 articles | PMID: 37374305 | PMCID: PMC10300859
Review Free full text in Europe PMC
Go to all (145) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (Showing 6 of 6)
- (3 citations) dbSNP - rs1876831
- (2 citations) dbSNP - rs279858
- (2 citations) dbSNP - rs563649
- (1 citation) dbSNP - rs242938
- (1 citation) dbSNP - rs1042173
- (1 citation) dbSNP - rs1799971
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Corticotropin-releasing factor: innocent until proven guilty.
Nat Rev Neurosci, 13(1):70; author reply 70, 20 Dec 2011
Cited by: 5 articles | PMID: 22183439 | PMCID: PMC3365568
Subjective response as a consideration in the pharmacogenetics of alcoholism treatment.
Pharmacogenomics, 16(7):721-736, 07 May 2015
Cited by: 10 articles | PMID: 25950242
Review
The role of opioidergic genes in the treatment outcome of drug addiction pharmacotherapy: A systematic review.
Am J Addict, 24(1):15-23, 01 Jan 2015
Cited by: 19 articles | PMID: 25823631
Review
Personalized Medicine of Alcohol Addiction: Pharmacogenomics and Beyond.
Curr Pharm Biotechnol, 18(3):221-230, 01 Jan 2017
Cited by: 5 articles | PMID: 28240173
Review
Funding
Funders who supported this work.
Intramural NIH HHS (3)
Grant ID: Z01 AA000301-10
Grant ID: ZIA AA000301-11
Grant ID: Z01 AA000301
NIAAA NIH HHS (2)
Grant ID: R01 AA017164
Grant ID: R01-AA017164-2
NIDA NIH HHS (11)
Grant ID: P20 DA025995
Grant ID: P20-DA-025995
Grant ID: R01-DA-024553
Grant ID: R01-DA-025201
Grant ID: P50 DA012756
Grant ID: P60 DA005186
Grant ID: R01 DA025201
Grant ID: R01 DA024553
Grant ID: 5-P50-DA- 012756-11
Grant ID: P60-DA 05186
Grant ID: P60-DA-005186-23