Abstract
Free full text

Serum contains a platelet-derived transforming growth factor.
Abstract
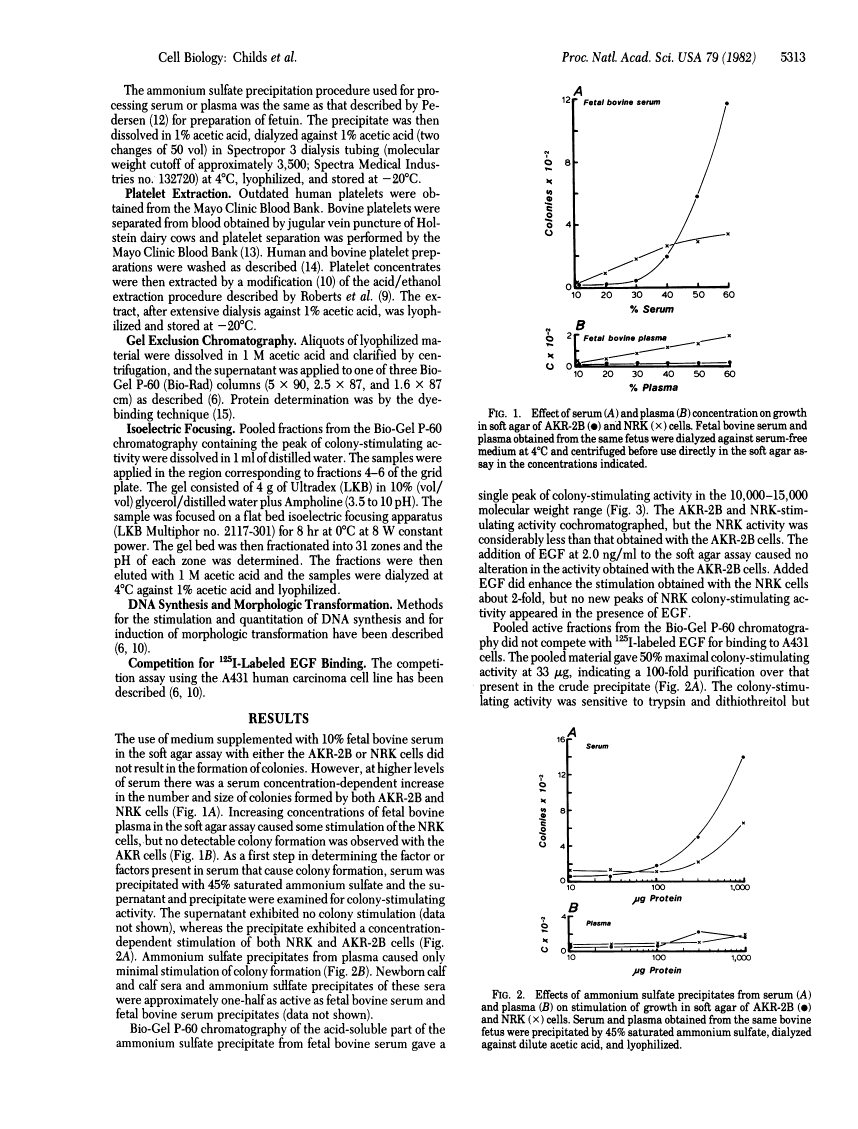
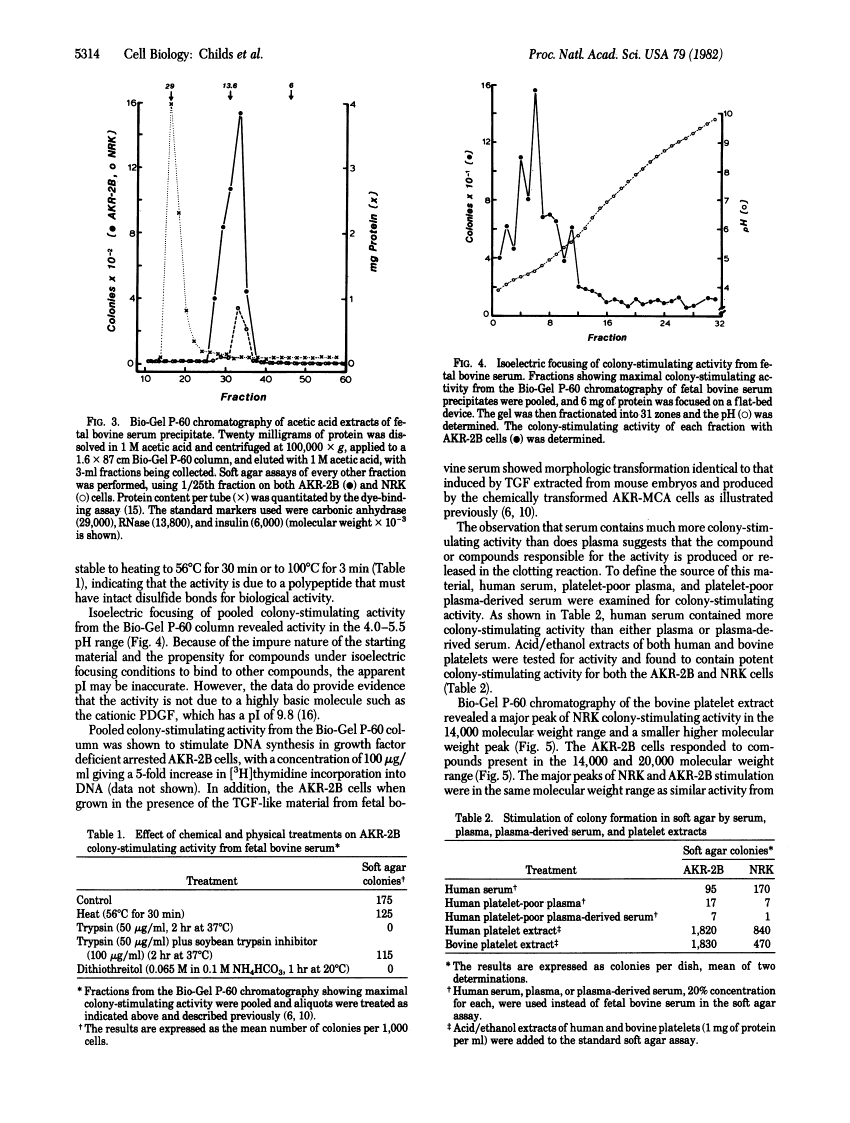
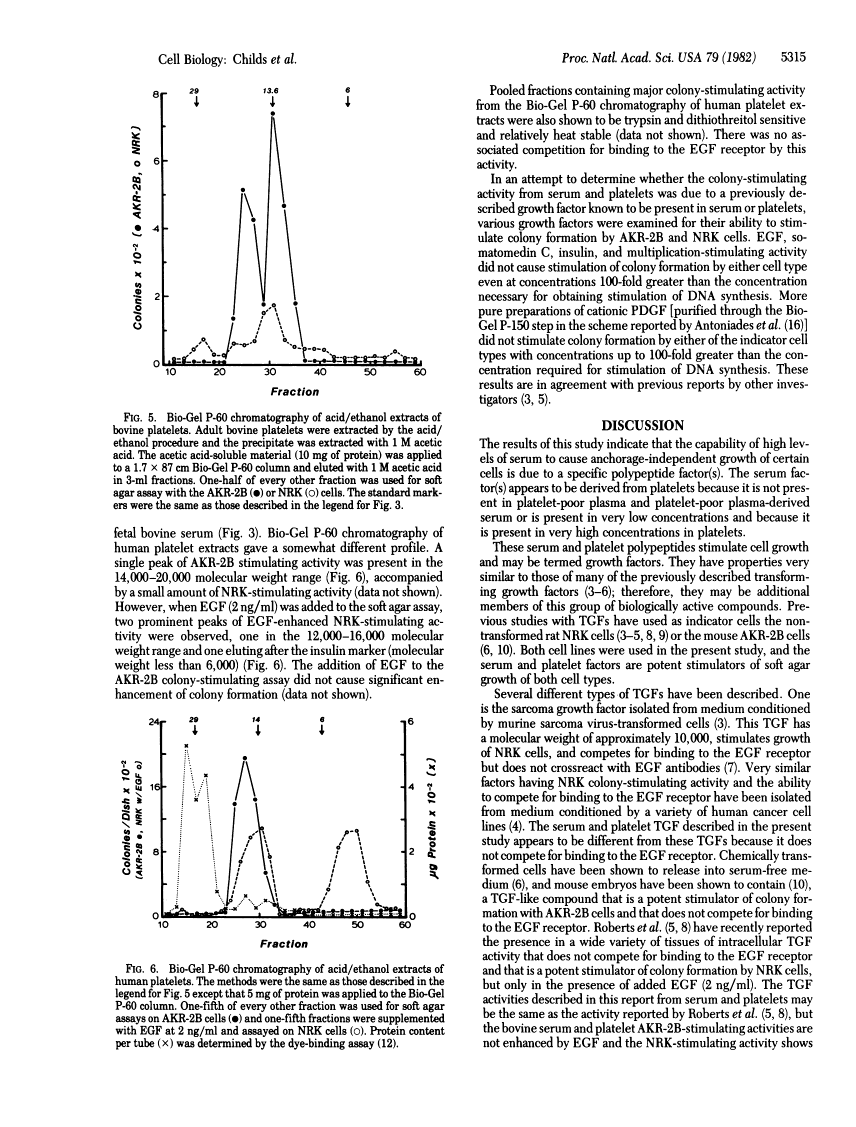
High concentrations of fetal bovine serum induced colony formation in soft agar by anchorage-dependent, nontransformed mouse AKR-2B and rat NRK cells. The colony-stimulating activity in fetal bovine serum was precipitated by 45% saturated ammonium sulfate and migrated in molecular sieve chromatography as a single peak of activity in the 10,000-15,000 molecular weight range. The colony-stimulating activity was heat and acid stable and was destroyed by trypsin and dithiothreitol, indicating the activity is due to a polypeptide that requires disulfide bonds for biological activity. No competition for binding to the epidermal growth factor receptor was associated with the colony-stimulating activity. Isoelectric focusing revealed activity in the pI 4-5 range. The colony-stimulating activity in serum appeared to be of platelet origin because platelet-poor plasma and platelet-poor plasma-derived serum contained little activity, whereas acid/ethanol extracts of bovine and human platelets had potent colony-stimulating activity. Chromatography of platelet extracts on Bio-Gel P-60 revealed peaks of AKR-2B colony-stimulating activity in the 12,000 and 20,000 molecular weight ranges. The other biological and chemical properties of the platelet colony-stimulating activity were the same as those for the serum activity. The data indicate the presence in serum of a platelet-derived growth factor(s) with properties similar to those of the transforming growth factors.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- O'Neill CH, Riddle PN, Jordan PW. The relation between surface area and anchorage dependence of growth in hamster and mouse fibroblasts. Cell. 1979 Apr;16(4):909–918. [Abstract] [Google Scholar]
- Peehl DM, Stanbridge EJ. Anchorage-independent growth of normal human fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3053–3057. [Europe PMC free article] [Abstract] [Google Scholar]
- de Larco JE, Todaro GJ. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. [Europe PMC free article] [Abstract] [Google Scholar]
- Todaro GJ, Fryling C, De Larco JE. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberts AB, Anzano MA, Lamb LC, Smith JM, Frolik CA, Marquardt H, Todaro GJ, Sporn MB. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982 Feb 4;295(5848):417–419. [Abstract] [Google Scholar]
- Moses HL, Branum EL, Proper JA, Robinson RA. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [Abstract] [Google Scholar]
- De Larco JE, Todaro GJ. Sarcoma growth factor (SGF): specific binding to epidermal growth factor (EGF) membrane receptors. J Cell Physiol. 1980 Feb;102(2):267–277. [Abstract] [Google Scholar]
- Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. [Europe PMC free article] [Abstract] [Google Scholar]
- Proper JA, Bjornson CL, Moses HL. Mouse embryos contain polypeptide growth factor(s) capable of inducing a reversible neoplastic phenotype in nontransformed cells in culture. J Cell Physiol. 1982 Feb;110(2):169–174. [Abstract] [Google Scholar]
- Rutherford RB, Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. [Europe PMC free article] [Abstract] [Google Scholar]
- Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976 Nov;34(3):395–402. [Abstract] [Google Scholar]
- Heldin CH, Wasteson A, Westermark B. Partial purification and characterization of platelet factors stimulating the multiplication of normal human glial cells. Exp Cell Res. 1977 Oct 15;109(2):429–437. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Antoniades HN, Scher CD, Stiles CD. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. [Europe PMC free article] [Abstract] [Google Scholar]
- Antoniades HN. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. [Europe PMC free article] [Abstract] [Google Scholar]
- Castor CW, Ritchie JC, Williams CH, Jr, Scott ME, Whitney SL, Myers SL, Sloan TB, Anderson BE. Connective tissue activation. XIV. Composition and actions of a human platelet autacoid mediator. Arthritis Rheum. 1979 Mar;22(3):260–272. [Abstract] [Google Scholar]
- Eastment CT, Sirbasku DA. Human platelet lysate contains growth factor activities for established cell lines derived from various tissues of several species. In Vitro. 1980 Aug;16(8):694–705. [Abstract] [Google Scholar]
- Cifone MA, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1039–1043. [Europe PMC free article] [Abstract] [Google Scholar]
- Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. [Abstract] [Google Scholar]
- Sporn MB, Harris ED., Jr Proliferative diseases. Am J Med. 1981 Jun;70(6):1231–1235. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.79.17.5312
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc346886?pdf=render
Citations & impact
Impact metrics
Article citations
TGF-β signaling in health, disease, and therapeutics.
Signal Transduct Target Ther, 9(1):61, 22 Mar 2024
Cited by: 36 articles | PMID: 38514615 | PMCID: PMC10958066
Review Free full text in Europe PMC
Advances and Challenges in Targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective.
Pharmaceuticals (Basel), 17(4):533, 20 Apr 2024
Cited by: 6 articles | PMID: 38675493 | PMCID: PMC11054419
Review Free full text in Europe PMC
Lyophilized Platelet-rich plasma for the management of thin endometrium and facilitation of in-vitro fertilization.
JBRA Assist Reprod, 27(1):55-59, 30 Mar 2023
Cited by: 4 articles | PMID: 35916459 | PMCID: PMC10065768
Isolation of Whole Cell Protein Lysates from Mouse Facial Processes and Cultured Palatal Mesenchyme Cells for Phosphoprotein Analysis.
J Vis Exp, (182), 01 Apr 2022
Cited by: 1 article | PMID: 35435918 | PMCID: PMC9093641
In Vitro Evaluation of the Influence of Substrate Mechanics on Matrix-Assisted Human Chondrocyte Transplantation.
J Funct Biomater, 11(1):E5, 18 Jan 2020
Cited by: 5 articles | PMID: 31963629 | PMCID: PMC7151603
Go to all (157) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transforming growth factors in solid human malignant neoplasms.
Cancer Res, 43(5):1966-1971, 01 May 1983
Cited by: 51 articles | PMID: 6299535
Transforming growth factor production by chemically transformed cells.
Cancer Res, 41(7):2842-2848, 01 Jul 1981
Cited by: 260 articles | PMID: 6265069
Comparison of intra- and extracellular transforming growth factors from nontransformed and chemically transformed mouse embryo cells.
Cancer Res, 43(4):1581-1586, 01 Apr 1983
Cited by: 75 articles | PMID: 6299525
The platelet-derived growth factor.
Cell, 14(2):203-210, 01 Jun 1978
Cited by: 342 articles | PMID: 352535
Review