Abstract
Free full text

Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound.
Abstract
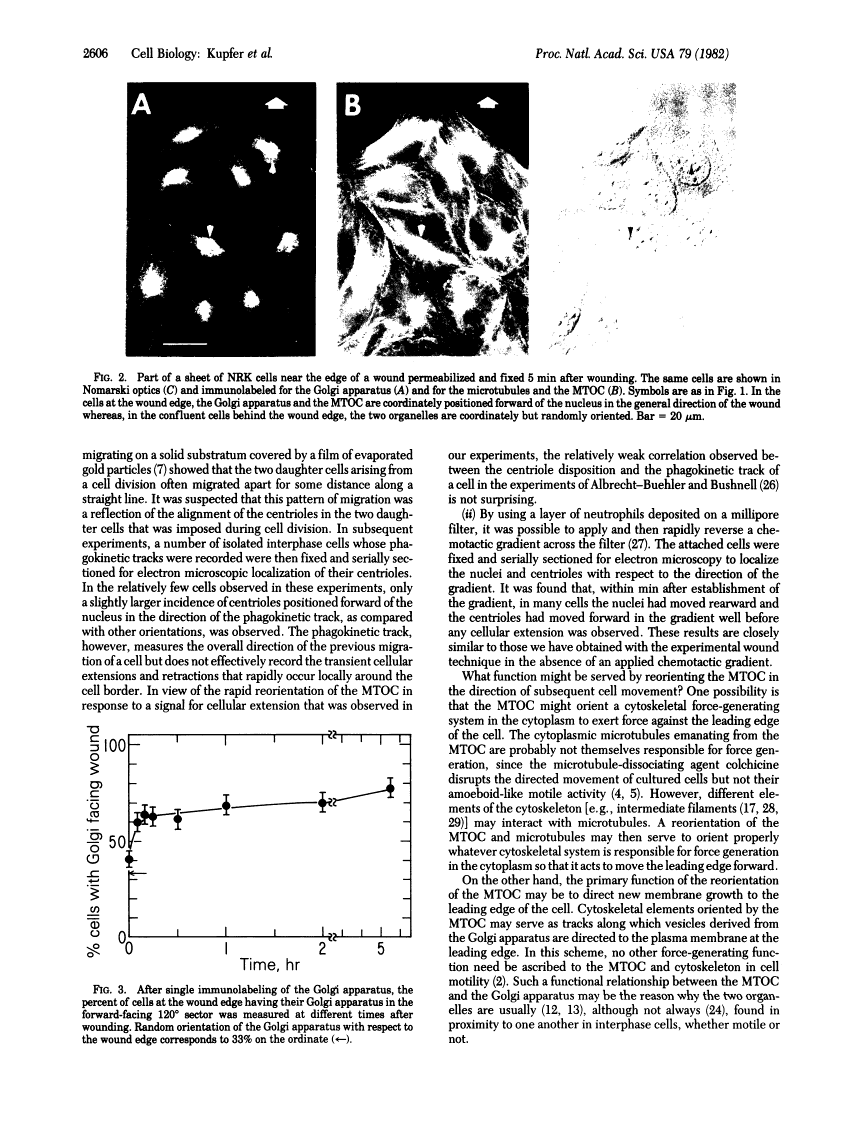
We have used the technique of experimental wounding of confluent monolayers of normal fibroblasts to induce essentially unidirectional and synchronous cell movement at the edge of the wound. The intracellular location of the Golgi apparatus and the microtubule-organizing center was determined by double indirect immunofluorescence microscopy, using antibodies specific for the membranes of the Golgi apparatus and antibodies specific for tubulin, respectively. In cells at the wound edge, the immunolabeled Golgi apparatus and microtubule-organizing center were in close proximity to one another and located predominantly forward of the cell nucleus facing the wound. In the same cultures in cells removed from the wound, the two organelles were also coordinately located; however, they were randomly oriented with respect to the wound edge. This reorientation of the two organelles in cells at the wound edge was evident within minutes after wounding and persisted as cell extension subsequently occurred into the wound. These results suggest that both the Golgi apparatus and the microtubule-organizing center may participate in directing cell movement. The possible mechanisms involved are discussed in the light of previous hypotheses and experimental evidence concerning cell motility.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970 Oct;62(2):389–398. [Abstract] [Google Scholar]
- Harris A, Dunn G. Centripetal transport of attached particles on both surfaces of moving fibroblasts. Exp Cell Res. 1972 Aug;73(2):519–523. [Abstract] [Google Scholar]
- Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [Abstract] [Google Scholar]
- Goldman RD. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. [Europe PMC free article] [Abstract] [Google Scholar]
- Huxley HE. Muscular contraction and cell motility. Nature. 1973 Jun 22;243(5408):445–449. [Abstract] [Google Scholar]
- Albrecht-Buehler G. Daughter 3T3 cells. Are they mirror images of each other? J Cell Biol. 1977 Mar;72(3):595–603. [Europe PMC free article] [Abstract] [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. [Europe PMC free article] [Abstract] [Google Scholar]
- Brinkley BR, Fuller EM, Highfield DP. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. [Europe PMC free article] [Abstract] [Google Scholar]
- Osborn M, Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. [Europe PMC free article] [Abstract] [Google Scholar]
- Louvard D, Reggio H, Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. [Europe PMC free article] [Abstract] [Google Scholar]
- Heggeness MH, Simon M, Singer SJ. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. [Europe PMC free article] [Abstract] [Google Scholar]
- Geiger B, Singer SJ. Association of microtubules and intermediate filaments in chicken gizzard cells as detected by double immunofluorescence. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4769–4773. [Europe PMC free article] [Abstract] [Google Scholar]
- Bergmann JE, Tokuyasu KT, Singer SJ. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. [Europe PMC free article] [Abstract] [Google Scholar]
- Gotlieb AI, Heggeness MH, Ash JF, Singer SJ. Mechanochemical proteins, cell motility and cell-cell contacts: the localization of mechanochemical proteins inside cultured cells at the edge of an in vitro "wound". J Cell Physiol. 1979 Sep;100(3):563–578. [Abstract] [Google Scholar]
- MARCUS PI. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1962;27:351–365. [Abstract] [Google Scholar]
- Hay ED. Extracellular matrix. J Cell Biol. 1981 Dec;91(3 Pt 2):205s–223s. [Europe PMC free article] [Abstract] [Google Scholar]
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970 Mar;59(3):393–398. [Abstract] [Google Scholar]
- Izzard CS, Lochner LR. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. [Abstract] [Google Scholar]
- Trelstad RL. The Golgi apparatus in chick corneal epithelium: changes in intracellular position during development. J Cell Biol. 1970 Apr;45(1):34–42. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmes LB, Trelstad RL. Patterns of cell polarity in the developing mouse limb. Dev Biol. 1977 Sep;59(2):164–173. [Abstract] [Google Scholar]
- Albrecht-Buehler G, Bushnell A. The orientation of centrioles in migrating 3T3 cells. Exp Cell Res. 1979 Apr;120(1):111–118. [Abstract] [Google Scholar]
- Malech HL, Root RK, Gallin JI. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977 Dec;75(3):666–693. [Europe PMC free article] [Abstract] [Google Scholar]
- Franke WW, Grund C, Osborn M, Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978 Aug;17(2):365–391. [Abstract] [Google Scholar]
- Ball EH, Singer SJ. Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6986–6990. [Europe PMC free article] [Abstract] [Google Scholar]
- Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.79.8.2603
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc346248?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
The receptor protein tyrosine phosphatase PTPRK promotes intestinal repair and catalysis-independent tumour suppression.
J Cell Sci, 137(14):jcs261914, 22 Jul 2024
Cited by: 0 articles | PMID: 38904097 | PMCID: PMC11298714
miR-29a-3p orchestrates key signaling pathways for enhanced migration of human mesenchymal stem cells.
Cell Commun Signal, 22(1):365, 17 Jul 2024
Cited by: 0 articles | PMID: 39020373 | PMCID: PMC11256664
NLRP3 is essential for neutrophil polarization and chemotaxis in response to leukotriene B4 gradient.
Proc Natl Acad Sci U S A, 120(35):e2303814120, 21 Aug 2023
Cited by: 4 articles | PMID: 37603754 | PMCID: PMC10468616
Ependymal polarity defects coupled with disorganized ciliary beating drive abnormal cerebrospinal fluid flow and spine curvature in zebrafish.
PLoS Biol, 21(3):e3002008, 02 Mar 2023
Cited by: 8 articles | PMID: 36862758 | PMCID: PMC10013924
StarD7 deficiency hinders cell motility through p-ERK1/2/Cx43 reduction.
PLoS One, 17(12):e0279912, 30 Dec 2022
Cited by: 0 articles | PMID: 36584213 | PMCID: PMC9803278
Go to all (248) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Reorientation of the Golgi apparatus and the microtubule-organizing center inside macrophages subjected to a chemotactic gradient.
Cell Motil, 5(1):17-29, 01 Jan 1985
Cited by: 38 articles | PMID: 3978702
Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies.
Proc Natl Acad Sci U S A, 80(14):4286-4290, 01 Jul 1983
Cited by: 74 articles | PMID: 6136036 | PMCID: PMC384022
Membrane insertion at the leading edge of motile fibroblasts.
Proc Natl Acad Sci U S A, 80(5):1367-1371, 01 Mar 1983
Cited by: 104 articles | PMID: 6298789 | PMCID: PMC393598
Interplay between microtubule dynamics and intracellular organization.
Int J Biochem Cell Biol, 44(2):266-274, 17 Nov 2011
Cited by: 137 articles | PMID: 22108200
Review