Abstract
Free full text

Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion
Associated Data
SUMMARY
Memory CD8+ T cells induced upon immunization exhibit improved functional features that contribute to protection of immunized hosts. Although both cognate antigen recognition and inflammation are important for memory CD8+ T cell reactivation, the relative contribution of these factors and the cell types providing these signals in vivo are poorly defined. Here, we show that Ly6C+CCR2+ inflammatory monocytes, a subset of monocytes, largely orchestrate memory CD8+ T and NK lymphocytes activation by differentiating into interleukin-18 (IL-18)- and IL-15-producing cells in an inflammasome and type I interferon-IRF3-dependent manner. Memory CD8+ T cells became potent effector cells by sensing inflammation from monocytes independently of their cognate antigen. Like NK cells, they underwent rapid mobilization, upregulated intense and sustained effector functions during bacterial, viral and parasitic infections, and contributed to innate responses and protection in vivo. Thus, inflammatory monocyte-derived IL-18 and IL-15 are critical to initiate memory CD8+ T and NK lymphocytes differentiation into antimicrobial effector cells.
INTRODUCTION
Understanding the differentiation of memory T cells is key to the rational design of effective and innovative strategies for developing better vaccines. Induction of long-lived memory CD8+ T cells that can mediate protective immune responses against microbial infections is largely programmed during the initial priming and requires three distinct signals, namely cognate antigen, costimulation and inflammation. While understanding the fine mechanisms of these processes is paramount to the design of better vaccination strategies, the cues that govern memory cell reactivation are poorly defined. Both cognate antigen recognition and inflammation were demonstrated to promote the activation of pathogen-specific memory CD8+ T cells. Earlier reports focusing on memory CD8+ T cells either in non-immunized or immunized hosts, documented that, similarly to natural killer (NK) cells (Chaix et al., 2008; Lucas et al., 2007; Nguyen et al., 2002), memory CD8+ T cells can respond to distinct inflammatory cytokines (Berg et al., 2003; Kambayashi et al., 2003; Kohlmeier et al., 2010; Liu et al., 2002; Yajima et al., 2005; Zhang et al., 1998). Upon incubation with a combination of interleukin (IL)-12 and IL- 18, memory CD8+ T cells can produce interferon gamma (IFN-γ) (Berg et al., 2003; Kambayashi et al., 2003); IL-15 is able to trigger their activation by mimicking T cell receptor (TCR) cross-linking (Liu et al., 2002; Yajima et al., 2005; Zhang et al., 1998) and type I IFN (IFN-I) directly enhances cytolytic activity of virus-specific memory CD8+ T cells (Kohlmeier et al., 2010). So far however, it is unclear whether, in comparison to cognate antigen-driven reactivation, such interleukin-driven mechanisms are critical and represent an important component of vaccine-induced memory CD8+ T cell reactivation in vivo. In fact, a recent report supported a strong role for inflammation in optimal reactivation of immunization-induced memory CD8+ T cells in vivo (Wirth et al., 2011).
Adding to this body of literature, it was also suggested that dendritic cells (DCs) play a key role in promoting robust memory CD8+ T cell proliferation during a recall infection (Zammit et al., 2005). However, the precise identity of the relevant cell(s) and the mechanisms through which they act, e.g., promoting inflammation and of which nature and/or presenting T cell cognate antigens, still remained unclear. Other myeloid-derived cells like monocytes, macrophages and neutrophils express Major Histocompatibility Complex (MHC) class I molecules and are also equipped with sets of pattern recognition receptors which efficiently sense pathogens-derived molecules, leading to the secretion of multiple inflammatory mediators (Iwasaki and Medzhitov, 2010). Therefore, establishing the contribution of antigen-dependent and independent signals to memory CD8+ T cell activation and the cell types orchestrating this process is a still unanswered question of considerable interest to unravel memory CD8+ T cell function and protective efficacy in vivo.
Using mice immunized with the intracellular bacterium Listeria monocytogenes (Lm), which develop long-lived protective CD8+ T cell memory, we had established that control of Lm growth during recall infection occurs within a few hours (Narni-Mancinelli et al., 2011). Consistent with others (Berg et al., 2003; Iwai et al., 2008; Kambayashi et al., 2003), we found that vaccine (Lm)-induced memory CD8+ T cells produced IFN-γ independently of cognate antigen recognition (Bajenoff et al., 2010), suggesting that initial activation of memory CD8+ T cells is regulated by other mechanisms. In the current study, we took advantage of the Lm immunization model to investigate the mechanisms of memory CD8+ T cell activation in vivo. We establish that pathogen-specific memory CD8+ T cells undergo initial reactivation inside tissues by expressing strong effector functions and activation markers without cognate antigen recognition requirements. Most importantly, we found that reactivation is mostly orchestrated by a subset of myeloid cells, the Ly6C+CCR2+ inflammatory monocytes, which provide inflammatory signals (IL-18, IL-15) to memory cells upon triggering of “danger” pathways (IFN-I, inflammasome) by molecules derived from several classes of microbial pathogens. These findings describe a unique role for inflammatory monocytes and provide a thorough understanding of the mechanisms that control the reactivation of memory CD8+ T cells induced upon immunizations.
RESULTS
Cognate antigen recognition is not required for early activation of memory CD8+ T cells in vivo
We first investigated whether antigen is required for the early activation of memory CD8+ T cells induced after a strong immunization. We grafted wild-type (WT) C57BL/6 (B6) mice with physiological numbers of naïve CD8+ T cells expressing (i) the monoclonal T cell receptor (TCR) OT-I specific for the model antigen Ovalbumin (Ova) and (ii) a traceable fluorescent protein (Green Fluorescent Protein (GFP) or Tomato). We subsequently immunized recipient animals with WT Lm-expressing Ova (Lm-Ova), which induced OT-I cells to differentiate into memory cells. Five weeks after primary immunization (and up to 3 months, not shown), mice were challenged with WT Lm either expressing or not the Ova antigen. The functional and cell-surface phenotypes of splenic OT-I memory cells were then analyzed ex vivo without further restimulation (Figure 1A–C). Control mice received naïve OT-I GFP+ cells. As early as 8 hours (hrs) post-infection and sustained over 48 hrs, OT-I memory cells, but not naïve, expressed major effector molecules such as IFN-γ, granzyme B (GrB) and perforin (Figure 1A–B). Concomitant upregulation of cell-surface markers reflecting T cell activation and trafficking (CD69, CCR5), adhesion and costimulatory molecules (CD11a, NKG2D) and effector differentiation (CD25 (Kalia et al., 2010; Pipkin et al., 2010)) were also observed (Figure 1B and not shown). Increased expression of T-bet and Eomesodermin, two major transcriptional master regulators of T cell differentiation into IFN-γ and cytolytic effectors (Intlekofer et al., 2005), confirmed that memory cells from challenged mice underwent a strong program of differentiation (Figure 1C). The kinetics and extent of modulation of all functional markers -in terms of proportion, absolute numbers (not shown) of memory cells and amounts of expression (Mean Fluorescence Intensity, MFI)- were equivalent whether cognate antigen was present or not, and even upon challenge with low numbers of bacteria (Figure S1A–C). Nevertheless, antigen was required for T cell-proliferation (Figure S1D). Of note, naïve OT-I cells showed no signs of activation without antigen (Figure 1B–C), implying that these functional characteristics were intrinsic to memory cells.

To generate memory CD8+ T cells, wild-type (WT) C57BL/6 (B6) mice adoptively transferred with 500 OT-I GFP+ cells (A–C) or BALB/c (D) mice were then immunized with WT Lm-Ova or WT Lm (BALB/c) and challenged 6 weeks (wks) later with WT Lm-Ova (blue), Lm (black), Lm-LLOser92 (D, dark blue) or left unchallenged (grey); labeled as “memory”. For “naïve” CD8+ T cells, 3×106 naïve OT-I GFP+ cells were transferred into WT B6 mice. At indicated times (A) or 8 hrs later (B–D), spleens cell suspensions incubated with Golgi Plug were stained for cell surface or functional intracellular markers as specified on the graphs. In (D), endogenous memory CD8+ T cells were tracked using LLO91–99-H-2Kd tetramers (tet). Data show expression of these markers after gating on memory (OT-I, endogenous) CD8+ T cells, with the relative proportion (%) and expression levels (Mean Fluorescence intensity, MFI) of memory cells. Representative flow cytometry histograms are shown. Data pool 3–4 independent replicate experiments (n=8–10 mice) with p-values.
To extend these results to endogenous non-TCR transgenic memory CD8+ T cells, WT mice were immunized with WT Lm and challenged with WT Lm or Lm lacking the dominant LLO91–99 epitope (Lm-LLOSer92), therefore providing equivalent inflammatory signals with or without the cognate epitope (Figure 1D). Similar to OT-I memory cells, endogenous LLO91–99-H-2Kd (tetramer+)-specific memory cells secreted IFN-γ, expressed GrB and the other activation markers (not shown) to equivalent extent, ruling out that these features were only attributes of OT-I memory cells. Therefore, memory CD8+ T cells induced upon strong pathogen immunization rapidly express a robust activation program that is comparable with or without T cell cognate antigen, indicating that antigen is not required for their initial activation in vivo.
Memory CD8+ T cells participate in innate immune responses
We next hypothesized that Lm-induced memory CD8+ T cells contribute to innate immune responses in immunized mice. We thus compared the activation of OT-I or endogenous LLO91–99-H2-Kd memory CD8+ T cells to that of innate NK lymphocytes in mice that received primary immunization with WT Lm and were challenged with Lm lacking the expression of either Ova257–264 or LLO91–99 cognate epitopes (Figures 2A and S2A). While NK cells rapidly expressed IFN-γ and GrB, the proportion of memory CD8+ T cells expressing these functions was markedly higher than NK cells (factor of 1.5–2). Because antigen was not required, we tested whether memory CD8+ T cells also expressed these effector functions in mice challenged with other non-OVA expressing microbial pathogens, including the murine Cytomegalovirus (MCMV), the extracellular bacterium Streptococcus pneumoniae (S. pneumoniae) and the murine parasite Plasmodium berghei (P. berghei). Mice (B6 and B6-Kd) respectively grafted with naïve OT-I or WP20 T cells (bearing another monoclonal TCR recognizing Lm-derived p60449–457 presented by H-2Kd) were immunized with WT Lm-Ova and challenged with each of the pathogens (Figures 2B–C and S2B–C). In all infections, and in WT BALB/c mice, the proportion and numbers of activated (IFN-γ+, GrB+) memory CD8+ T cells was either equivalent or higher than that of NK cells. Furthermore, activated memory CD8+ T cells generally expressed higher amounts (MFI) of these functions compared to activated NK cells (up to a factor of 2). The proportion of IFN-γ+ cells in infected organs, e.g., spleen and liver (MCMV, P. berghei) or lung (S. pneumoniae), of unimmunized versus Lm-immunized mice also showed that CD8+ T cells account for at least 37% of IFN-γ+ cells (Figures 2D–E; S2B–C and not shown). Thus in immunized animals only, CD8+ T cells which include Lm-specific memory cells, participate in IFN-γ+ innate immune responses similarly to NK cells. The transfer of purified OT-I memory cells into naïve recipient animals that were subsequently challenged with non-Ova expressing WT Lm conferred modest (factor of 3–5) but significant bacteriocidal immunity (Figure 2F).
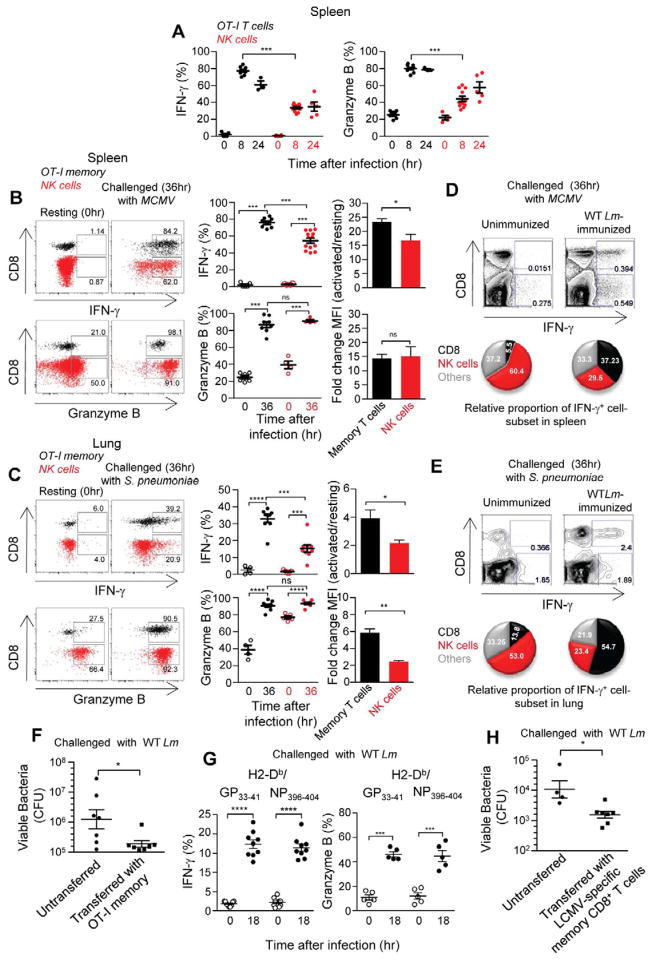
WT B6 mice transferred with OT-I GFP+ cells were immunized with WT Lm-Ova (A–F) or left unimmunized (D, E). Animals were challenged 6 wks later with WT Lm (A, F), MCMV (B, D) or Streptococcus pneumoniae (C, E). In (G, H) mice were immunized with the lymphochorionmeningitis virus (LCMV). Sixty days later, animals were challenged with WT Lm (G) or LCMV-specific H2-Db/GP33–41 and H2-Db-NP396–404 memory CD8+ T cells were purified and transferred into naïve B6 mice subsequently challenged with WT Lm (H). Spleens (A, B, D, G) and lungs (C, E) were harvested at indicated times, incubated with Golgi Plug and stained for CD8, CD3, LCMV tetramers and NKp46 (NK cell) cell surface markers and intracellular IFN-γ and GrB. In (A–C, G), data show the relative proportion (%) and fold increase in MFI (activated to resting cells) of IFN-γ+ or GrB+ cells amongst OT-I (black), NK (red) cells, and LCMV tet+ cells after gating on each cell type. Representative dot-plots and compilation of 2–3 independent replicate experiments with each dot featuring one individual mouse are shown (n=4–13). In (D, E) are shown representative dot-plots of % IFN-γ+ cells in unimmunized versus WT Lm-immunized mice challenged with either the murine Cytomegalovirus, MCMV (spleen) or S. pneumoniae (lung). The relative proportion of NK, CD8+ T and other cells amongst IFN-γ+ cells is shown in the pie-charts with p-values. In (F, H), flow-purified OT-I memory (~2×104) (F) or LCMV-specific (~105) (H) cells were transferred or not into naïve WT mice subsequently challenged with WT Lm (F:5×104, H:2,000). CFUs in spleens were determined by plating 48 hrs later. Data pool 2–4 independent replicate experiments (n=4–8) with p-values.
Finally, to generalize our findings to memory CD8+ T cells induced by other immunizations, we infected mice with a distinct microbial pathogen, the lymphochoriomeningitis virus (LCMV Armstrong), and challenged them with WT Lm 60 days later (Figure 2G and S2D). Similarly to Lm-specific memory CD8+ T cells, endogenous LCMV-specific memory CD8+ T cells recognizing 2 distinct epitopes derived from the LCMV glycoprotein (GP33–41) and nucleoprotein (NP396–404) produced IFN-γ (~17%) and GrB (~45%) and upregulated activation markers (CD69, CD25, not shown), therefore extending these results to memory CD8+ T cells induced by acute viral infection. It is also noticeable that the proportion of memory and NK cells undergoing IFN-γ and GrB expression was equivalent (Figure S2D). Importantly and in line with these observations, naïve recipient mice adoptively transferred with LCMV-specific memory CD8+ T cells and subsequently challenged with WT Lm exhibited lower bacterial burden (factor of ~3) as compared to untransferred mice (Figure 2H). Thus pathogen-induced memory CD8+ T cells can participate to innate immunity and immunological protection.
Triggering of inflammasome and IFN-I pathways promote memory CD8+ T cell differentiation into effectors
We next investigated the molecular mechanisms regulating the activation of memory CD8+ T cells. Since activation was observed across infections with various microbial pathogens, we hypothesized that common cytokine signals are likely to control these processes. In line with others studies (Berg et al., 2003; Kambayashi et al., 2003; Kohlmeier et al., 2010; Liu et al., 2002; Yajima et al., 2005; Zhang et al., 1998), addition of combinations of IL-12 and IL-18, IFN-I or IL-15, induced the activation of memory CD8+ T cells in vitro with no TCR cross-linking requirement (Figure 3A). Incubation of antigen-specific memory CD8+ T cells (OT-I and endogenous) with IL-12 and IL-18 induced their secretion of IFN-γ, while incubation with IL-15 or IFN-I led to their expression of GrB, consistent with the idea that a combination of these cytokines is required for IFN-γ and GrB expression in vivo. Secretion of these mediators involves non-overlapping “danger” pathways; production of bioactive IL-18 from its pro-IL-18 precursor requires inflammasome-caspase-1 proteolytic cleavage (Kuida et al., 1995), while the trans-presentation of IL-15 (Burkett et al., 2004) upon IFN-I stimulation requires IRF-3 and IRF-7 transcription factors (Beuneu et al., 2011; Lucas et al., 2007). To investigate which danger pathways control memory CD8+ T cell activation in vivo, we adoptively transferred memory CD8+ T cells into WT chimera mice reconstituted with either Casp1−/− or Irf3−/− bone-marrow cells or into Il15−/− mice and monitored their activation after WT Lm infection (Figure 3B and S3). A significantly lower proportion of OT-I memory cells expressed IFN-γ in Casp1−/−, Irf3−/− and Il15−/− recipient mice compared to WT animals, demonstrating that the inflammasome pathway and IL-15 contributed to IFN-γ production by memory CD8+ T cells in vivo. As importantly, upregulation of GrB expression was abrogated in the Irf3−/− chimera and Il15−/− recipient mice compared to WT mice. The proportion of GrB+ memory cells in both Irf3−/− and Il15−/− mice was comparable to unchallenged controls, suggesting that IL-15 regulates the expression of early cytolytic effector functions in memory cells, possibly in an IRF3-dependent manner. Altogether, this suggested that memory CD8+ T cells express IFN-γ and cytolytic effector functions in vivo through inflammasome- and IRF-3-pathways independently of cognate antigen recognition.
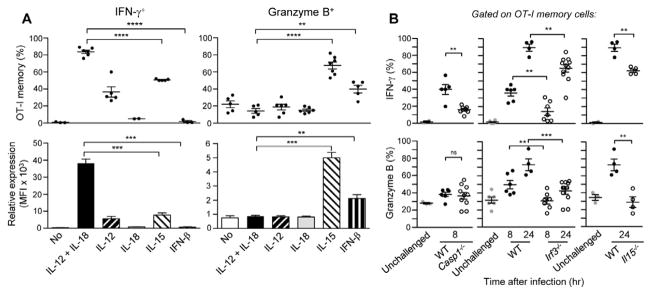
CD8+ T cells were purified from WT B6 mice grafted with OT-I GFP+ cells and immunized with WT Lm-Ova 6 wks prior. (A) CD8+ T cells were cultured with indicated recombinant cytokines. The proportion (%) of OT-I GFP+ memory cells expressing IFN-γ/GrB and the fold increase in MFI (activated/resting) are shown. Each dot represents OT-I memory CD8+ T cells from a different donor mouse and data pool 3 independent replicate experiments (n=4-7). (B) Il15−/− or irradiated WT bone-marrow chimera of Casp1−/− or Irf3−/− mice were challenged or not (grey) with WT Lm and transferred with purified CD8+ T cells either into WT (closed) or knockout (open) mice. At 8 or 24 hrs, OT-I memory cells from spleens were analyzed for expression of IFN-γ and GrB. Data are pool of 3 independent replicate experiments (n=4-11) with p-values. Error bars on graphs represent Mean+/−SEM.
Inflammatory monocytes provide IL-18 and IL-15 activating cytokines to memory CD8+ T cells
We next sought to define the cells providing these cytokines in vivo. Sentinel cells of the immune system such as DCs, macrophages, neutrophils and Ly6C+ monocytes, a subset of monocytes involved in antimicrobial host defenses (Shi and Pamer, 2011), were potential candidates. By flow-cell sorting, we purified conventional DCs (cDCs), neutrophils and Ly6C+ monocytes from mice infected with WT Lm and incubated them with resting memory CD8+ T cells in vitro (Figures 4A and S4A). A significant proportion of memory cells secreted IFN-γ (30–40%) in presence of inflammatory monocytes while weaker (<10%) activation was measured with activated cDCs or neutrophils. A similar trend was also observed for expression of GrB. We further analyzed whether these cells could provide IL-15 and IL-18 activating cytokines to the memory cells. Since direct measure of IL-18 or of active caspase-1 inside ex-vivo isolated innate cells was below level of detection, we performed the same experiment as in Figure 4A, except that memory CD8+ T cells were either coated or not with an anti-IL-18 receptor (IL-18R) blocking serum prior to incubation with the distinct subsets of in vivo activated myeloid cells (Figure 4B and not shown). Whereas expression of GrB on OT-I memory cells remained unchanged in all groups, secretion of IFN-γ, which was only measurable in presence of activated Ly6C+ monocytes (Figure 4A), was inhibited by ~80%. Thus Ly6C+ monocytes most likely promoted IFN-γ-secretion from the memory cells through inflammasome-mediated release of IL-18. Next, we monitored the cell-surface expression of IL-15Rα by various myeloid splenic cell subsets that could trans-present bioactive IL-15 (Sandau et al., 2004). Ly6C+ monocytes (CD3−B220−NK1.1−CD11bhi, Figure S4A) upregulated this receptor at far superior amounts than cDCs, macrophages or neutrophils (Figure 4C and S4B). Strong upregulation of IL-15Rα was true not only in mice infected with Lm, but also with MCMV, S. pneumoniae, P. berghei and upon poly(I:C) injection (Figure 4D). Therefore, in all organs targeted by the different microbes and in which Ly6C+ monocytes are rapidly recruited (Shi and Pamer, 2011), these cells exhibited a functional advantage in expressing high cell-surface bioactive IL-15Rα-IL-15 complexes over other myeloid cell types.
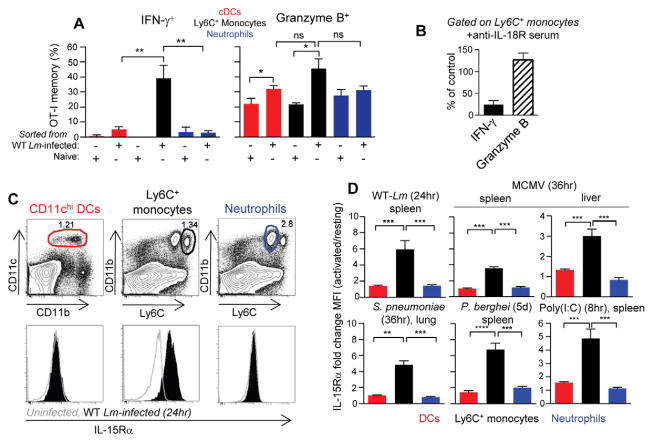
(A–B) CD8+ T cells, cDCs, neutrophils or Ly6C+ monocytes were purified by flow-cell sorting respectively from WT B6 mice that (i) contained OT-I GFP+ memory cells or (ii) were naive or immunized with WT Lm 20 hrs earlier. Purified CD8+ T cells were incubated with each innate cell subset before staining for cell surface expression of CD8 and intracellular IFN-γ and GrB. The proportion (%) of OT-I GFP+ memory cells expressing either marker is shown. (B) CD8+ T cells were incubated with anti-IL-18R prior to adding activated Ly6C+ monocytes. (A–B) represent 3 independent replicate experiments (n=3–6) with p-values. (C) Spleen cells from WT B6 mice immunized with WT Lm (with 105 or 106 Lm) 24 hrs prior were stained with mAbs against cell surface markers discriminating conventional dendritic cells (cDC, CD11chi), neutrophils (CD11bhi, Ly6Cdim, ly6G+) and Ly6C+ monocytes (CD11bhi, Ly6Chi, T/B/NK cell exclusion), and for expression of IL-15Rα. Black gates shown on dot plots define the different cell populations and histograms overlay IL-15Rα expression levels on the distinct cell subsets in uninfected (grey) versus infected (dark) mice. (D) Relative increase of IL-15Rα expression on resting versus activated DC (red), Ly6C+ monocytes (black) and neutrophils (blue) from spleen, lung and liver of B6 WT mice immunized with WT Lm, MCMV, S. pneumoniae, P. berghei or injected with poly(I:C) at indicated times. Data are representative of 2 independent replicate experiments (n=6) with p-values.
Inflammatory monocytes efficiently respond to IFN-I and trans-present bioactive IL-15
We further characterized the origin and fate of activated Ly6C+ monocytes. For this, we took advantage of a genetically engineered mouse model that express the CFP fluorescent reporter protein fused to the diphtheria toxin receptor (DTR) under the control of the CCR2 promoter (CCR2-DTR-CFP+ (Hohl et al., 2009)). In these mice, Ly6C+ monocytes, which also represent the major CCR2+ cell subset in vivo (Geissmann et al., 2003; Serbina et al., 2003), could not only be traced (CFP+) but also selectively eliminated (DTR+) upon diphtheria toxin (DT) injection (Figure S4C). In line with current knowledge (Geissmann et al., 2010), CFP+CD11bhi cells (CCR2+Ly6Chi monocytes) in non-infected animals are clearly distinct from cDCs (CD11chiCD11blo/+), macrophages that could be extracted from the spleen (defined as F4/80+CD11bint), neutrophils (CD11bhiLy6Cdim) and plasmacytoid DCs (pDCs) (SiglecH+CD11bint) (Figure 5A). Although CFP+ cells remain phenotypically distinct from all of the previous cell populations in mice challenged with Lm or MCMV, they upregulated cell-surface markers that define other myeloid cell types such as CD11c (DC), F4/80 (macrophages) and pDCs (PDCA-1), suggesting that they may differentiate into functional DCs and/or macrophages during microbial-driven activation. In support of this hypothesis, activated CCR2+Ly6C+ monocytes upregulated cell-surface expression of MHC-II molecules, CD40 and CD86 costimulatory molecules, the C-type lectin CD209b, Sca-1 (that marks IFN-I signaling (Essers et al., 2009)) as well as IL-15Rα in the course of the various infections (Figures 5A, B and S4C, D).
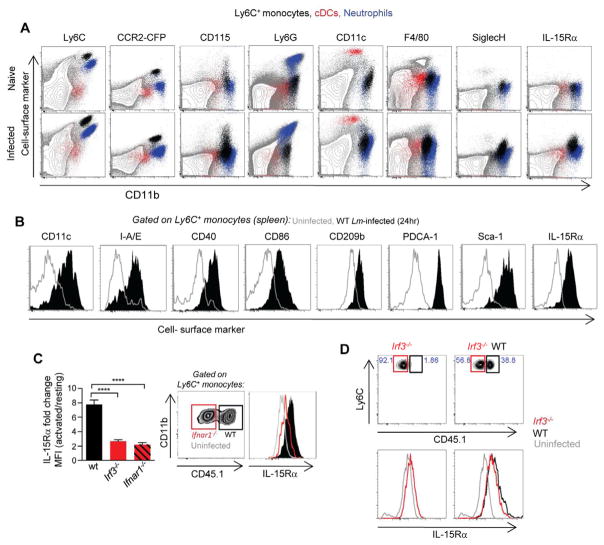
(A) Phenotypic analysis of Ly6C+ monocytes (black dots), cDCs (red) and neutrophils (blue) overlaid amongst whole splenocytes (grey) in naïve and WT Lm-infected mice. (B) Histograms show expression of indicated cell surface markers on Ly6C+ splenic monocytes from uninfected (grey) versus WT Lm-infected (black) mice. Data pool 2 replicate experiments (n=6). (C) Bar graphs show cell surface expression of IL-15Rα on Ly6C+ monocytes from the spleen of WT (black), Irf3−/− (red), and Ifnar1−/− (hatched) bone-marrow chimera infected with WT Lm for 24 hrs. Histogram shows IL-15Rα expression on Ly6C+ monocytes from a WT mixed bone-marrow chimera of WT CD45.1+ (black) and Ifnar1−/− (red) mice. Data represent 3 independent replicate experiments (n=4) with p-values. (D) Histograms are gated on WT CD45.1+ or Irf3−/− Ly6C+ monocytes and show IL-15Rα expression on Ly6C+ monocytes from irradiated WT mixed bone-marrow chimera of WT CD45.1+ (black) and Irf3−/− (red) bone-marrow cells. Data represent 1 experiment (n=4).
Results in Figure 3B suggested that IFN-I signaling acts by enhancing IL-15 trans-presentation. In line with this hypothesis, Ly6C+ monocytes responded to recombinant IFN-I in vivo and induced downstream phosphorylation of STAT1, greater than in cDCs or neutrophils (factor of 1.5–3 (MFI), Figure S5). We next investigated whether expression of IL-15Rα on activated Ly6C+ monocytes required IFN-I signaling within the monocytes. For this, we generated mixed chimera mice reconstituted with bone-marrow cells from IFN-I receptor- deficient (Ifnar1−/−) and WT CD45.1+ mice and monitored IL-15Rα expression after WT Lm infection (Figure 5C). Upregulation was severely impaired (factor of 3) in the WT-Ifnar1−/− chimera, suggesting that signaling through the IFN-I receptor is a major pathway of IL-15 trans-presentation by inflammatory monocytes in vivo. However, Irf3−/− Ly6C+ monocytes could differentiate into IL-15Rα+ monocytes in mixed chimera of Irf3−/− and WT CD45.1+ bone-marrow cells (Figure 5D), demonstrating that IFN-I may be secreted by other cell types, which still induced Ly6C+ monocytes to express IL-15Rα. Thus, IFN-I directly regulates the ability of inflammatory monocytes to trans-present bioactive IL-15 in vivo, possibly in a paracrine manner.
Inflammatory monocytes drive memory CD8+ T and NK cells differentiation into effectors during Lm infection
To prove that inflammatory monocytes regulated pathogen-induced memory CD8+ T cell activation in vivo, we treated the CCR2-DTR mice with DT, which eliminated Ly6C+ monocytes (Figure S4C), prior to OT-I and endogenous memory CD8+ T cell transfer, and before infection with WT Lm (Figure 6A). DT-treated mice had significantly less activated memory CD8+ T cells, e.g., expressing IFN-γ (52%) and GrB (34%) compared to untreated control mice (74 and 60%), both in frequencies and numbers at 8 hrs post-challenge infection, further supporting a role for inflammatory CCR2+ monocytes in optimal activation of memory CD8+ T cells in vivo. Likewise, in Ccr2−/− mice in which Ly6C+ monocytes are mostly retained in the bone-marrow (Serbina and Pamer, 2006), we measured half as many IFN-γ+ memory CD8+ T cells than in WT control mice (not shown), consistent with our previous observations. In support of this hypothesis, we also found that the transient clusters of OT-I memory cells -some of which were IFN-γ+- that we previously described in the red pulp area of infected spleens by 6–12 hrs post challenge infection (Bajenoff et al., 2010) also contained massive numbers of interacting CCR2+ inflammatory monocytes (Figure 6B). This was not observed in unchallenged mice containing resting OT-I memory cells.

WT CCR2-DTR−/− or CCR2-DTR+/− littermates were injected with DT prior to infections and transfers with OT-I and endogenous memory CD8+ T cells purified from WT B6 mice grafted with OT-I GFP+ cells and immunized with WT Lm-Ova 6 wks earlier. At indicated times after WT Lm (105 or 106) challenge, the activation (IFN-γ, GrB) of OT-I (A) and endogenous (C) memory CD8+ T cells, and NK cells (D) was monitored in the spleen of mice after staining for the appropriate cell-surface markers (CD8, CD3, NKp46). Grey dots depict resting memory CD8+ T cells, each dot representing an individual mouse in 2 independent replicate experiments (n=6-11) with p-values. (B) CCR2-DTR mice were transferred with OT-I-tomato+ cells, immunized with WT Lm-Ova and challenged or not (resting) with WT Lm 6 wks later. After 8 hrs, spleens were fixed, sectioned and stained to reveal IFN-γ, CFP (CCR2+ monocytes) and tomato (OT-I memory), and analyzed by confocal microscopy. Images are representative of 4 mice in 2 independent replicate experiments. Error bars on graphs represent Mean+/−SEM.
By 24 hrs, the proportion and numbers (not shown) of IFN-γ+ OT-I (6.9%) and endogenous (non-OT-I) memory cells in CCR2-DTR-depleted mice was equivalent to that of unchallenged mice (0.2%), and significantly reduced in comparison to non-DT treated mice (60.8%) (Figure 6A, C). At this time, endogenous GrB+ memory CD8+ T cells (12.4%) were also significantly diminished and at equivalent proportions (5.8%) to that of unchallenged mice (4%) (Figure 6C). However, the frequencies of OT-I memory cells expressing GrB was comparable in both experimental groups, suggesting that other cell types such as cDCs or macrophages (Lucas et al., 2007; Mortier et al., 2009) could compensate for the loss of Ly6C+ monocytes and trans-present sufficient levels of bioactive IL-15 to OT-I memory cells (Figure 6A).
Since activation of NK cells depends on IFN-I, IL-15 and IL-18 cytokines (Beuneu et al., 2011; Chaix et al., 2008; Lucas et al., 2007), we reasoned that inflammatory monocytes also regulated NK cell activation. As hypothesized, the proportion of IFN-γ+ and GrB+ NK cells decreased substantially in DT-treated CCR2-DTR mice challenged with WT Lm respectively at 8 and 24 hrs, and was close to that of unchallenged mice (Figure 6D). Thus, collectively Ly6C+ monocytes not only control memory CD8+ T cell, but also NK cell differentiation into robust effector cells.
Sustained cytolytic marker expression by memory CD8+ T and NK cells requires Ly6C+ monocytes and macrophages
Since eliminating Ly6C+ monocytes only had a partial and transient effect on the expression of GrB by both categories of lymphocytes (Figure 6A, D), we hypothesized that other myeloid cell types must be involved. Several reports using the CD11c-DTR transgenic mouse model, in which the DTR is expressed under the CD11c promoter, allowing for selective depletion of CD11c+ cells (Jung et al., 2002), suggested that cDCs orchestrate the activation of NK and memory CD8+ T cells in vivo (Lucas et al., 2007; Mortier et al., 2009; Zammit et al., 2005). To investigate this possibility, we transferred OT-I memory cells 12 hrs post-DT treatment into CD11c-DTR and CCR2-DTR along with WT littermate animals as controls, and monitored the expression of GrB by OT-I memory and NK cells 24 hrs after the challenge infection (Figure S6). GrB was still upregulated on the distinct lymphocytes from DT-treated DTR-expressing animals and was equivalent to that of non-depleted animals. Analysis of the populations eliminated upon DT injection in WT Lm infected and uninfected DTR-expressing mice revealed that in CD11c-DTR mice, cDCs (CD11chi CD8α+ and CD8α- subsets) and splenic macrophages (F4/80+CD11b+) were efficiently eliminated (80 and 96% respectively) whereas in CCR2-DTR mice, depletion mostly targeted Ly6C+ monocytes (>95%) (Figure S7 and not shown). This suggested that inflammatory monocytes or cDCs and subsets of splenic macrophages compensated for each other’s respective loss and provided a sufficient amount of trans-presented IL-15 to lymphocytes.
To assess this hypothesis further, we used clodronate liposomes, a well-established non-genetic method that eliminates a wide array of phagocytic cells (Chow et al., 2011). Clodronate liposome injection depleted Ly6C+ monocytes (by~90%), splenic macrophages (by~80%), as well as cDCs (by~50%) and neutrophils (~20%) (Figure S7). However, myeloid cells exhibiting high turnover, such as monocytes or neutrophils, underwent rapid replacement and fully restored their homeostasis by 5–7 days ((van Rooijen et al., 1989) and Figure S7). We injected WT and CCR2-DTR B6 mice with clodronate or PBS control liposomes 1 or 6 days prior to transfer of purified OT-I memory cells, DT treatment and infection with WT Lm (Figures 7A and S7). Twenty-four hrs post-challenge infection, upregulation of GrB expression and secretion of IFN-γ by OT-I memory CD8+ T cells was fully abrogated in day 1-clodronate liposome-depleted mice compared to untreated control mice (GrB:28 versus 63%; IFN-γ: 4 versus 55%) both in frequencies and numbers (Figure 7A and not shown). Likewise, upregulation of GrB and secretion of IFN-γ by NK cells was prevented (Figure 7B). Thus, as hypothesized, eliminating these subsets of phagocytic cells efficiently abrogated the upregulation of GrB by memory CD8+ T and NK cells, suggesting that they are required for sustained expression of bioactive IL-15 (Lucas et al., 2007; Mortier et al., 2009). In WT and CCR2-DTR mice treated with clodronate liposomes 6 days prior, in which Ly6C+ monocytes and neutrophils levels were largely restored but splenic macrophages were still absent and the proportion of cDC stayed comparable to day 1-treated mice (Figure S7), memory CD8 and NK lymphocytes still differentiated into effector cells (Figure 7A, B). When clodronate liposome-treated CCR2-DTR mice received DT to further deplete Ly6C+ monocytes, lymphocyte activation was fully abrogated. This suggested that inflammatory monocytes and splenic macrophages indeed contribute to sustain memory CD8+ T and NK lymphocytes differentiation into cytolytic effector cells. Thus altogether, inflammatory monocytes, and to a lesser extent splenic macrophages, most critically orchestrate the activation of both categories of lymphocytes in vivo.

B6 or B6-Kd+/− WT mice were respectively transferred with 500 naïve OT-I-tomato+ and WP20-GFP+ T cells and immunized with WT Lm-Ova and Lm. Six wks later, purified OT-I or WP20 memory cells were adoptively transferred to CCR2-DTR mice. (A–C) Mice were further injected or not with clodronate liposomes and treated or not with DT prior to WT Lm or MCMV infection. At indicated times after the challenge infection, expression of IFN-γ and/or GrB by splenic OT-I and NK cells was monitored in the different experimental groups. Dot plot are representative FACS profiles after gating on OT-I or NK cells and numbers correspond to the % of IFN-γ+ or GrB+ cells in the gate shown. Scattered plots represent the pool of 2–3 independent replicate experiments (n=3–9) with p-values. In (D), WP20 memory cells were transferred to CCR2-DTR mice (+/−DT) expressing or not expressing the MHC-I molecule Kd prior to WT Lm infection. Grey dots depict resting memory CD8+ T cells. In (E), OT-I memory cells were transferred into CCR2-DTR mice (+/−DT) further challenged with WT ΔActA Lm-Ova and their frequency in the spleen of mice was measured 3 days later. Results represent 2–3 independent replicate experiments (n=2–5) with p-values.
Early activation of memory CD8+ T and NK cells requires Ly6C+ monocytes both during viral infection and cognate antigen stimulation
To extend the role of Ly6C+ monocytes to other infections, we inoculated MCMV into CCR2-DTR mice grafted with memory CD8+ T cells (Figure 7C). The proportion of IFN-γ+ memory CD8+ T (OT-I:81.3%, endogenous, not shown) and NK (63.5%) cells was significantly decreased in DT-treated animals (OT-I:49.2 and NK:31.8%) demonstrating that also in the case of a viral infection, inflammatory monocytes exhibited a critical role in activating these lymphocytes. Thus, these cells are important master regulators of memory CD8+ and NK lymphocytes activation in vivo.
We next assessed whether inflammatory monocytes modulated the early activation of cognate antigen-specific memory CD8+ T cell-responses. For this, CCR2-DTR mice either expressing or not the MHC-I Kd molecule were transferred with WP20 memory cells; treated or not with DT and challenged with WT Lm (Figure 7D). Twenty-four hrs later, the frequency of WP20 memory cell secreting IFN-γ was significantly lower in DT-treated compared to untreated mice whether cognate antigen was presented or not. Therefore, Lm-specific memory CD8+ T cells underwent comparable differentiation into effector cells with or without cognate antigen stimulation and this process was largely controlled by Ly6C+ monocytes. Thus Ly6C+ monocyte-derived inflammatory signals IL-18 and IL-15 drive the early phases of memory CD8+ T cell activation.
Finally, to assess whether Ly6C+ monocytes also regulated cognate antigen-specific memory CD8+ T cell-responses, we measured OT-I memory cell proliferation in CCR2-DTR mice treated or not with DT 3 days after challenge infection with ΔActA Lm-Ova, a highly attenuated strain of Lm (Figure 7E). We used ΔActA Lm-Ova instead of WT Lm-Ova to prevent the uncontrolled bacterial growth that occurs in Ly6C+ monocyte-depleted mice (Shi and Pamer, 2011). We observed a significant loss of proliferation (factor of >2), suggesting that beyond their effect on early T cell-differentiation into effector cells, inflammatory monocytes also impacted subsequent cognate antigen-dependent proliferative responses, reinforcing their important role during CD8+ T cell-memory responses.
DISCUSSION
This study demonstrates that Ly6C+CCR2+ inflammatory monocytes are critical mediators of memory CD8+ T and NK lymphocyte activation in vivo. Upon detection of microbial pathogen-derived “danger signals” through triggering of type I interferon and inflammasome activation pathways, these cells directly provide important inflammatory cytokines -e.g., IL-18, IL-15 and possibly IFN-I- that directly mediate the activation of immunization-induced memory CD8+ T and NK lymphocytes. Along these lines, we established that the initial steps of activation of memory CD8+ T cells do not require and are independent of cognate antigen recognition by the memory cells. In response to this restricted set of inflammatory cytokines, memory CD8+ T cells undergo expression of a robust program of activation (CD69+, CD25+, CD11a+) and effector differentiation (IFN-γ+, GrB+, Perforin+, T-bet+, Eomesodermin+). This promotes a protective Th1-type environment through the rapid release of high levels of IFN-γ that participate in the innate immune defense of the host. Our results also uncouple the pathways that orchestrate the differentiation of memory and NK cells into IFN-γ+ versus GrB+ effector cells.
We highlight a unique function of inflammatory monocytes as main providers of bioactive IL-18 and IL-15 that promote rapid memory CD8+ T cell activation in vivo. Other cell types have been shown to produce these cytokines in vitro and in vivo (Lucas et al., 2007; Mortier et al., 2009; Mortier et al., 2008). Cells from the macrophage phagocyte system, DCs and neutrophils express specialized innate immune receptors, allowing for early detection of potential danger signals (Iwasaki and Medzhitov, 2010). A recent study suggested that cDCs, via NLRC4 inflammasome activation triggered by distinct microbial infections, can regulate non-cognate IFN-γ secretory activity of pathogen-specific memory CD8+ T cells through an IL-18-IL-18 receptor dependent mechanism (Kupz et al., 2012). While both sets of data suggest a comparable inflammasome-IL-18-dependent molecular mechanism to control memory CD8+ T cell-differentiation into IFN-γ-secreting effector cells, our results favor an essential role for inflammatory monocytes and possibly splenic macrophages rather than cDCs. This could be explained by differences in the experimental systems that have been used, particularly as the CD11c-DTR-expressing mouse not only induces elimination of cDC (Jung et al., 2002), but also of subsets of splenic macrophages including F4/80+ macrophages and likely other resident macrophages from the spleen (Chow et al., 2011; Probst et al., 2005). In the CCR2-DTR mouse, Ly6C+ monocytes, which represent the major immune cell expressing CCR2, underwent approximately 95% elimination upon DT injection while other splenic myeloid cell populations were largely preserved (Hohl et al., 2009). With the combined use of the CD169-DTR mouse model that eliminates CD169+Moma-1+ marginal metallophilic macrophages (MMM), ER-TR9+ marginal zone macrophages (MZM) and 50% of Ly6C+ monocytes (our unpublished data), but not F4/80+ red-pulp macrophages or cDCs (Miyake et al., 2007), our data suggest that neither MMM nor MZM tissue-resident macrophages are likely to have a major contribution in memory CD8+ T and NK cell activation. Thus, distinct genetic and non-genetic loss of function experiments in vivo, in vitro activation assays of memory CD8+ T cells, and visualization of in situ interactions between Ly6C+ monocytes and memory CD8+ T cells altogether provide strong support to inflammatory monocytes rather than cDCs as most critical players of IL-18-driven IFN-γ-secretion by pathogen-specific memory CD8+ T cells. Another possible explanation for the discrepancy with our study may be that impaired non-cognate T cell activation is subsequent to the loss of cDCs which were shown to be critical for recovering and shuttling blood-inoculated bacteria in the spleen (Edelson et al., 2011; Neuenhahn et al., 2006).
While cDCs and macrophages can trans-present IL-15 to CD8+ T cells, they exhibit distinct functional roles: IL-15 derived from cDCs contributes to central memory cell maintenance, whereas that from macrophages supports vaccine-induced effector CD8+ T cell transition to the memory stage (Mortier et al., 2009). Likewise, DC-derived IL-15 is a critical mediator of inflammatory responses in vivo (Ohteki et al., 2006) and prime NK cells (Lucas et al., 2007; Mortier et al., 2008). We provide new evidence that activated Ly6C+ monocytes rapidly upregulate and trans-present high levels of bioactive IL-15 during acute infections with various classes of microbial pathogens. IL-15 contributes to both GrB expression and to lesser extent IFN-γ secretion by memory CD8+ T and NK cells. In line with cited studies demonstrating a role for cDCs and macrophages in sustaining IL-15-dependent memory CD8+ T and NK cell activation and/or homeostasis, clodronate liposome-mediated depletion that eliminates a large variety of phagocytic cells including monocytes, tissue-macrophages and half of the cDCs, prevented the expression of cytolytic effector markers on memory and NK cells. However, using DT-treated CCR2-DTR and CD11c-DTR mice, lymphocytes still differentiated into GrB+ cells, suggesting that loss of Ly6C+ monocytes or alternately of cDCs and subsets of splenic macrophages could compensate for each other. Likewise, mice treated with clodronate liposome 7 days earlier provided the signals of differentiation to lymphocytes, even though they lacked splenic macrophages. Importantly, elimination of Ly6C+ monocytes in CCR2-DTR mice treated with clodronate liposomes 7 days prior, prevented GrB expression in memory CD8+ T and NK lymphocytes, consistent with the idea that inflammatory monocytes, while likely the main source of bioactive IL-15, can also be compensated by F4/80+ splenic red pulp macrophages (Mortier et al., 2009). Indeed, inflammatory monocytes form important clusters with memory CD8+ T cells after the challenge infection in splenic red pulp, in which they likely provide bioactive IL-15, but also interact with F4/80+ macrophages mostly found in the red pulp, supporting the idea that both cell subsets contribute to IL-15-dependent activation, and reflecting the spatio-temporal specialization of IL-15 trans-presentation in vivo (Mortier et al., 2009).
A previous study suggested an essential role for cDCs in optimal cognate-antigen driven memory CD8+ T cell-responses against Lm, vesicular stomatitis and influenza viruses during the first 24 to 48 hrs after challenge infection (Zammit et al., 2005). We found that inflammatory monocytes provided essential stimuli promoting robust Lm-specific memory CD8+ T cell-proliferative responses. In this study too, the apparent discrepancy with our data may result from the CD11c-DTR genetic conditional depletion system that was used (Chow et al., 2011; Jung et al., 2002; Probst et al., 2005). With regards to cognate antigen-driven memory CD8+ T cell responses, both our results and this report show an impact on effective memory CD8+ T cell proliferation. We found that whereas inflammatory cytokines, but not T cell cognate antigens, are essential to initiate memory CD8+ T cell reactivation, the expansion of memory cells requires the presence of cognate antigen, which may be presented by cDCs and/or inflammatory monocytes upon differentiation into potent antigen-presenting cells (Shi and Pamer, 2011). Indeed, one possible model is that inflammatory monocytes provide inflammatory stimuli, while cDC present cognate T cell antigens.
The cytokines that rapidly lead to efficient mobilization of memory CD8+ T and NK lymphocytes are released upon triggering of pathways which require the recognition of “danger” molecules by specialized sets of innate immune receptors (Iwasaki and Medzhitov, 2010). These innate sensors represent a very first line of alert of the immune system that is switched on only in potentially harmful situations. These pathways are stimulated by almost every microbial pathogen and must have co-evolved with the host immune defenses; they involve the recognition by the host immune system of highly conserved pathogen-derived molecules such as DNA and pore-forming toxins like listeriolysin O, hemozoin, pneumolysin and ESAT-6 (Dostert et al., 2009; Hara et al., 2008; Shoma et al., 2008). Monocytes and their functional progeny, which represent immune cells of high motility and astonishing plasticity, are equipped to detect and provide high levels of these inflammatory mediators.
Importantly, this mechanism also provides a Th1 cell-type (IFN-γ-dependent) protective environment (Berg et al., 2003; Kambayashi et al., 2003) while cognate antigen-specific responses take place. The release of substantial amounts of IFN-γ promotes expression of antimicrobial functions by effector cells such as macrophages and monocytes (Barton et al., 2007), which not only contribute to early pathogen destruction but also to the priming, polarization and proliferation of Th1-type T cells (Das et al., 2001). Cognate antigen recognition by non-cognate antigen-activated memory CD8+ T cells in vivo leads to significantly stronger IFN-γ response by these T cells (factor of ~3, not shown). Upregulation of cytolytic markers by memory cells make them ready to kill cells that present their cognate antigen. Similarly, expression of the CD25 marks their differentiation into potent effector cells (Kalia et al., 2010; Pipkin et al., 2010), and is required for their proliferation which only occurs upon actual recognition of their cognate antigen.
In summary, we describe a general mechanism of lymphocyte activation optimized through the lack of requirement for antigen specificity, yet controlled via tight cellular compartmentalization of IL-15 and IL-18 signals. These findings may open novel therapeutic perspectives in which targeted stimulation of Ly6C+ monocytes or its human equivalent to selectively provide bioactive IL-18 and IL-15 could boost the efficacy of antimicrobial and antitumoral protective immune responses.
EXPERIMENTAL PROCEDURES
Mice
All mice were housed and bred in our SPF animal facility; detailed description is provided in the online supplemental data.
Microbial pathogens, mice infections and measure of protective immunity
Listeria monocytogenes (Lm): Mice were inoculated with Lm 10403s strain, either WT Lm, Lm-Ova, LLOSer92 Lm, for primary immunization with 0.1×LD50 Lm (3×103 or 104 WT Lm and Lm-OVA respectively) and for secondary infections 4–6 wks later with 106 WT Lm or Lm-Ova unless otherwise specified. Streptococcus pneumoniae: 5×105 CFU of the ST3 strain A66.1 (A66) grown in tryptic soy broth to midlog phase was inoculated i.n. to mice in 25 μl/nare. Murine cytomegalovirus: 2×104 PFU/mouse of the Smith strain derived from a salivary gland stock was injected i.v to mice. Plasmodium Berghei (Pb): 5×105 Pb ANKA infected red blood cells from seeder mice were injected i.v. to mice. Lymphocytic Choriomeningitis Virus: 2×105 PFU/mouse of the Armstrong strain were injected i.p. to mice.
Generation of memory CD8+ T cells in vivo
Lm: WT B6 mice were adoptively transferred with ~500 OT-I or 1,000 WP20 cells isolated from spleen and lymph nodes of OT-I RAG-1−/− or WP20 Kd+ RAG-1−/− expressing GFP and/or Tomato (Td). One day later, mice were immunized i.v. with 104 WT Lm-Ova or Lm, and were used 4–6 wks later when they had developed memory cells. LCMV: WT B6 mice were immunized with 2×105 PFU of virus i.v. and used at least 8 wks after the primary immunization.
In vivo memory T cell and NK cell activation assays
In immunized mice: Mice containing Lm-specific memory CD8+ T cells, e. g. immunized 30 days prior, were challenged with WT Lm or Lm-OVA, 50μg poly(I:C), S. Pneumoniae, MCMV, or Pb, and organs analyzed by FACS at the indicated time points in the text/figures/legends. In adoptively transferred mice: CD8+ T cells were purified from the spleens of primary immunized mice and 3–5×106 cells transferred into mice that were either infected with WT Lm 30 min before or left uninfected. In MCMV experiments, mice were infected for 24 hrs before transfer. After 8 or 24 hrs, spleens were recovered and OT-I, WP20, or endogenous memory cells activation was monitored. For antigen-specific proliferation, 6×105 purified CD8+ T cells were transferred into recipients and proliferation analyzed 72 hrs after ΔActA Lm-Ova infection.
Statistics
Statistical significance was calculated using an unpaired Student t test and two-tailed P values are given as: (*) P<0.1; (**) P<0.01; and (***) P<0.001; (ns) P>0.1. All p values of 0.05 or less are significant.
Acknowledgments
H2-Kd/LLO91–99 tetramers came from the NIH Tetramer Facility. We are grateful to W. Jacobs Jr. for privileged access to the HHMI Aria II, and thank L. Tesfa (FACS Core). We thank F. Sutterwala (University of Iowa) and R. Flavell (Yale University) for providing Casp1−/− bone-marrow cells and mice; D. Woodland and J. Kohlmeier (Trudeau Institute) for providing Ifnar1−/− bone marrow cells; T. Moran (Mount Sinai School of Medecine) for Irf3−/− mice; and E. Pamer (Memorial Sloan-Kettering Cancer Center) for CCR2-DTR and WP20 mice. We thank C. Biron (Brown University) for providing stocks of MCMV. We thank M. Bajenoff (CIML, France), S. Porcelli (AECOM, NY), L. Chorro, C. Chandrabos, E. Spaulding for helpful discussions. Work was supported by Institutional Funds of the Albert Einstein College of Medicine of Yeshiva University (GL), the National Institute of Health (R21AI095835, GL), the Agence Nationale pour la Recherche (ANR-10-LABX-61, JM), InCa Atip/Avenir (JM), and the fundation Bettencourt-Schueller (JM). Core flow cytometry were supported by the Albert Einstein Cancer Center (NCI Grant 2P30CA013330).
Footnotes
Author Contributions
S.M.S. designed the study, performed experiments and analyzed data. A.R. and J.M. performed, analyzed the LCMV experiments and commented on the manuscript. G.L. designed the study, analyzed the data and wrote the paper.
Author Information
The authors declare that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajenoff M, Narni-Mancinelli E, Brau F, Lauvau G. Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS One. 2010;5:e11524. [Europe PMC free article] [Abstract] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. [Abstract] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. [Europe PMC free article] [Abstract] [Google Scholar]
- Beuneu H, Deguine J, Bouvier I, Di Santo JP, Albert ML, Bousso P. A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J Immunol. 2011;187:2084–2088. [Abstract] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. [Abstract] [Google Scholar]
- Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. [Abstract] [Google Scholar]
- Das G, Sheridan S, Janeway CA., Jr The source of early IFN-gamma that plays a role in Th1 priming. J Immunol. 2001;167:2004–2010. [Abstract] [Google Scholar]
- Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. [Europe PMC free article] [Abstract] [Google Scholar]
- Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, et al. CD8alpha(+) Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35:236–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. [Abstract] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. [Abstract] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. [Europe PMC free article] [Abstract] [Google Scholar]
- Hara H, Tsuchiya K, Nomura T, Kawamura I, Shoma S, Mitsuyama M. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J Immunol. 2008;180:7859–7868. [Abstract] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. [Europe PMC free article] [Abstract] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. [Abstract] [Google Scholar]
- Iwai Y, Hemmi H, Mizenina O, Kuroda S, Suda K, Steinman RM. An IFN-gamma-IL-18 signaling loop accelerates memory CD8+ T cell proliferation. PLoS One. 2008;3:e2404. [Europe PMC free article] [Abstract] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. [Europe PMC free article] [Abstract] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. [Europe PMC free article] [Abstract] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. [Abstract] [Google Scholar]
- Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170:2399–2408. [Abstract] [Google Scholar]
- Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. [Abstract] [Google Scholar]
- Kupz A, Guarda G, Gebhardt T, Sander LE, Short KR, Diavatopoulos DA, Wijburg OL, Cao H, Waithman JC, Chen W, et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat Immunol 2012 [Abstract] [Google Scholar]
- Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc Natl Acad Sci U S A. 2002;99:6192–6197. [Europe PMC free article] [Abstract] [Google Scholar]
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. [Europe PMC free article] [Abstract] [Google Scholar]
- Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. [Abstract] [Google Scholar]
- Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. [Abstract] [Google Scholar]
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. [Europe PMC free article] [Abstract] [Google Scholar]
- Narni-Mancinelli E, Soudja SM, Crozat K, Dalod M, Gounon P, Geissmann F, Lauvau G. Inflammatory Monocytes and Neutrophils Are Licensed to Kill During Memory Responses In Vivo. PLoS Pathog. 2011:29. [Europe PMC free article] [Abstract] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. [Abstract] [Google Scholar]
- Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. [Abstract] [Google Scholar]
- Ohteki T, Tada H, Ishida K, Sato T, Maki C, Yamada T, Hamuro J, Koyasu S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 2006;203:2329–2338. [Europe PMC free article] [Abstract] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. [Europe PMC free article] [Abstract] [Google Scholar]
- Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. [Abstract] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. [Abstract] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. [Abstract] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. [Abstract] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. [Europe PMC free article] [Abstract] [Google Scholar]
- Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama R, Daim S, Mitsuyama M. Critical involvement of pneumolysin in production of interleukin-1alpha and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect Immun. 2008;76:1547–1557. [Europe PMC free article] [Abstract] [Google Scholar]
- van Rooijen N, Kors N, Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989;45:97–104. [Abstract] [Google Scholar]
- Wirth TC, Martin MD, Starbeck-Miller G, Harty JT, Badovinac VP. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur J Immunol. 2011;41:1321–1333. [Europe PMC free article] [Abstract] [Google Scholar]
- Yajima T, Nishimura H, Sad S, Shen H, Kuwano H, Yoshikai Y. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J Immunol. 2005;174:3590–3597. [Abstract] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.immuni.2012.05.029
Read article for free, from open access legal sources, via Unpaywall:
https://www.cell.com/article/S1074761312003688/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.immuni.2012.05.029
Article citations
DAMP-ing IBD: Extinguish the Fire and Prevent Smoldering.
Dig Dis Sci, 04 Jul 2024
Cited by: 0 articles | PMID: 38963463
Review
Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.
NPJ Vaccines, 9(1):66, 21 Mar 2024
Cited by: 2 articles | PMID: 38514656 | PMCID: PMC10957963
IL-15 in T-Cell Responses and Immunopathogenesis.
Immune Netw, 24(1):e11, 16 Feb 2024
Cited by: 2 articles | PMID: 38455459
Review
CD8+ Tissue-Resident Memory T Cells: Versatile Guardians of the Tissue.
J Immunol, 212(3):361-368, 01 Feb 2024
Cited by: 2 articles | PMID: 38227907
Evaluating immunological and inflammatory changes of treatment-experienced people living with HIV switching from first-line triple cART regimens to DTG/3TC vs. B/F/TAF: the DEBATE trial.
Front Immunol, 14:1279390, 16 Oct 2023
Cited by: 2 articles | PMID: 37908359 | PMCID: PMC10613634
Go to all (178) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
CD8 T cells regulate allergic contact dermatitis by modulating CCR2-dependent TNF/iNOS-expressing Ly6C+ CD11b+ monocytic cells.
J Invest Dermatol, 134(3):666-676, 23 Sep 2013
Cited by: 16 articles | PMID: 24061165
Memory-T-cell-derived interferon-γ instructs potent innate cell activation for protective immunity.
Immunity, 40(6):974-988, 12 Jun 2014
Cited by: 65 articles | PMID: 24931122 | PMCID: PMC4105986
NK1.1+ CD8+ T cells escape TGF-β control and contribute to early microbial pathogen response.
Nat Commun, 5:5150, 06 Oct 2014
Cited by: 33 articles | PMID: 25284210 | PMCID: PMC4836950
Mechanisms of Memory T Cell Activation and Effective Immunity.
Adv Exp Med Biol, 850:73-80, 01 Jan 2015
Cited by: 21 articles | PMID: 26324347 | PMCID: PMC4836952
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: 2P30CA013330
Grant ID: P30 CA013330
NIAID NIH HHS (3)
Grant ID: R01 AI103338
Grant ID: R21 AI095835
Grant ID: R21AI095835





