Abstract
Free full text

Direct transfer of cloned genes from bacteria to mammalian cells.
Abstract
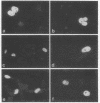
Induction of a virus infection by cloned simian virus 40 DNA was chosen as a test system to detect transfer of genes from bacteria to cultured mammalian cells. Escherichia coli cells containing a recombinant plasmid with three tandem inserts of simian virus 40 DNA were able to infect CV-1 monkey cells under various conditions. The gene transfer was resistant to DNase I and therefore seems not to occur via free DNA but most likely via uptake of whole bacteria, followed by release of plasmid DNA and generation of infectious circular simian virus 40 DNA in a recombination-excision process. Spontaneous transfer was found to be infrequent, 4 x 10(9) bacteria yielding one infection per 10(7) monkey cells. The frequency was greatly increased by adding bacteria as a calcium phosphate coprecipitate or by fusion of lysozyme-treated bacteria (protoplasts) with monkey cells in the presence of polyethylene glycol. With the latter technique, 10(4) protoplasts gave rise to one infection per 15 monkey cells. Experiments with other cell lines of human, monkey, and mouse origin, and also with bacteria harboring another recombinant plasmid, indicate that DNA transfer from bacteria to mammalian cells is a general phenomenon.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
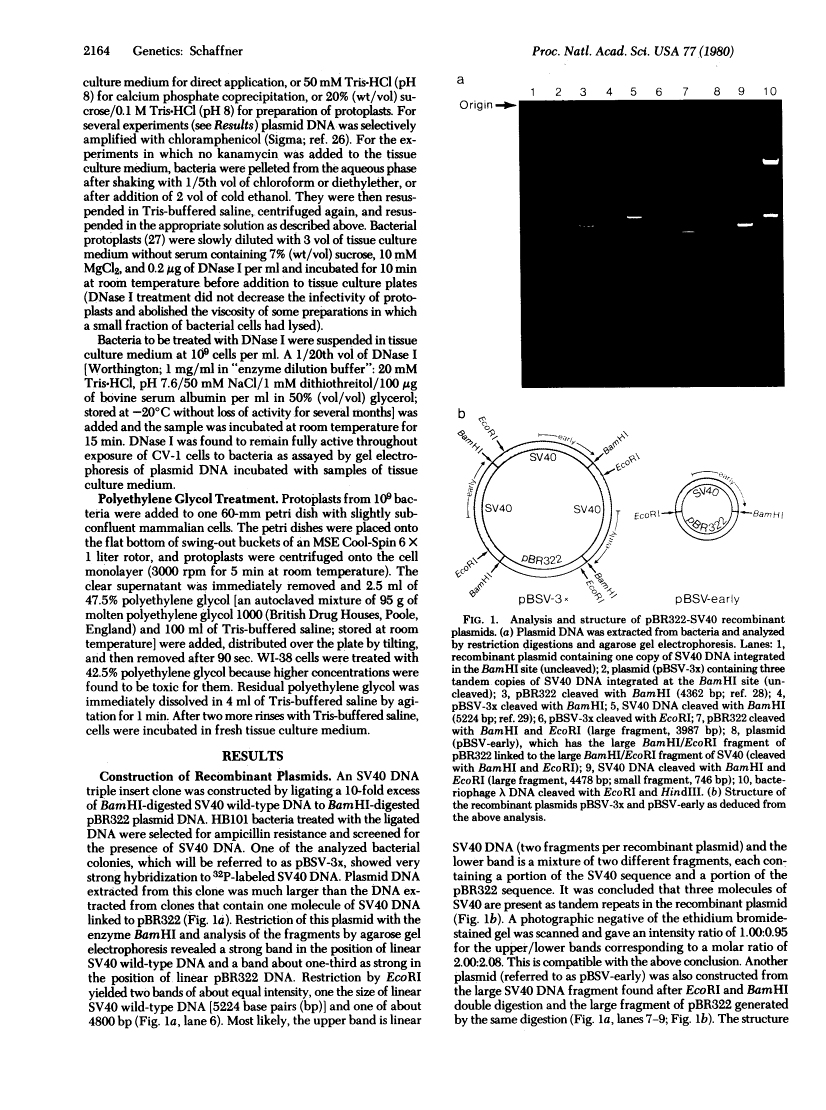
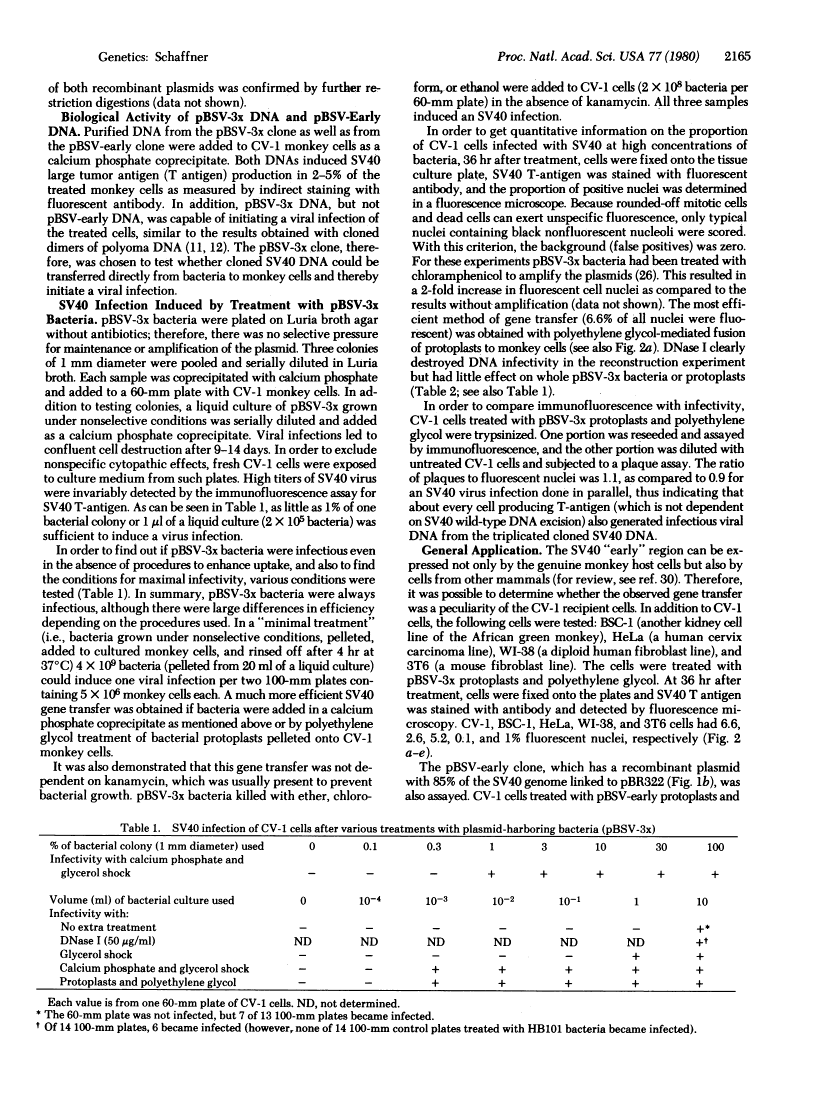
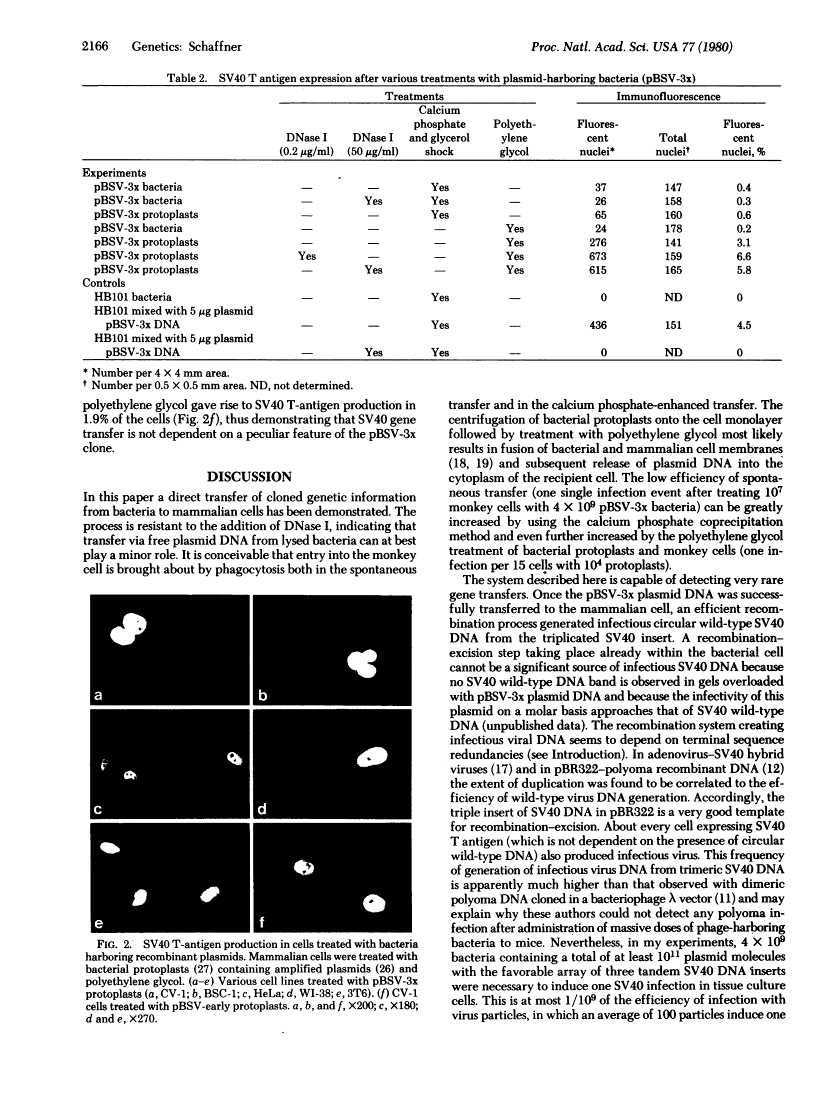
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimayorca GA, Eddy BE, Stewart SE, Hunter WS, Friend C, Bendich A. ISOLATION OF INFECTIOUS DEOXYRIBONUCLEIC ACID FROM SE POLYOMA-INFECTED TISSUE CULTURES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1805–1808. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. [Abstract] [Google Scholar]
- Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. [Abstract] [Google Scholar]
- Mantei N, Boll W, Weissmann C. Rabbit beta-globin mRNA production in mouse L cells transformed with cloned rabbit beta-globin chromosomal DNA. Nature. 1979 Sep 6;281(5726):40–46. [Abstract] [Google Scholar]
- ORTH G, ATANASIU P, BOIRON M, REBIERE JP, PAOLETTI C. INFECTIOUS AND ONCOGENIC EFFECT OF DNA EXTRACTED FROM CELLS INFECTED WITH POLYOMA VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:1090–1095. [Abstract] [Google Scholar]
- Israel MA, Chan HW, Rowe WP, Martin MA. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. [Abstract] [Google Scholar]
- Chan HW, Israel MA, Garon CF, Rowe WP, Martin MA. Molecular cloning of polyoma virus DNA in Escherichia coli: lambda phage vector system. Science. 1979 Mar 2;203(4383):887–892. [Abstract] [Google Scholar]
- Fried M, Klein B, Murray K, Greenaway P, Tooze J, Boll W, Weissmann C. Infectivity in mouse fibroblasts of polyoma DNA integrated into plasmid pBR322 or lambdoid phage DNA. Nature. 1979 Jun 28;279(5716):811–816. [Abstract] [Google Scholar]
- Botchan M, Topp W, Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. [Abstract] [Google Scholar]
- Botchan M, Topp W, Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. [Abstract] [Google Scholar]
- Birg F, Dulbecco R, Fried M, Kamen R. State and organization of polyoma virus DNA sequences in transformed rat cell lines. J Virol. 1979 Feb;29(2):633–648. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelly TJ, Jr, Lewis AM, Jr, Levine AS, Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. [Abstract] [Google Scholar]
- Kennett RH. Cell fusion. Methods Enzymol. 1979;58:345–359. [Abstract] [Google Scholar]
- Mertz JE, Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. [Abstract] [Google Scholar]
- Copeland NG, Cooper GM. Transfection by exogenous and endogenous murine retrovirus DNAs. Cell. 1979 Feb;16(2):347–356. [Abstract] [Google Scholar]
- Kimura G, Dulbecco R. Isolation and characterization of temperature-sensitive mutants of simian virus 40. Virology. 1972 Aug;49(2):394–403. [Abstract] [Google Scholar]
- Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [Abstract] [Google Scholar]
- Morrow JF, Cohen SN, Chang AC, Boyer HW, Goodman HM, Helling RB. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. [Europe PMC free article] [Abstract] [Google Scholar]
- Grunstein M, Hogness DS. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. [Europe PMC free article] [Abstract] [Google Scholar]
- Hershfield V, Boyer HW, Yanofsky C, Lovett MA, Helinski DR. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss RL. Methods for protoplast formation in Escherichia coli. Methods Cell Biol. 1978;20:141–147. [Abstract] [Google Scholar]
- Sutcliffe JG. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. [Abstract] [Google Scholar]
- Fiers W, Contreras R, Haegemann G, Rogiers R, Van de Voorde A, Van Heuverswyn H, Van Herreweghe J, Volckaert G, Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. [Abstract] [Google Scholar]
- Butel JS, Tevethia SS, Melnick JL. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res. 1972;15:1–55. [Abstract] [Google Scholar]
- Graham FL, Abrahams PJ, Mulder C, Heijneker HL, Warnaar SO, De Vries FA, Fiers W, Van Der Eb AJ. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. [Abstract] [Google Scholar]
- Graham FL. Biological activity of tumor virus DNA. Adv Cancer Res. 1977;25:1–51. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.77.4.2163
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc348672?pdf=render
Citations & impact
Impact metrics
Article citations
Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives.
Vaccines (Basel), 12(9):968, 27 Aug 2024
Cited by: 0 articles | PMID: 39340000 | PMCID: PMC11435753
Review Free full text in Europe PMC
Bacteria and cells as alternative nano-carriers for biomedical applications.
Expert Opin Drug Deliv, 19(1):103-118, 25 Jan 2022
Cited by: 5 articles | PMID: 35076351 | PMCID: PMC8802895
Review Free full text in Europe PMC
Mycoplasma pneumoniae Infections: Pathogenesis and Vaccine Development.
Pathogens, 10(2):119, 25 Jan 2021
Cited by: 45 articles | PMID: 33503845 | PMCID: PMC7911756
Review Free full text in Europe PMC
Recent advances in delivery of veterinary DNA vaccines against avian pathogens.
Vet Res, 50(1):78, 10 Oct 2019
Cited by: 24 articles | PMID: 31601266 | PMCID: PMC6785882
Review Free full text in Europe PMC
Increasing the bactofection capacity of a mammalian expression vector by removal of the f1 ori.
Cancer Gene Ther, 26(7-8):183-194, 13 Aug 2018
Cited by: 8 articles | PMID: 30100607 | PMCID: PMC6760541
Go to all (123) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Methods for molecular cloning in eukaryotic cells.
Methods Enzymol, 68:357-375, 01 Jan 1979
Cited by: 0 articles | PMID: 232221
Isolation of mutants of an animal virus in bacteria.
Science, 209(4463):1392-1396, 01 Sep 1980
Cited by: 237 articles | PMID: 6251547
Characterization and biological activity of cloned simian virus 40 DNA fragments.
J Biol Chem, 256(12):6469-6474, 01 Jun 1981
Cited by: 58 articles | PMID: 6263916
Gene transfer and gene mapping in mammalian cells in culture.
In Vitro, 16(1):55-76, 01 Jan 1980
Cited by: 10 articles | PMID: 6245032
Review