Abstract
Free full text

PNAS Plus
Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing
Abstract
Knowledge of the rate and nature of spontaneous mutation is fundamental to understanding evolutionary and molecular processes. In this report, we analyze spontaneous mutations accumulated over thousands of generations by wild-type Escherichia coli and a derivative defective in mismatch repair (MMR), the primary pathway for correcting replication errors. The major conclusions are (i) the mutation rate of a wild-type E. coli strain is ~1 × 10−3 per genome per generation; (ii) mutations in the wild-type strain have the expected mutational bias for G:C > A:T mutations, but the bias changes to A:T > G:C mutations in the absence of MMR; (iii) during replication, A:T > G:C transitions preferentially occur with A templating the lagging strand and T templating the leading strand, whereas G:C > A:T transitions preferentially occur with C templating the lagging strand and G templating the leading strand; (iv) there is a strong bias for transition mutations to occur at 5′ApC3′/3′TpG5′ sites (where bases 5′A and 3′T are mutated) and, to a lesser extent, at 5′GpC3′/3′CpG5′ sites (where bases 5′G and 3′C are mutated); (v) although the rate of small (≤4 nt) insertions and deletions is high at repeat sequences, these events occur at only 1/10th the genomic rate of base-pair substitutions. MMR activity is genetically regulated, and bacteria isolated from nature often lack MMR capacity, suggesting that modulation of MMR can be adaptive. Thus, comparing results from the wild-type and MMR-defective strains may lead to a deeper understanding of factors that determine mutation rates and spectra, how these factors may differ among organisms, and how they may be shaped by environmental conditions.
Mutations are the source of variation upon which natural selection acts; thus, a complete understanding of evolutionary processes must include an accurate assessment of mutation rates and of the molecular spectrum of mutational events. In addition, we need to know whether, and how, intrinsic and extrinsic factors influence mutational processes. This understanding must be founded on baseline parameters established by analyzing mutations that accumulate in a neutral fashion, unbiased by selective pressures. Much of our knowledge of spontaneous mutation is based on mutations that occur in nonessential reporter genes during short-term laboratory culture of microorganisms (1). An alternative approach is to compare presumably neutral mutations that have accumulated over evolutionary time periods in diverged species (2). Both methods have substantial uncertainties. The experimental approach may use reporter loci that are not representative of the whole genome and necessarily incorporates assumptions about the expression and neutrality of the mutant phenotypes. The historical approach relies on estimated divergence times and the absence of selective pressure on synonymous sequence changes. High-throughput whole-genome sequencing allows some of these limitations to be overcome. For example, a recent study analyzed the base-pair substitutions (BPSs) that arose in Escherichia coli strains that had been subjected to long-term evolution studies (3). Although the contribution of selection was minimized by considering only synonymous BPSs, selection based on codon usage nonetheless may have biased the results (4).
The mutation-accumulation (MA) strategy combined with whole-genome sequencing overcomes many of these limitations. The MA protocol is designed to allow mutations to occur in a neutral manner, devoid of selective pressure (5). The general strategy is to establish a number of clonal populations from a founder individual and then to take each population through repeated single-individual bottlenecks for thousands of generations. Because the effective population size of each line is one, genetic drift prevents selection from eliminating all but the most deleterious mutations, which typically are less than 1% of mutations (6). For microorganisms, streaking for single colonies on agar medium accomplishes the bottleneck, allowing around 30 generations of growth between passages. Selection within a colony is minimal because most new mutations arise during the last few generations of growth when the population is large. Furthermore, the great majority of mutations have no fitness effects, and of those that do, the effects are small (6–9). The application of whole-genome sequencing to MA lines has made this protocol an extremely valuable way to determine a complete and nearly unbiased picture of mutation profiles. Recent results from applying this protocol to microbes include the genome-wide spontaneous mutational spectrum in Saccharomyces cerevisiae (10) and the mutational consequences of loss of DNA oxidative damage repair in Salmonella typhimurium (11).
Because of its decades of use as a model for genetic and physiological research, Escherichia coli is the ideal microorganism for investigations at the genomic level. However, there still are questions about mutational processes in this best-known-of-all microbe. For example, in the papers cited above (1, 3) estimates of the mutation rate vary by an order of magnitude. The experiments reported here were designed to yield a highly accurate estimate of the spontaneous mutation rate of E. coli. In addition, by obtaining a large number of neutral mutations, we anticipated determining the spectrum of mutagenic changes across the whole genome at a greater density than has been possible previously.
The remarkably low rates at which mutations occur result from both the high intrinsic accuracy of DNA replication and various enzymatic activities that survey and repair DNA. Chief among these is mismatch repair (MMR), which surveys newly replicated DNA, detects mismatched bases, recruits enzymes to destroy the new DNA strand, and forces repolymerization using the old DNA strand as the template (reviewed in ref. 12). MMR is highly conserved and found in all domains of life. However, bacterial strains isolated from nature often lack MMR capacity (13, 14), and MMR-defective strains frequently become dominant in long-term evolution experiments (15). Indeed, the presence or absence of various DNA repair pathways can be quite sporadic among microbial species (16–18). In addition, MMR proteins are subject to regulation (19, 20), and thus the activity of MMR is responsive to both intrinsic and extrinsic factors. For these reasons, we have included in this report the results of an MA experiment with an isogenic MMR-defective strain. The resulting large pool of mutations gives a picture of genomic mutagenic processes hitherto unseen.
Results
Spontaneous Mutation Rate of Wild-Type E. coli Is Lower than Expected.
The wild-type strain we used for the MA protocol is a prototrophic derivative of the reference E. coli strain, MG1655. The parameters of a two-part MA experiment with this strain are given in Table 1. In the first part (designated “wild-type 3K”), MA lines were passaged for about 3,000 generations before sequencing; in the second part (designated “wild-type 6K”), unsequenced lines continued to be passaged for an additional 3,000 generations and then were sequenced. Surprisingly, the 6K lines accumulated more mutations per generation than did the 3K lines. Thus, we have two estimates of the spontaneous mutation rate that differ by 30% but are within 95% confidence limits (CL) of each other. This result illustrates the inherent uncertainty of estimating mutation rates from sparse data. Nonetheless, because the MA protocol minimizes selection (see below), and because we have a fairly accurate measure of the number of generations that the lines experienced, we believe that these two values put accurate limits on the spontaneous mutation rate of E. coli. Both parts of the experiment gave results that appear to be random and unbiased (see below); thus, the best estimate of the spontaneous mutation rate is the midpoint of these limits, 2.2 × 10−10 mutations per nucleotide per generation or 1.0 × 10−3 mutations per genome per generation (Table 1). This rate is about threefold lower than Drake’s value of 3–4 × 10−3 per genome per generation for all DNA-based microbes (1, 21). Possible reasons for this difference are considered in the Discussion.
Table 1.
Parameters of MA experiments
| Strain | No. of BPSs | No. of indels* | No. of lines | Generations per line | Total no. of generations | BPSs per line | Indels per line | Mutation rate per nucleotide (× 1010) | 95% CL† | Mutation rate per genome‡ (× 103) | 95% CL† |
| Wild-type 3K | 93 | 9 | 38 | 3,080 | 117,040 | 2.45 | 0.24 | 1.88 | ±0.46 | 0.87 | ±0.21 |
| Wild-type 6K | 140 | 12 | 21 | 6,356 | 133,476 | 6.67 | 0.57 | 2.45 | ±0.49 | 1.14 | ±0.23 |
| MutL− | 1625 | 306 | 34 | 375 | 12,750 | 47.8 | 9.00 | 326 | ±153 | 151 | ±71 |
*Insertion or deletion of ≤4 nt.
†Critical values of the t distribution were used to calculate 95% CLs for the means of the Poisson distributions shown in Fig. 1. These values then were used to compute the CLs for the mutation rates.
‡Genome = 4,639,675 nt.
Table 2 gives mutation rates for the wild-type strain estimated using classical fluctuation tests, scoring for resistance to rifampicin (RifR) and to nalidixic acid (NalR). When normalized to the number of nucleotides that, when mutated, give the resistance phenotypes (22–24), the two mutation rates are 0.33 and 0.21 × 10−10 mutations per nucleotide per generation, respectively. The mean rate for BPS mutations from the MA experiment was 1.99 × 10−10 per nucleotide per generation, six- to nine-fold higher than that obtained from fluctuation tests. Low values obtained in the fluctuation tests probably are responsible for this difference; several cell generations may be required for drug-sensitive molecules to be replaced by resistant ones before newly arisen RifR and NalR mutations are expressed. Such phenotypic lag will result in low apparent mutation rates (25).
Table 2.
BPS mutation rates
| Phenotype | Mutation rate per locus (× 1009) | Mutation rate per nucleotide (× 1010) | 95% CL | Mutation rate per genome (× 103) | 95% CL | |
| Fluctuation test results* | ||||||
 Wild type Wild type | RifR | 2.6 | 0.33 | 0.22–0.46 | 0.15 | 0.10–0.21 |
| NalR | 0.43 | 0.21 | 0.11–0.34 | 0.10 | 0.05–0.16 | |
 MutL– MutL– | NalR | 99 | 49 | 44–55 | 23 | 21–25 |
 Increase caused by loss of MutL– Increase caused by loss of MutL– | 233 | |||||
| MA results | ||||||
 Wild-type 3K Wild-type 3K | 1.71 | ±0.44† | 0.80 | ±0.20† | ||
 Wild-type 6K Wild-type 6K | 2.26 | ±0.45† | 1.05 | ±0.21† | ||
 Wild type mean Wild type mean | 1.99 | 0.92 | ||||
 MutL– MutL– | 275 | ±14† | 127 | ±6† | ||
 Increase caused by loss of MutL– Increase caused by loss of MutL– | 138 | |||||
*Mutation rates and 95% CL were calculated as described in SI Materials and Methods. Values were normalized assuming RifR is conferred by 79 BPSs in the rpoB gene (22) and NalR is conferred by 18 BPSs in the gyrA gene (23) plus two BPSs in the gyrB gene (24).
†Critical values of the t distribution were used to calculate 95% CLs for the means of the Poisson distributions of the numbers of mutations per MA line.These values then were used to compute the CLs for the mutation rates.
Selection is Minimal During MA Experiments.
There are several ways to evaluate the degree to which selective pressures may have biased the mutational profile obtained from the MA experiments. Assuming that selective pressure on noncoding regions is minimal, if mutations accumulate in a neutral manner, the ratio of the number of mutations occurring in coding versus noncoding DNA should reflect the ratio of the number of nucleotides that are in coding versus noncoding DNA, which is 5.74 for the MG1655 genome (3.95 × 106 nucleotides in protein-coding sequences and 6.88 × 105 in noncoding sequences). The observed ratio from the wild-type data was 3.31 (179/54), which is significantly less than 5.74 (χ2 = 5.0, P = 0.03). However, because of possible differences in nucleotide content in coding and noncoding DNA, the ratio of mutations in these regions could be biased by the types of mutational events that occur. To address this issue, we performed Monte Carlo simulations using the actual spectrum of BPSs observed from the wild-type results; the results from 1,000 simulations gave a ratio of 5.93 ± 1.04 (mean ± SD), close to the theoretical ratio. Thus it appears that the actual ratio is less than expected for an unbiased distribution of mutations. This result may reflect the inherent variability of sparse data or, more interestingly, may indicate that coding DNA is less susceptible to mutation than noncoding DNA (see below).
Another commonly used measure of bias is the ratio of nonsynonymous to synonymous BPSs, under the assumption that synonymous mutations are relatively neutral. Given the codon usage in MG1655, the expected ratio is 3.25. The ratio observed from the MA experiments with the wild-type strain was 2.25 (55/124), which is not significantly different from expected (χ2 = 2.3, P = 0.13). One thousand Monte Carlo simulations using the observed spectrum of BPSs from the wild-type results gave a ratio of 2.71± 0.41, which is not significantly different from the actual ratio (χ2 = 0.5, P = 0.47). Thus, the MA protocol resulted in little or no apparent bias against nonsynonymous mutations.
Even synonymous BPSs could be selected against if they result in less favorable codons. Based on the codon usage in MG1655, of the 55 codon changes resulting from synonymous BPSs in the wild-type strain, 16 resulted in a more commonly used codon, four changes were neutral (<10% difference in usage), 11 changes resulted in a less commonly used codon (10–30% difference in usage), and 24 resulted in a relatively rarely used codon (>30% difference in usage) (Table S1). Whether these codon changes would affect fitness depends on whether the genes are highly expressed; of the 55 genes affected, three are considered to be highly expressed (26). In one of these, guaA, the mutation resulted in a more commonly used codon, and in two, aspS and metQ, the mutation resulted in a less commonly used codon. Thus, synonymous BPSs creating less favorable codons occurred both in highly expressed genes and in less highly expressed genes, indicating that these mutations were not purged by selection during the MA experiment.
Finally, most mutations creating chain-terminating (nonsense) codons are expected to have deleterious consequences (21). Of the 1.2 × 107 possible codon-changing BPSs in the E. coli genome, 423,094 will create a chain-terminating codon. Thus, genome-wide, 3% {423,094 ÷ [(3 × (4.6 × 106)]} of all BPS mutations should be nonsense mutations. This calculation predicts that seven of the 233 BPSs observed in the wild-type strain should have been nonsense; eight were recovered.
Previous studies estimated the rate of deleterious mutations in wild-type E. coli to be 0.05–0.2 × 10−3 per genome per generation (7, 27), which is, at most, only a fourth of the total mutation rate observed here. The rate of beneficial mutations is even lower, and, in addition, most beneficial and deleterious mutations have only small (<3%) effects on fitness (7, 27). Taken together, our results with the wild-type strain are consistent with these findings and support the basic premise that the MA protocol minimizes selection and allows mutations to accumulate in a nearly neutral fashion.
Loss of MMR Increases the BPS Mutation Rate and Changes the Mutational Bias.
Loss of MMR typically increases mutation rates 100- to 200-fold (12). The MMR-defective strain we used for the MA experiment has a deletion of the mutL gene, which encodes MutL, one of the key enzymes required for MMR. As shown in Table 2, the MutL− strain had a 233-fold increase in mutation rate to NalR as measured by a fluctuation test. The 375-generation MA experiment resulted in 1,625 BPS mutations in 34 lines, giving a BPS mutation rate of 2.75 × 10−8 per nucleotide per generation, a 138-fold increase relative to the wild-type strain.
The observed ratio of BPSs in coding versus noncoding regions in the MutL− strain was 6.63 (1,412/213), which is greater than but not significantly different from the theoretical ratio of 5.74 (χ2 = 2.13, P = 0.14). One thousand Monte Carlo simulations using the observed spectrum of BPSs from the MutL− strain gave a ratio of 5.29 ± 0.36; a value as large as 6.63 was not recovered. Thus, it appears that in the absence of MMR, BPSs are more likely to occur in coding regions than in noncoding regions, suggesting that MMR preferentially prevents errors in coding DNA.
In contrast to the wild-type strain, the ratio of nonsynonymous to synonymous BPSs was only 1.92 (589/307) in the MutL− strain, significantly less than the expected 3.25 (χ2 = 39; P < 0.0001). Loss of MMR changes the spectrum of BPSs (see below), which change accounts for most, if not all, of this relative increase in synonymous BPSs. One thousand Monte Carlo simulations using the observed spectrum of BPSs from the MutL− strain gave a ratio of 1.66 ± 0.09; values of 1.92 or higher were obtained twice. Thus it appears that the MutL− results are at the low end of the expected result for an unbiased distribution of nonsynonymous and synonymous BPSs.
Distribution of Mutations Is Random among Wild-Type Lines but Not Among MutL− Lines.
If the mutation rate were constant throughout the MA experiment, then the numbers of mutations among the lines should have a Poisson distribution. As shown in Fig. 1A, this hypothesis was well supported for the wild-type strain; the two mutation datasets fit very well to Poisson distributions with means of 2.68 and 7.24 mutations per line (χ2 = 1.3, P = 0.97 and χ2 = 3.7, P = 0.89, respectively). However, the distribution of mutations per line in the MutL− strain clearly deviated from expected (χ2 = 299, P = <0.0001) (Fig. 1B), suggesting that some lines may have accumulated mutation-rate modifiers. Indeed, the high and low sections of the MutL− data for BPSs can be fit to two Poisson distributions with means of 37 and 54 mutations per line (χ2 = 1.6, P = 0.99 and χ2 = 2.9, P = 0.99, respectively). However, fluctuation tests performed at the end of the MA protocol with MutL− lines whose numbers of accumulated mutations spanned the distribution shown in Fig. 1B showed no consistent differences in mutation rates. Thus, if mutation modifiers appeared, they had been eliminated by the end of the experiment.
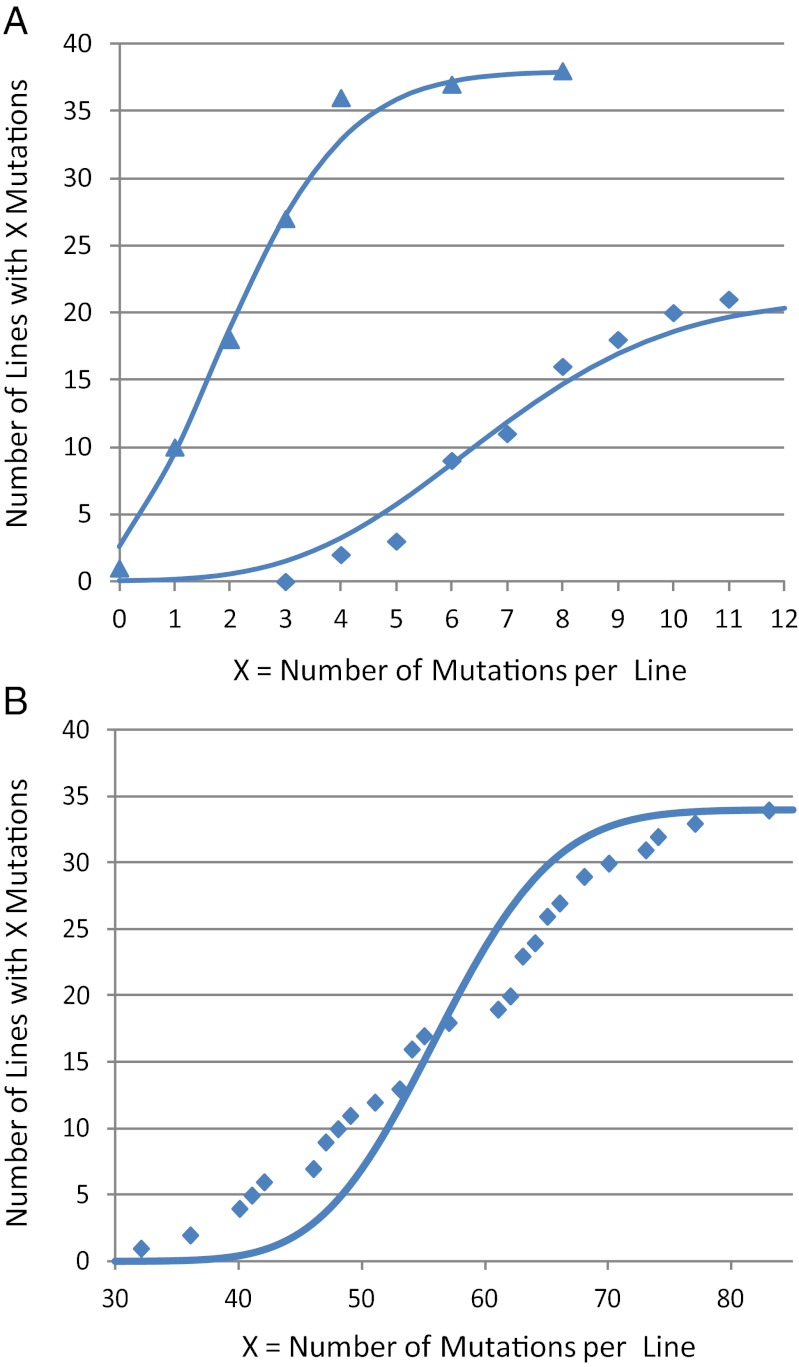
Distribution of mutations among MA lines. The number of lines with a given number of mutations is plotted against the number of mutations per line and compared with the Poisson distribution expected for the mean number of mutations per line (solid traces). (A) Wild-type 3K lines (38 lines; triangles) and wild-type 6K lines (21 lines; diamonds). (B) MutL− lines (34 lines).
Spectrum of BPSs Is Shifted by Loss of MMR.
The spectrum of BPSs in the wild-type strain is given in Table 3, and the rates of each type of BPSs are shown in Fig. 2A. The wild-type 3K and 6K datasets resulted in slightly different proportions of the different types of BPSs, but none of the differences are statistically significant at the 95% level. In addition, in 1,000 Monte Carlo simulations of the two spectra the proportions of most of the mutational events from the two datasets fell within one SD of each other; the exceptions were A:T > C:G and G:C > T:A, which fell within the 95% CL. Transitions, particularly G:C > A:T mutations, dominated the wild-type spectrum, comprising 56% of the BPSs; of the transversions, G:C > C:G and A:T > T:A were the rarest. This spectrum is the expected one for wild-type strains based on both locus-specific and genome-wide experiments (see refs. 2, 3, and 28 and references therein).
Table 3.
Spectra of BPS in wild-type and MutL− strains
| Wild type | MutL− | ||||
| Number | Fraction | Number | Fraction | Increase caused by MutL–* | |
| Type of substitution | |||||
| Transitions | 131 | 0.56 | 1,588 | 0.97 | 240 |
 A:T > G:C A:T > G:C | 49 | 0.21 | 1,141 | 0.70 | 465 |
 G:C > A:T G:C > A:T | 82 | 0.35 | 447 | 0.28 | 108 |
| Transversions | 102 | 0.44 | 37 | 0.02 | 7 |
 A:T > T:A A:T > T:A | 17 | 0.07 | 14 | 0.009 | 5 |
 A:T > C:G A:T > C:G | 38 | 0.16 | 10 | 0.006 | 5 |
 G:C > T:A G:C > T:A | 30 | 0.13 | 10 | 0.006 | 6 |
 G:C > C:G G:C > C:G | 17 | 0.07 | 3 | 0.002 | 4 |
| A:T sites | 104 | 0.45 | 1,165 | 0.71 | 224 |
| G:C sites | 129 | 0.55 | 460 | 0.28 | 70 |
| Total | 233 | 1,625 | 138 | ||
| Consequences of substitutions | |||||
| Position | |||||
 Noncoding Noncoding | 54 | 0.23 | 213 | 0.13 | 77 |
 Coding Coding | 179 | 0.77 | 1,412 | 0.87 | 157 |
| Within coding sequences | |||||
 Synonymous Synonymous | 55 | 0.31 | 482 | 0.34 | 176 |
 Nonsynonymous Nonsynonymous | 124 | 0.69 | 930 | 0.66 | 149 |
| Amino acid changes | |||||
 Conservative Conservative | 57 | 0.46 | 644 | 0.69 | 224 |
 Nonconservative Nonconservative | 67 | 0.54 | 286 | 0.31 | 85 |
*Mutation rates normalized to the relevant genomic nucleotide composition, compared with the average of the rates for wild-type 3K and 6K.
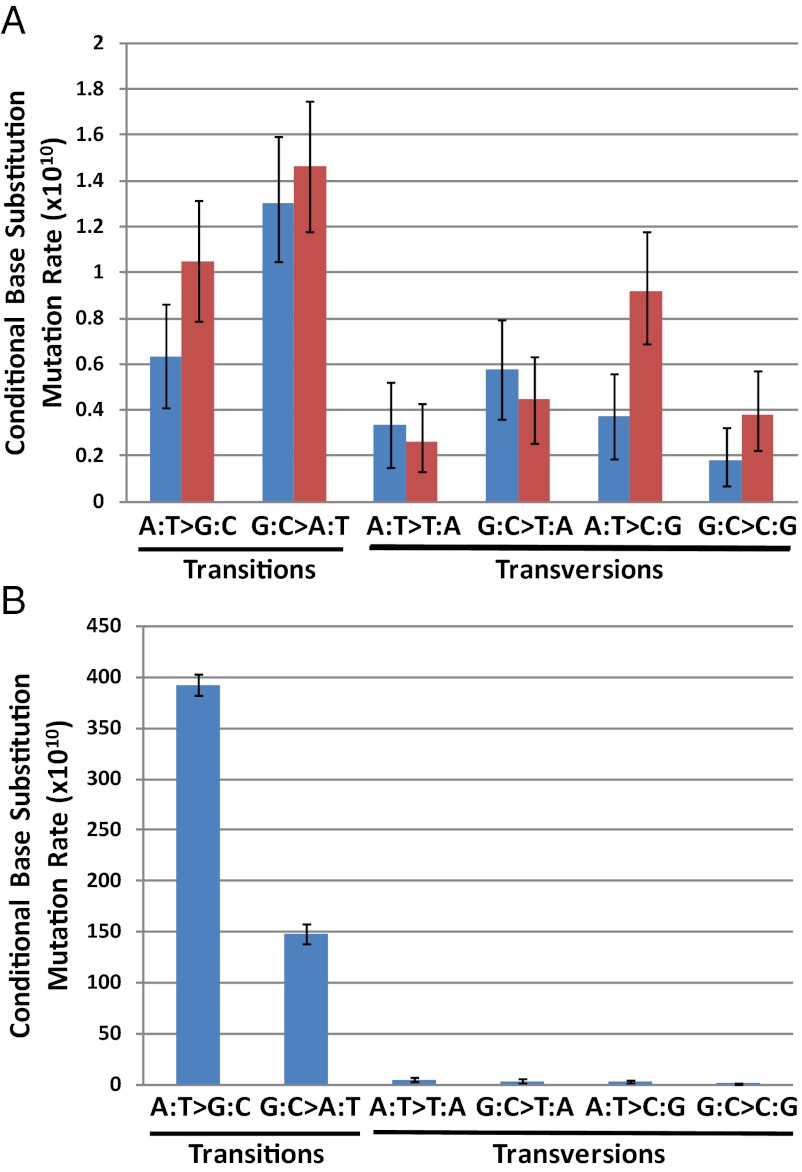
Mutation rates of each of the six BPSs. Bars represent the mutation rate of each type of BPS normalized to the number of AT or GC base pairs in the genome. (A) Wild-type 3K dataset (93 mutations; blue) and wild-type 6K dataset (140 mutations; red). (B) MutL− dataset (1,625 mutations). Error bars represent the fifth percentile and 95th percentile values from 1,000 Monte Carlo simulations of a random distribution with the mutational spectra observed for each dataset.
The spectrum of the MutL− strain also was strongly biased toward transitions, which made up 98% of the BPSs. However, in contrast to the wild-type strain, A:T > G:C transitions were by far the dominant class, accounting for 70% of all of the BPSs (Table 3 and Fig. 2B). Overall, the effect of loss of MMR was to shift the BPS bias from changing G:C to A:T base pairs to changing A:T to G:C base pairs. The increase in the proportion of A:T > G:C transitions in the MutL− strain was not the result of decreases in the rates of other mutational events. As shown in Table 3 and Fig. 2B, the mutation rates of all types of BPSs were increased in the MutL− strain, but A:T > G:C events rose disproportionally. This change in mutational bias has been seen in some, but not all, previous studies of MMR defective strains (Discussion).
Occurrence of BPSs Has a Strong DNA-Strand Bias.
Assuming that the BPSs observed are the result of replication errors, A:T > G:C mutations result from C mispaired with a template A or G mispaired with a template T. The E. coli genome is divided into two replichores, each replicated in opposite directions starting at the origin of replication centered on nucleotide 3,923,883 and ending at the terminus approximately half-way around the chromosome [nucleotide 1,604,045 is the exact half-way point, but termination is imprecise, usually occurring between TerA at nucleotide 1,339,769 and TerC at nucleotide 1,607,200 (29)]. Thus, the leading and lagging strands are switched in the two replichores relative to the conventional 5′-to-3′ nucleotide numbering system defining the “top” strand. In the right replichore A:T > G:C transitions in the MutL− strain were twice as likely to occur with A rather than T in the top strand (390 As versus 175 Ts); in the left replichore this bias was reversed: A:T > G:C transitions were twice as likely to occur with T rather than A in the top strand (394 Ts versus 182 As). This bias held true when the mutations were normalized to the A and T content of the strands. Thus, in both the right (clockwise) and left (counterclockwise) replichores, A:T > G:C transitions were twice as likely to occur with A templating the lagging strand and T templating the leading strand during replication than in the opposite orientation. In contrast, G:C > A:T transitions were twice as likely to occur (305/142) with C templating the lagging strand and G templating the leading strand during replication than in the opposite orientation (also true when normalized to G and C content of the strands). The bias for transitions occurring with C templating the lagging strand also was evident in the wild-type strain (58/24), but the bias for A templating the lagging strand was not as strong (29/20).
BPSs in the MutL− Strain Are Strongly Biased by the Local Sequence Context.
Of the BPSs that occurred at A:T sites in the MutL− strain, 77% (899/1,165) occurred in the sequence 5′ApC3′ or the equivalent 5′GpT3′ (the two bases indicated are on the same DNA strand with the mutated base in bold; the “p” represents the phosphate linking the two nucleosides). The influence of the neighboring C or G was particularly prominent at the A:T sites that gave rise to A:T > G:C mutations: of these, 79%, (897/1,141) were at 5′ApC3′ or 5′GpT3′ (Table S2). G:C > A:T transitions in the MutL− strain also were biased by the local sequence context: 53% (238/447) were at 5′GpC3′ or 5′GpC3′ sites (Table S2). Transition mutations in the wild-type strain were not as strongly biased by neighboring Gs or Cs, suggesting that MMR is relatively efficient at preventing mutations at these sites (Table S2).
Methylated Bases Are Mutational Hotspots.
The internal Cs in the sequences CCAGG and CCTGG are methylated at the 5 position by the Dcm methylase (30). Because 5meC can deaminate, creating thymine in the DNA, Dcm sites are hotspots for G:C > A:T transitions (31). Of the 82 G:C > A:T transitions that occurred in the wild-type strain, eight (about 10%) occurred at Dcm sites. There are 12,045 Dcm sites in the E. coli genome, so only 2% of the Cs potentially are methylated. Thus, our data confirm that Dcm sites are mutational hotspots. However, only one of the 447 G:C > A:T transitions in the MutL− strain occurred at a Dcm site; because the rate of this event is only threefold higher than in the wild-type strain, MMR appears to be poor at preventing these mutations (Discussion).
The A in GATC sites is methylated at the 6 position by the Dam methylase (30); 6meA is prone to depurination, producing transversions (32, 33). Of the 24 A:T transversions that occurred in the MutL− strain, 17 occurred at Dam sites; 13 of those were A:T > T:A, and four were A:T > C:G transversions. Four A:T > G:C transitions also occurred at Dam sites. A similar but less prominent pattern was observed in the wild-type strain: of 55 A:T transversions, 11 occurred at Dam sites; four were A:T > T:A, and seven were A:T > C:G. Two A:T > G:C transitions also occurred at Dam sites in the wild-type strain. There are 19,120 Dam sites in the E. coli genome, so only about 3% of the As potentially are methylated. Therefore, the number of A:T transversions occurring at Dam sites far exceeds the expected number, indicating that these sites are mutational hotspots.
The Dam methylase has some activity at noncanonical target sites, particularly GACC sequences but also CATC, TATC, AATC, and GATT sequences (34), but these do not appear to be hotspots for transversions (see SI Text for further discussion).
BPSs Are Not Biased Toward Highly Expressed Genes.
Of E. coli’s 4,146 protein-coding genes, 253 are considered to be highly expressed (26). These genes account for 6% of the nucleotides in the genome and 7% of the nucleotides in the coding DNA. In the wild-type strain 16 BPSs were in highly expressed genes, representing 6% of all of the BPSs and 9% of BPSs in coding DNA. In the MutL− strain 102 BPSs (6% of all of the BPSs and 7% of the BPSs in coding DNA) were in highly expressed genes. None of these values is significantly different from expected (P ≥ 0.42 in every case). Thus, our results do not support previous observations that highly expressed genes have either high (11, 35, 36) or low (37–39) mutation rates. Unlike a previous report (39), we found no bias for any type of base change occurring preferentially on either the transcribed or the nontranscribed DNA strand (Table S3).
Sequence Repeats are Hotspots for Indels.
Insertions and deletions ≤4 nt accounted for the vast majority of the indels in both the wild-type and the MutL− strain; the few larger indels and insertion-sequence element movements will be treated in a separate analysis. When normalized to the whole genome, small indels occurred at about 1/10th the rate of BPSs in both the wild-type and the MutL− strain (Table 4). Loss of MMR resulted in a 288-fold increase in the rate of indel formation, with the increase biased toward additions of G:C base pairs. The majority of indels were gain or loss of a single nucleotide in runs of identical nucleotides; the few multiple-nucleotide indels were gains or losses of 2- or 3-nt units in runs of identical units.
Table 4.
Indel mutation rates
| Single-nucleotide indels | Multiple-nucleotide indels* | ||||||
| Strain | Mutation rate per genome (× 105) | Plus | Minus | A:T | G:C | Plus | Minus |
| Wild-type 3K | 7.69 | 5 | 3 | 6 | 2 | 0 | 1 |
| Wild-type 6k | 8.99 | 1 | 10 | 4 | 7 | 0 | 1 |
| MutL− | 2,400 | 177 | 120 | 97 | 200 | 3 | 6 |
| Increase caused by loss of MutL† | 288 | 553 | 187 | 187 | 451 | — | 59 |
*2–4 nt.
†Mutation rates for the MutL− strain compared with the average of the rates for wild-type 3K and 6K.
The rate at which indels occurred normalized to the whole genome obscures the true mutation rate at sequence repeats, which are known hotspots for indel formation (40). As shown in Fig. 3A, the rate at which indels occurred as a function of the length of the run increased exponentially in both the wild-type and the MutL− strain. The slope of the least-squares line fitted to the MutL− data is 25% greater than that fitted to the wild-type data, suggesting that the ability of MMR to prevent indels increases with increasing run length. Alternatively, the wild-type data can be fit to two lines, with the rate of increase of mutations in runs ≥5 nt being about 20% greater than that of the MutL− strain. In either case, although the increase in the rate of indel formation caused by the loss of MMR is high overall, the increase in runs of a given length is at most 70-fold.
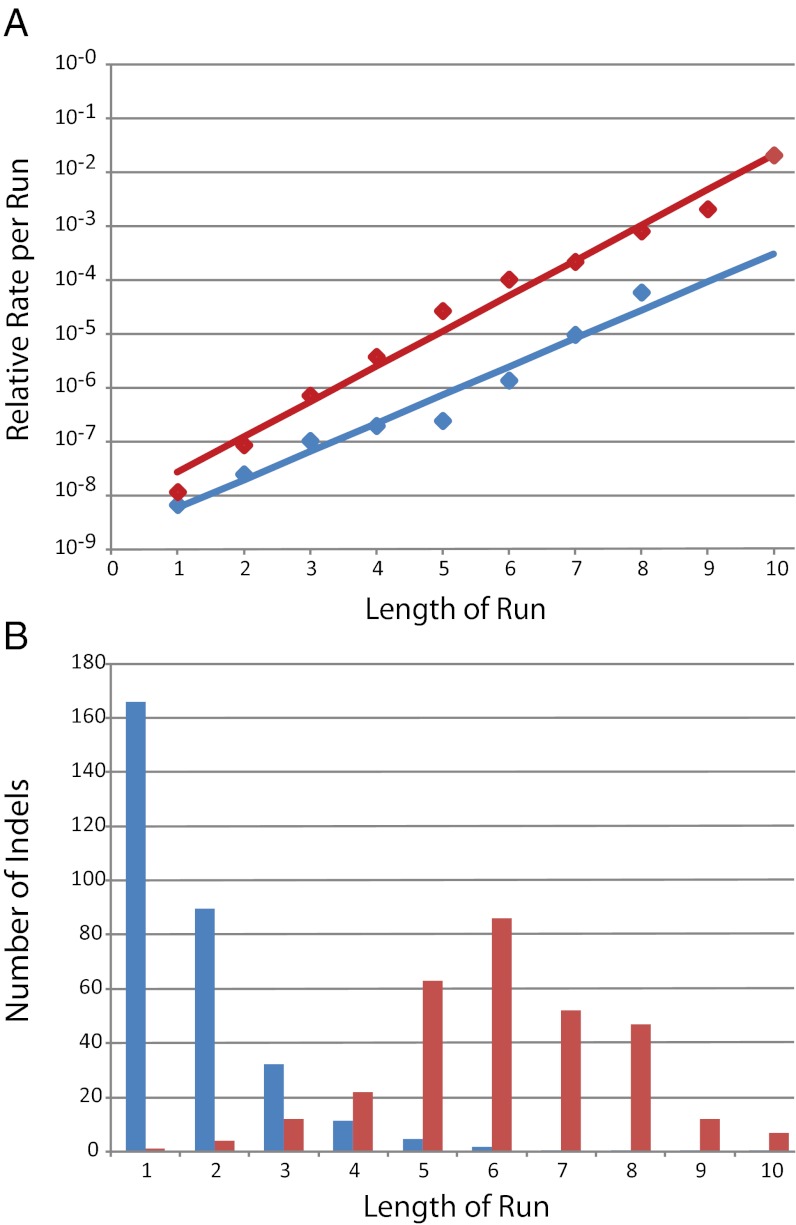
Indel formation at runs. (A) The relative mutation rate of indels in a run of a given length is plotted against the length of the run. The relative mutation rate of indels in each run length is the number of observed indels divided by the total number of target nucleotides (= nucleotides in the run × the number of runs of that length in the genome × the number of MA lines in the analysis). Blue diamonds represent the combined wild-type 3K and 6K datasets (21 indels); red diamonds represent the MutL− dataset (306 indels). Lines are the least-squared fits to the data. (B) Values for the MutL− dataset (306 indels). Blue bars represent the expected number of indels in a run of a given length calculated as the total number of indels obtained × the fraction of runs in the genome of that length. Red bars represent the actual number of indels observed in each run length.
To determine the probability of an indel occurring at a run of a given length, we multiplied the total number of indels observed by the fraction of nucleotides that are in runs of each length in the genome. Compared with the actual number of indels observed, any run ≥4 nt was a hotspot (Fig. 3B). However, certain runs may be more likely to sustain an indel than other runs of the same length. To identify these potential hotspots, we looked for runs in which more than one indel occurred, and these are given in Table S4.
Discussion
The major conclusions of the study presented here are (i) the best estimate of the spontaneous mutation rate of a wild-type E. coli strain growing on rich medium is 1 × 10−3 per genome per generation; (ii) in the absence of MMR, mutations are dominated by A:T > G:C transitions; (iii) A:T > G:C transitions occur preferentially with A templating the lagging strand and T templating the leading strand, whereas G:C > A:T transitions occur preferentially with C templating the lagging strand and G templating the leading strand; and (iv) there is a strong bias for transitions to occur at 5′ApC3′/3′TpG5′ sites and, to a lesser degree, at 5′GpC3′/3′CpG5′ sites.
In addition to these major points, our results identify GATC sites as hotspots for A:T transversion mutations. We extend to the whole genome the previous findings that CCAGG and CCTGG sites are hotspots for G:C transitions (41) and that indels occur preferentially at sequence repeats (40). However, although the rate of indels at repeats is high and increases exponentially with the length of a sequence run, the overall genomic rate of indels is only 1/10th that of BPSs.
Our finding that the mutation rate of this model prokaryote is one third of Drake’s estimate based on analyses of specific-locus experiments (1, 21) was unexpected. As pointed out by Drake (4), different estimates obtained from MA experiments and specific-locus methods may reflect selection against the mutations that accumulate during the former protocol, which, by the use of a carefully chosen reporter gene, are minimized in the latter. However, by several criteria the MA protocol followed here minimized selection: (i) the ratio of synonymous to nonsynonymous BPSs in the wild-type strain did not differ significantly from that expected by chance; (ii) the wild-type mutation rate based solely on synonymous BPSs differs little from that calculated based on all BPSs (see below); (iii) most synonymous BPSs resulted in less commonly used codons, indicating that these mutations were not selected against; and (iv) nonsense mutations were recovered at the expected frequency, indicating that these mutations also were not selected against.
Alternative explanations for the discrepancy are that the specific loci used by Drake (1, 21) to estimate the genomic mutation rate were not representative of the genome as a whole or that some of the approximations used were not appropriate for E. coli. In addition, the growth conditions used in the two types of experiments are very different. Most obviously, during MA experiments the cells grow in colonies, which can become microaerobic, whereas for specific-locus experiments cells typically are grown in well-aerated liquid cultures. Given these differences, perhaps it is remarkable that the two estimates are within threefold.
Recently Wielgoss et al. (3) estimated the E. coli genomic mutation rate based on synonymous BPSs occurring during long-term evolution experiments. Their value, 0.41 × 10−3 per genome per generation, is less than half the rate that we observed. The mutation rate based solely on the 55 synonymous BPSs in our wild-type dataset is 1.1 x10−3 per genome per generation. This value is close to the rate we obtained considering all the BPSs, and is nearly threefold higher than the value calculated by Wielgoss et al. (3). Drake (4) has argued that selection against synonymous codon changes in the long-term evolution experiments was sufficient to account for the low rate obtained by Wielgoss et al. We believe that the higher mutation rate that we obtained reflects the fact that the MA protocol truly minimizes selective pressure against mutations. The lowest mutation rate estimate for E. coli, 0.1–0.2 × 10−3 per genome per generation, comes from comparative genomics (42). Although the various contributions of selection, mutational bias, and experimental conditions appear sufficient to explain differences in experimentally determined mutation rates, there is as yet no consistent explanation for the difference between estimates based on comparative genomics and those derived from experimental evidence (2).
Although whole-genome sequencing can give an unparalleled view of mutational events across the entire genome, it cannot supersede the results obtained with more traditional methods. For example, except for highly frequent events, such as indels at repeat sequences, sequencing unselected genomes reveals only single mutational events. Cataloging events at similar sites in the genome allows aspects of mutagenic specificity to be deduced (see below) but does not identify true hotspots, i.e., specific sites that are particularly mutation-prone. Thus, the mutational topography revealed by single-locus studies is a necessary component of our understanding of mutational processes.
A striking result from our MA experiments is that, in the absence of MMR, the BPS spectrum of cells growing on rich medium is dominated by A:T > G:C transitions (Table 3 and Fig. 2). Because of individual idiosyncrasies of each mutational target, this bias had not been seen in many previous studies of MMR-defective microbes. Indeed, the observed bias in MMR-defective E. coli often was toward G:C > A:T mutations (43–45). Only when collections of mutations were sequenced, as in the mnt gene (46), the lacI gene (47), the rpoB gene (22), and the gyrA gene (23), was an A:T > G:C bias in MMR-defective strains revealed. Our data confirm and extend these results to the entire genome.
Mutational data from a variety of sources have confirmed that in almost all organisms spontaneous mutations are dominated by G:C > A:T transitions, a bias that tends to drive genomes toward greater A:T content (28). However, the G:C content of genomes varies widely, so some selective pressure or nonadaptive mechanism must drive genomes back toward G:C-richness. Because MMR corrects replication errors, the mutational spectrum in its absence reveals that errors made during replication would, if uncorrected, increase the genomic G:C content. Thus, the MMR system serves not only to keep mutation rates low but also to prevent genomes from drifting toward ever-higher G:C content. This observation suggests that the G:C/A:T balance of the genomes of different organisms might be determined, at least in part, by the functioning of their MMR system. In a recent survey of 699 bacterial genomes, about 20% lacked one or more genes in the MMR pathway, but there was no correlation with the G:C content of the genomes (48). Two caveats apply to these results: (i) other repair activities in the cell may compensate for the absence of MMR; and (ii), the presence of the MMR genes does not mean that the system is functioning to the same extent in each organism. Our results also have a forensic implication. Because the activity of the MMR varies by growth phase and is influenced by environmental conditions (19, 20), an organism’s mutational spectrum might be a fingerprint of its recent history.
Loss of MMR caused a larger increase in mutations in coding than in noncoding DNA (Table 3), suggesting that the MMR system is more active on the DNA that encodes genes. This difference could reflect the participation of the MMR proteins, including MutL, in transcription-coupled repair (49). However, contrary to a recent report (39), we found no bias for any specific mutational event to occur on the transcribed versus the nontranscribed DNA strand (Table S3); such a bias could reflect the activity of transcription-coupled repair. Nor did our data support previous conclusions that highly expressed genes are either vulnerable or resistant to mutation (37–39).
Our results shed light on the origins of spontaneous mutations. Deamination of cytosine to uracil frequently is cited as a cause of G:C > A:T transitions. The mutation rate of G:C > A:T transitions in the MutL− strain was 0.035 per genome per generation, which is 60 times the estimated deamination rate of cytosine in dsDNA and 10–20 times the estimated deamination rate of cytosine in ssDNA (Table S5). Adding to the low level of deamination are the activities of uracil glycosylase, which removes uracil from DNA, and mismatch uracil DNA glycosylase, which removes uracils paired with guanines. Thus, unless the rate of cytosine deamination is greatly underestimated, it cannot be responsible for the number of G:C > A:T mutations observed. However, the creation of thymines by deamination of 5meC is important. In the wild-type strain, 10% of the G:C > A:T transitions occurred at the C that is methylated by the Dcm methylase. Thus, our results support and extend to the whole genome the prescient finding that Dcm sites are mutational hotspots (41).
Mutations at Dcm sites can be prevented by very short patch (VSP) repair, an enzyme system that removes the T mispaired with C at these sites (reviewed in ref. 50). MutL participates in VSP repair, so it was surprising that the rate of G:C > A:T mutations at Dcm sites was increased only threefold in the MutL− strain. However, MutL facilitates but is not required for the relatively inefficient VSP repair (51, 52). Because G:C >A:T mutations were increased 100-fold overall in the MutL− strain, other sources of G:C > A:T mutations must occur at much higher frequencies than deamination of 5meC. Because MMR normally prevents these G:C > A:T transitions, mutations at Dcm sites become prevalent in wild-type cells.
Transitions arise from A:C and G:T mismatches, both of which are well corrected by the E. coli MMR system in vitro (53) and in vivo (54, 55). If both these mismatches occurred with equal frequency and irrespective of DNA strand, then loss of MMR would elevate both A:T > G:C and G:C > A:T transitions to the same extent. Therefore, the observed bias for A:T transitions in the MutL− strain implies either that the mismatches that lead to A:T transitions are replication errors that occur more frequently than those that lead to G:C transitions or that a separate error-correcting pathway efficiently and preferentially prevents G:C transitions. One candidate for this error-correcting function is the proofreading activity of the E. coli replicase DNA polymerase III, which resides in a separate subunit, epsilon. Whether error correction by epsilon is biased has been debated for years, but a recent study (56) showed a bias toward correcting G:C > A:T mutations that would account for our results. Another candidate is MutY, which can remove an A mispaired with C, preventing G:C > A:T mutations if C is the template (and promoting A:T > G:C mutations if A is the template) (57). Future MA studies will reveal if these enzymes contribute to mutational specificity in the absence of MMR.
Transitions arise when a purine mispairs with the wrong pyrimidine during replication. Long ago, such mispairings were postulated to result from tautomeric shifts in the bases (58, 59). Others have proposed mispairs involving ionized bases (60). Crucial to these schemes is that the postulated mispair match the geometry of the normal base pair, allowing it to fit within the DNA polymerase-active site for catalysis. Recently both A:C and the G:T mispairs have been captured in the active site of a crystallized polymerase (61, 62); the former involved amino:imino tautomers, and the latter probably involved ionized bases. In both cases the mismatched base pair had the canonical Watson–Crick geometry of the cognate base pair. One of the striking aspects of the MutL− data is that 79% of the A:T transitions occurred at sites with the sequence 5′ApC3′/3′TpG5′ (Table S2), a preference that has been observed in previous studies (22, 63). We favor the hypothesis that these transitions are templated by the A for the following reason: because polymerase approaches the template base from the 3′ side, a C:G base pair would be established first and, because of its strong base-pairing and stacking interactions, could stabilize the A:C mispair. In the alternative configuration, requiring a T:G mispair, the stabilizing G:C base pair would not be established until after the T:G mispair formed. The strong preference for C, not G, 3′ to the A, further suggests that the stabilizing base pair must have the correct orientation.
Another striking aspect of the MutL− data is the 2:1 bias for A:T transitions occurring with A in the top strand on the right replichore and T in the top strand in the left replichore. The purine:pyrimidine strand-bias for G:C transitions was the reverse: they were twice as likely to occur with C in the top strand on the right replichore and G in the top strand in the left replichore. This mutational pattern would tend to produce the well-known “keto-skew” of the E. coli chromosome (64); i.e., in the first half of the chromosome the top strand has more Gs and Ts, the bases with keto groups, and in the second half of the chromosome, the top strand has more As and Cs, the bases with amino groups. A similar mutational bias and keto-skew near replication origins has been described recently in S. cerevisiae (65). However, the bias in itself does not tell us which base gives rise to the mutation.
If A is the templating base for A:T transitions, as hypothesized above, the A/T strand bias would require that the mutational event be A templating the insertion of a C during lagging-strand synthesis. If the G/C strand bias reflects the same molecular constraints, then the G:C transitions would be produced by C templating insertion of an A during lagging-strand synthesis. Arguing against this hypothesis is the fact that G:C transitions did not show a strong bias for a particular base 3′ to the C. However, 74% of the G:C transitions occurred at sites with either C or G 3′ to the C (Table S2), suggesting that the C:A mispair could be stabilized by the formation of a 3′G:C base pair regardless of its strand orientation.
Although we favor the hypothesis that A and C are the templating bases and that the A:C mispair forms during lagging-strand synthesis, the alternative is that T and G template the G:T mispair during leading-strand synthesis. Then the mismatch would be stabilized by the G:C base pair formed after the mismatch. In support of this hypothesis, extension past a mismatch by only 4 nucleotides protects the mismatch from exonucleolytic proofreading (66). The hypothesis that the mismatch occurs during leading-strand synthesis also would agree with the often-stated conclusion that leading-strand synthesis is less accurate than lagging-strand DNA synthesis (67). However, although the fidelity of synthesis of the two strands may not be equal, the conclusion that synthesis of the leading strand is the less accurate one is based on primer-extension reactions in vitro and may not pertain in vivo.
Transversions made up 44% of the mutations in the wild-type strain. Because loss of MMR increased transversions only about 10-fold, compared with 200-fold for transitions, transversions were only a minor component of the MutL− spectrum. This result means that MMR is relatively inefficient in correcting the mismatches that lead to transversions and/or that other repair pathways are active in preventing transversions. For example, mutations that inactivate the enzymes that deal with oxidized guanines are powerful mutators, greatly increasing both A:T > C:G and G:C > T:A mutations (68). These two transversions accounted for 29% of the BPSs recovered in the wild-type strain, but their rates were increased only sixfold in the MutL− strain, suggesting that MMR is relatively blind to the mismatches that cause these mutations. As mentioned above, A:T transversions occurred preferentially at GATC sites where the A is methylated at the 6 position by the Dam methylase. GATC sites also were hotspots in the mutational spectrum of the rpoB gene (69). Depurinated bases lead to transversion mutations because for many DNA polymerases the preferred order of base insertion opposite a missing base is A followed by G, although this order is far from strict (33, 70). The estimated rate of depurination of 6meA is about 3 × 10−3 per E. coli genome per generation (Table S5), which is sufficient to account for the observed rate of A:T transversions at GATC sites in both the wild-type and the MutL− strains (0.09 × 10−3 and 2.4 × 10−3 per genome per generation, respectively).
Our data show that the rate of indel formation at mononucleotide runs increases exponentially with the length of the run and that the ability of MMR to correct these indels may increase with run length (Fig. 3A). Although we identified indel hotspots based on repeated occurrence, any run of 4 nucleotides or more is a potential hotspot (Fig. 3B). Because an indel is likely to inactivate a gene, there should be selection against runs in genes encoding important cell functions. On the other hand, genes that have significant runs could be dispensable; indeed, such genes may be “contingency loci,” able to be activated and inactivated by frequently occurring mutations (71). We inspected the list of genes in which indels were recovered to find those whose activation or inactivation might have a selective advantage under stressful conditions. Interestingly, we recovered two indels in dinB and one in umuC, the genes that encode E. coli’s two error-prone polymerases. The two runs in the dinB gene, of four and six Gs respectively, occur early in the coding sequence and thus indels in these runs certainly inactivate the gene. These runs are conserved in most E. coli and Shigella genomes in the database but are not conserved in Salmonella. The mutated run in the umuC gene, consisting of five As late in the coding sequence, likewise is conserved in most E. coli and Shigella genomes but not in Salmonella. Surprisingly, an indel was isolated midway in the mutT gene in a run of six Cs that is not well conserved even among E. coli strains. The phenotype of mutT mutants is an increase in G:C > TA mutations (68); because the MA strain carrying this mutation did not accumulate any G:C > TA mutations, the indel must have occurred late in the MA experiment. Other mutated genes of interest are xthA, which encodes an exonuclease involved in base-excision repair, and alkB, which encodes an enzyme involved in the repair of alkylation DNA damage. A recent survey of bacterial genomes found that hundreds of species had repeat sequences in MMR genes in which indels could act as genetic switches to change the mutation rates of these microbes (72). Thus, in addition to mechanisms that modulate the activity of DNA repair enzymes, microbes also may have adaptive or serendipitous mechanisms that genetically alter their DNA repair capabilities, and thus their mutation rates, allowing them to respond to changes in environmental conditions.
Materials and Methods
Bacterial Strains and Media.
The wild-type strain PFM2 is a prototrophic derivative of the E. coli K12 reference strain MG1655 (73, 74). The MutL strain PFM5 is a derivative of PFM2 with the mutL gene replaced by an in-frame scar sequence (75). Details of the genetic techniques and media used are given in SI Materials and Methods.
Estimation of Mutation Rates by Fluctuation Tests.
Mutation rates to NalR or RifR were estimated using fluctuation tests as described (76). Further details are given in SI Materials and Methods.
MA Procedure.
MA lines originated from single colonies isolated on LB agar plates; each day each line was streaked for single colonies on an LB agar plate, two lines per plate, and incubated at 37 °C for 24 h. To avoid bias, the colony chosen for passage was a well-isolated one closest to a line drawn down the center of the plate. Originally 100 lines of the wild-type strain and 70 lines of the MutL− strain were started, but losses occurred during passage (when a single colony was not obtained), DNA preparation, library construction, and because of lineage ambiguities (see Shared Mutation Analysis below). Each of these steps accounted for a reduction of about 20%, resulting in the number of lines given in Table 1. The losses were random and so should not affect the results. In no case was a line lost because it went extinct. Further details are given in SI Materials and Methods.
Estimation of Generations and Cell Viability in Colonies.
The number of generations per passage, estimated from the number of cells in colonies of a given diameter, usually was 28 generations. The fraction of dead cells in resuspended colonies determined with the Live/Dead BacLight Bacterial Viability Kit (Invitrogen, Inc) was 0.05 ± 0.01 (mean ± SEM). Further details are given in SI Materials and Methods.
Genomic DNA Preparation.
The PureLink Genomic DNA purification kit (Invitrogen Corp.) was used to purify genomic DNA from 0.5–1 mL of overnight LB cultures inoculated from the freezer stocks. DNA concentration and purity were assessed using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Inc.).
Sequencing and Quality Control.
Sequencing was performed at Beijing Genome Institute (BGI) using the Illumina HiSeq2000 platform. For each MA line, ~101 (470 Mbp) paired-end reads (2 × 90 bp) were retained after discarding reads that did not meet BGI’s quality control. Reads with any one of the following characteristics were discarded: (i) ≥10% unreadable bases; (ii) ≥20% low-quality (≤Q20) bases; (iii) adapter contamination (≥15-bp overlap allowing up to 3-bp mismatch); or (iv) duplicate read-pairs. After such filtering an average of 91.1% of reads were retained.
SNP and Short Indel Calling.
The reference genome sequence was NCBI Reference Sequence NC_000913.2. For each sample, Illumina reads were aligned to the E. coli K12 (strain MG1655) genome with the short read alignment tool, BWA (ver. 0.5.9) (77). Short indels (≤4 bp) were called based on the read mapping of the SNP calling procedures. Further details are given in SI Materials and Methods.
Mutation Confirmation by Conventional Sequencing.
We randomly selected 19 BPSs from the wild-type 3K MA lines (~20% of the total) for confirmation. Because indels generally are more challenging to call accurately than SNPs (78), we checked all 10 of the small indels called from the wild-type 3K MA line. There were no false calls in the BPS dataset. One indel that had been called as −G actually was a deletion of 24 nt in a repeat element; all other indel calls were correct. Further details are given in SI Materials and Methods.
Mutation Annotation.
Several custom scripts were written to annotate candidate variants. The coordinates of protein-coding genes were obtained from the GenBank page of NCBI reference sequence NC_000913.2. BPSs were determined to be synonymous, nonsynonymous, or noncoding based on the genetic code. Nonsynonymous BPSs were designated as conservative or nonconservative based on the BLOSUM62 matrix (79); a value ≥0 was considered conservative.
Shared Mutation Analysis.
The wild-type and MutL− strains experienced ~80 and ~75 generations of growth, respectively, as their phenotypes were checked and as they were frozen and regrown before the first bottleneck for the MA protocol. Thus, MA lines could share mutations that occurred during this period. Sequence alignment and lineage analysis were used to distinguish mutations that arose independently from those that shared a common ancestor. Further details are given in SI Materials and Methods.
Monte Carlo Simulations.
A custom script was written to simulate a random distribution of BPSs corresponding to the observed mutational spectra. For simplicity, the number of mutations was fixed at the observed numbers; 1,000 trials were simulated.
Acknowledgments
We thank D. Osiecki, D. Simon, K. Storvik, J. Townes, N. Yahaya, and A. Ying Yi Tang for valuable technical assistance; members of the Lynch laboratory for useful discussions; and E. Eisenstadt, S. Finkel, A. Hanson, M. Lynch, and the anonymous reviewers for helpful comments on this manuscript. This research was supported by Multidisciplinary University Research Initiative Award W911NF-09-1-0444 from the US Army Research Office (to P.L.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences and mutations reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive, http://www.ncbi.nlm.nih.gov/sra (accession nos. SRA054030 and SRA054031).
See Author Summary on page 16416 (volume 109, number 41).
This article contains supporting information online at www.pnas.org/lookup/suppl/10.1073/pnas.1210309109/-/DCSupplemental.
References
Author Summary
Author Summary
Mutations, heritable changes in the sequence of the genome, are the source of variation upon which natural selection acts. To understand evolution fully, one needs to know the rate at which mutations occur and the molecular nature of spontaneous mutational changes. Additional important questions include (i) is the genome uniformly susceptible to mutation, or are there mutable and immutable sites, and (ii) are mutation rates constant or are they driven by changing intrinsic and extrinsic factors. The goal of the present study was to establish baseline parameters in the model prokaryote Escherichia coli by analyzing the spontaneous mutations that accumulate in a neutral fashion, unbiased by selective pressures. We also compared these mutations with those accumulating in a strain defective in mismatch repair (MMR), an important DNA repair pathway. We reached the following major conclusions: (i) the spontaneous mutation rate is about threefold lower than previously reported; and (ii) MMR is a major determinant of the types of mutational changes that occur and of the base composition of the genome.
To analyze spontaneous mutations free of selective pressures, we used a mutation-accumulation (MA) strategy combined with whole-genome DNA sequencing. The basic MA protocol starts by establishing a number of lines of clonal populations derived from a founder individual; each line then is passed through repeated single-individual bottlenecks for thousands of generations. With microorganisms, streaking for single colonies accomplishes the bottleneck. Because the effective population size of each line is one, genetic drift prevents selection from eliminating all but the most deleterious mutations (which are rare in any case). By sequencing the genomes of many such MA lines grown on rich medium, we determined the rate of spontaneous mutation for E. coli to be 1 × 10−3 per genome per generation (Table P1). This rate is a third of that expected based on mutation rates at specific genetic loci (1) but is more than twice the rate estimated from synonymous mutations that accumulate during long-term evolution experiments (2).
The activities of various enzymes that repair DNA are major determinants of the spontaneous mutation rate. Chief among these activities is MMR, which surveys newly replicated DNA, detects mismatched bases, recruits enzymes that destroy the new DNA strand, and forces repolymerization using the old DNA strand as template. Thus, the mutations that occur in the absence of MMR are likely to be the direct result of replication errors. For this reason, we performed a parallel MA experiment with an MMR-defective strain in which the mutation rate is increased more than 100-fold (Table P1). The result is a collection of mutations large enough to produce a detailed picture of genomic mutagenic processes. An important conclusion from the analysis of nearly 2,000 mutations is that in the absence of MMR mutations are biased toward changing A:T base pairs to G:C base pairs, the opposite of the bias seen in the wild-type bacteria.
Table P1.
Parameters and results of the mutation accumulation experiments
| Strain | No. of BPSs | No. of Indels* | No. of lines | Generations per line | Total no. of generations | BPSs per line | Indels per line | Mutation rate per nucleotide (×1010) | Mutation rate per genome (×103) |
| Wild-type 3K | 93 | 9 | 38 | 3,080 | 117,040 | 2.45 | 0.24 | 1.88 | 0.87 |
| Wild-type 6K | 140 | 12 | 21 | 6,356 | 133,476 | 6.67 | 0.57 | 2.45 | 1.14 |
| Wild-type, mean | — | — | — | — | — | — | — | 2.2 | 1.0 |
| MutL | 1,625 | 306 | 34 | 375 | 12,750 | 47.8 | 9.00 | 326 | 151 |
The first two rows give the results of a two-part MA experiment with the wild-type strain. In the first part (labeled “Wild-type 3K”), MA lines were passaged on rich medium for about 3,000 generations; in the second part (labeled “Wild-type 6K”), additional lines were passaged similarly for another 3,000 generations. Although the 6K lines accumulated more mutations per generation than did the 3K lines, the two estimates of the spontaneous mutation rate are within 95% confidence limits of each other. Both parts of the experiment gave results that appear to be random and nonbiased; thus, the best estimate of the mutation rate per generation is the midpoint, 1.0 × 10−3 mutations per genome or 2.2 × 10−10 mutations per nucleotide. The fourth row gives the parameters and results of a parallel MA experiment with the MMR-defective strain, MutL, whose mutation rate was increased 150 times relative to the wild-type strain. BPSs, base-pair substitutions.
*Insertion or deletion of four or fewer nucleotides.
The molecular spectrum of spontaneous base-pair substitutions in almost all organisms is dominated by G:C to A:T changes, which tend to drive genomes toward higher AT content (3). Because the GC content of genomes varies widely, there must be some selective pressure or nonadaptive mechanism that drives genomes back toward increased GC content. Our results suggest that MMR not only serves to keep mutation rates low but also may be a major determinant in maintaining the GC/AT balance of the genome.
MMR is highly conserved and is found in all domains of life, but bacterial strains isolated from nature sometimes lack MMR capacity, suggesting that modulation of MMR may be adaptive under certain environmental conditions. In addition, the activity of MMR is regulated in response to intrinsic and extrinsic factors. Thus, by combining the results of the MMR-defective strain with those of wild type, we begin to achieve a deeper understanding of the factors that determine mutation rates, mutational spectra, genomic base composition, how these factors may differ among organisms, and how they may be shaped by environmental conditions.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.1210309109
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/109/41/E2774.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.1210309109
Article citations
Evolution of pH-sensitive transcription termination in <i>Escherichia coli</i> during adaptation to repeated long-term starvation.
Proc Natl Acad Sci U S A, 121(39):e2405546121, 19 Sep 2024
Cited by: 0 articles | PMID: 39298488 | PMCID: PMC11441560
The Effect of the Presence and Absence of DNA Repair Genes on the Rate and Pattern of Mutation in Bacteria.
Genome Biol Evol, 16(10):evae216, 01 Oct 2024
Cited by: 0 articles | PMID: 39376054 | PMCID: PMC11493085
Mutant fate in spatially structured populations on graphs: Connecting models to experiments.
PLoS Comput Biol, 20(9):e1012424, 06 Sep 2024
Cited by: 0 articles | PMID: 39241045 | PMCID: PMC11410244
Synonymous rpsH variants: the common denominator in Escherichia coli adapting to ionizing radiation.
NAR Genom Bioinform, 6(3):lqae110, 24 Aug 2024
Cited by: 0 articles | PMID: 39184377 | PMCID: PMC11344242
Clinically relevant mutations in regulatory regions of metabolic genes facilitate early adaptation to ciprofloxacin in Escherichia coli.
Nucleic Acids Res, 52(17):10385-10399, 01 Sep 2024
Cited by: 0 articles | PMID: 39180403 | PMCID: PMC11417348
Go to all (398) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (2)
- (2 citations) ENA - SRA054031
- (2 citations) ENA - SRA054030
RefSeq - NCBI Reference Sequence Database
- (2 citations) RefSeq - NC_000913.2
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
DNA Replication-Transcription Conflicts Do Not Significantly Contribute to Spontaneous Mutations Due to Replication Errors in Escherichia coli.
mBio, 12(5):e0250321, 12 Oct 2021
Cited by: 5 articles | PMID: 34634932 | PMCID: PMC8510543
Determinants of Base-Pair Substitution Patterns Revealed by Whole-Genome Sequencing of DNA Mismatch Repair Defective Escherichia coli.
Genetics, 209(4):1029-1042, 15 Jun 2018
Cited by: 19 articles | PMID: 29907647 | PMCID: PMC6063221
Directionality of DNA replication fork movement strongly affects the generation of spontaneous mutations in Escherichia coli.
J Mol Biol, 307(5):1195-1206, 01 Apr 2001
Cited by: 36 articles | PMID: 11292335
Insights from a decade of biophysical studies on MutL: Roles in strand discrimination and mismatch removal.
Prog Biophys Mol Biol, 117(2-3):149-156, 18 Feb 2015
Cited by: 21 articles | PMID: 25701376
Review





