Abstract
Free full text

The abscopal effect associated with a systemic anti-melanoma immune response
Summary
We report a case of metastatic melanoma treated with palliative radiotherapy to the primary tumor. The patient also experienced regression of non-irradiated lesions, demonstrating the abscopal effect. Importantly, serology showed anti-MAGEA3 antibodies, documenting an association between the abscopal effect and a systemic anti-tumor immune response. Whereas the literature suggests immune activation after tumor irradiation, this case documents an anti-tumor response seen in direct association with abscopal clearance. Implications for radiation in melanoma immunotherapy are discussed.
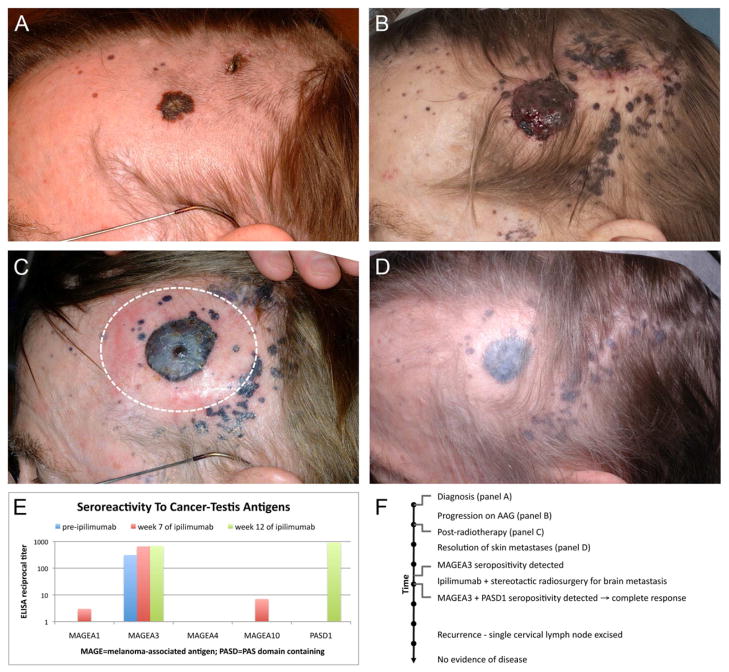
A 67-year-old man presented with pigmented lesions on the head and neck (Fig 1A). Biopsies revealed a 2.2 mm thick stage IIIC malignant melanoma, with multiple satellite metastases. PET scan showed no additional metastatic disease. Despite chemotherapy the primary lesion progressed (Fig 1B). The patient then underwent localized radiation to the primary tumor with a 2 centimeter margin (dashed line, Fig 1C) using 6 MeV electrons at 2,400 cGy delivered in 3 fractions. Six weeks after radiation there was flattening of the primary tumor but no change in the untreated metastases on the forehead, scalp, or neck (Fig 1C). However, 8 months after radiotherapy, all in-transit metastases had resolved (Fig 1D). Dermal pigmentation remained at the site of prior tumors, but no clinical evidence of residual tumor mass. The patient remained free of recurrent skin disease, however after 36 months he developed nodal and brain metastases. He was treated with intracranial stereotactic radiosurgery (SRS) and immunotherapy with ipilimumab. Pre-ipilimumab serology detected autoantibodies against melanoma antigen A3 (MAGEA3) at 1:300 by ELISA (Fig 1E), demonstrating an existing systemic anti-tumor immune response. After SRS and ipilimumab, the patient’s MAGEA3 titer rose to 1:700 and he mounted a new response to the cancer antigen PAS domain containing 1 (PASD1) while achieving a complete remission. Aside from a recurrence in a cervical lymph node that was surgically removed, the patient has remained disease-free and is alive 7 years after cutaneous radiation (Fig 1F).
This patient received palliative radiation to his primary melanoma, yet there was a delayed but robust response in all untreated cutaneous metastases. This type of response in distant tumors after local radiotherapy is known as the abscopal effect.1 The abscopal effect has been reported in several malignancies, including one prior case of malignant melanoma.2 It is hypothesized that irradiating the tumor induces antigen release and cytokine production, which mediate a systemic antitumor immune response. However, little evidence has been reported demonstrating immune activation in association with the phenomenon. One hepatocellular carcinoma patient experienced increased serum TNFα following radiotherapy of bone metastasis along with regression of non-irradiated tumor.3 Although this signifies immune activation after irradiation, it falls short of demonstrating a tumor-specific immune response.
In the present case, the abscopal effect was associated with post-radiation anti-melanoma antibodies, suggesting that irradiation induced or potentiated a systemic antitumor immune response. This finding is consistent with mouse melanoma studies where localized radiation augmented antitumor immune effector cells.4 In other mouse studies, CD8+ T cells were required for reduction of melanoma burden following radiotherapy, and immunotherapy enhanced the response.5 Moreover, adding Flt3-ligand immunotherapy to radiation induced the abscopal effect in non-irradiated mouse mammary tumors.6 Together these observations support the immune hypothesis for the abscopal effect.
Further evidence that radiation can induce a systemic anti-melanocyte immune response is that vitiligo can arise in non-irradiated skin after treating melanoma with radiation.7 Like the abscopal effect, radiation likely induces an immune response to tumor melanocytes leading to loss of normal melanocytes at distant sites.7, 8
Immunotherapy is a mainstay in treating melanoma. Three FDA-approved therapies for melanoma target the immune system — interferon-alpha-2b, interleukin-2, and ipilimumab. In addition, vaccines9 and imiquimod10 are used for melanoma immunotherapy. Synergy between radiation and the immune system is seen in mouse models,4–6 and now in a patient with melanoma. If radiation effectively primes immune responses, then combining radiotherapy with immunotherapy should be beneficial by inducing an abscopal-like anti-melanoma response. The long-term remission achieved with SRS plus ipilimumab for melanoma recurrence in the above patient further supports this concept. Ongoing trials combining radiotherapy with immunotherapy should advance our understanding of this promising treatment approach.
Acknowledgments
The authors thank Ms. Erika Ritter for technical support and Ms. Daphne Demas for medical photography.
Footnotes
Conflicts of Interest Notification: JDW has been a consultant to and received grants from Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ijrobp.2012.03.017
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3415596?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ijrobp.2012.03.017
Article citations
Enhanced systemic tumor suppression by in situ vaccine combining radiation and OX40 agonist with CpG therapy.
J Transl Med, 21(1):619, 12 Sep 2023
Cited by: 0 articles | PMID: 37700338 | PMCID: PMC10498626
Peptide-based vaccine for cancer therapies.
Front Immunol, 14:1210044, 16 Aug 2023
Cited by: 12 articles | PMID: 37654484 | PMCID: PMC10467431
Review Free full text in Europe PMC
Therapeutic Efficacy, Radiotoxicity and Abscopal Effect of BNCT at the RA-3 Nuclear Reactor Employing Oligo-Fucoidan and Glutamine as Adjuvants in an Ectopic Colon Cancer Model in Rats.
Life (Basel), 13(7):1538, 11 Jul 2023
Cited by: 1 article | PMID: 37511913 | PMCID: PMC10381875
Successful targeting in situ of an oncogenic nuclear antigen by hapten induced tumor associated autoantibodies (iTAA).
Sci Rep, 13(1):9902, 19 Jun 2023
Cited by: 0 articles | PMID: 37336938 | PMCID: PMC10279707
Awaken Immune Cells by Hapten Enhanced Intratumoral Chemotherapy with Penicillin Prolong Pancreatic Cancer Survival.
J Cancer, 14(8):1282-1292, 08 May 2023
Cited by: 3 articles | PMID: 37283801 | PMCID: PMC10240667
Go to all (231) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Abscopal Effect in the Era of Checkpoint Inhibitors.
Int J Mol Sci, 22(13):7204, 04 Jul 2021
Cited by: 13 articles | PMID: 34281259 | PMCID: PMC8267720
Review Free full text in Europe PMC
Immunologic correlates of the abscopal effect in a patient with melanoma.
N Engl J Med, 366(10):925-931, 01 Mar 2012
Cited by: 1234 articles | PMID: 22397654 | PMCID: PMC3345206
Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies.
Eur J Cancer, 48(16):3045-3051, 22 Jun 2012
Cited by: 32 articles | PMID: 22727601
Role of radiation therapy as immune activator in the era of modern immunotherapy for metastatic malignant melanoma.
Am J Clin Oncol, 38(1):119-125, 01 Feb 2015
Cited by: 27 articles | PMID: 23648438
Review
Funding
Funders who supported this work.
Intramural NIH HHS (1)
Grant ID: Z99 CA999999
NCI NIH HHS (2)
Grant ID: Y99 CA999999
Grant ID: P30 CA008748