Abstract
Free full text

Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end.
Abstract
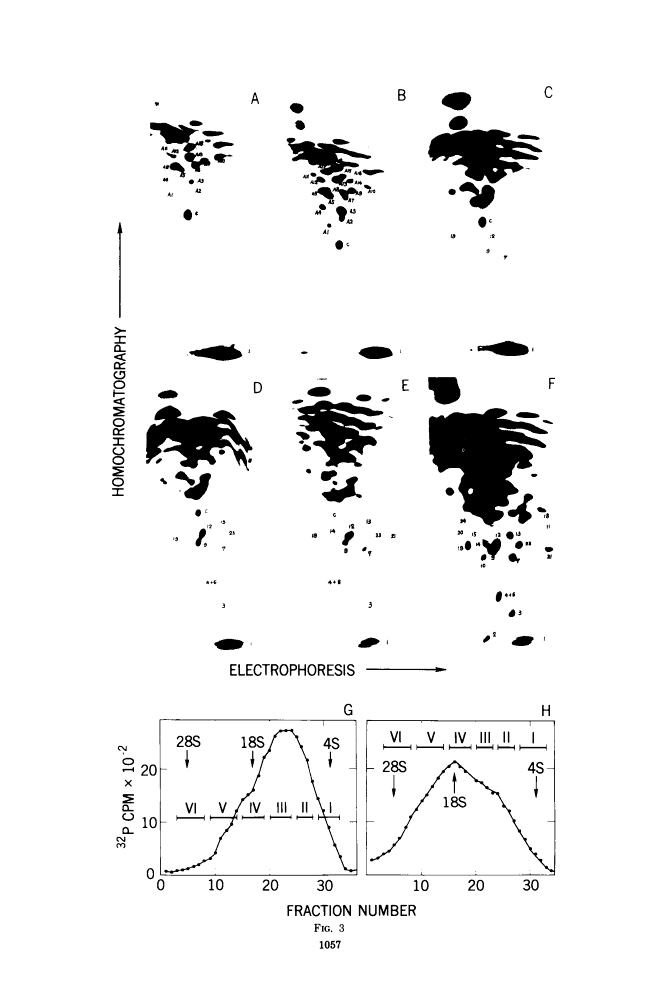
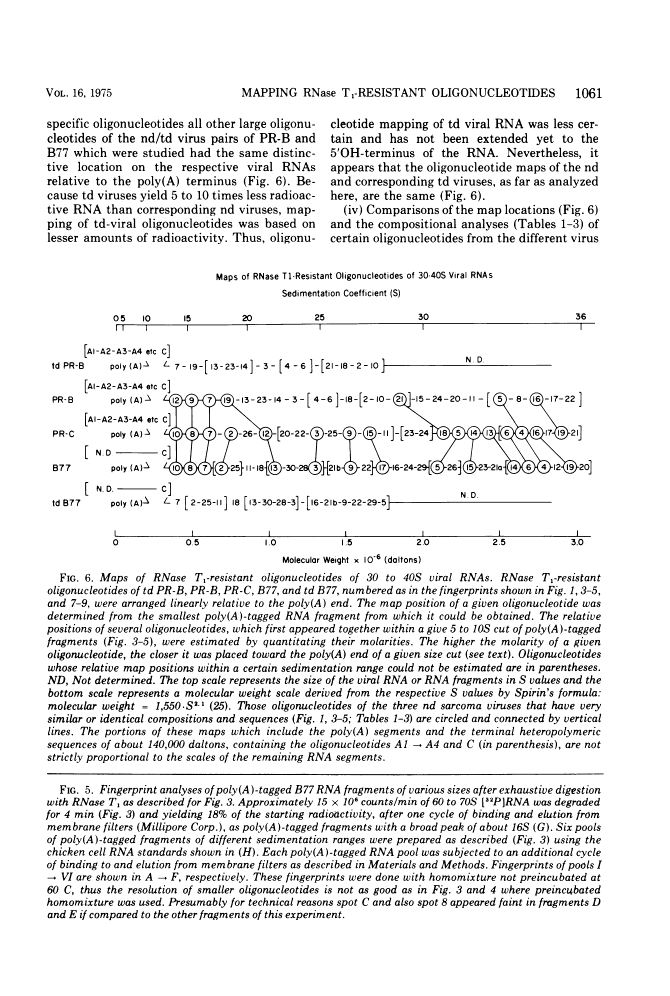
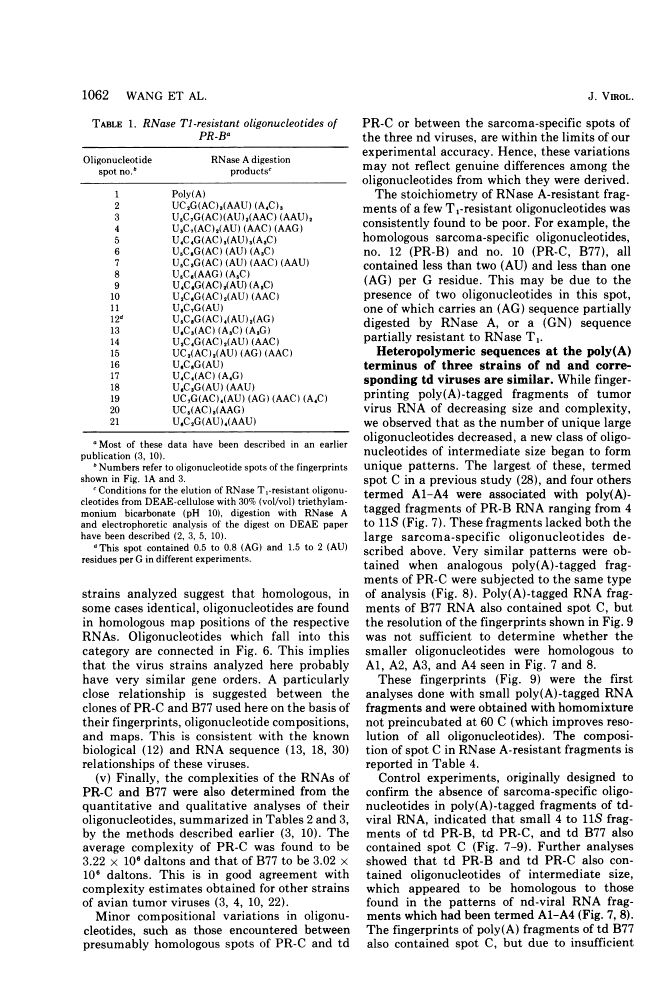
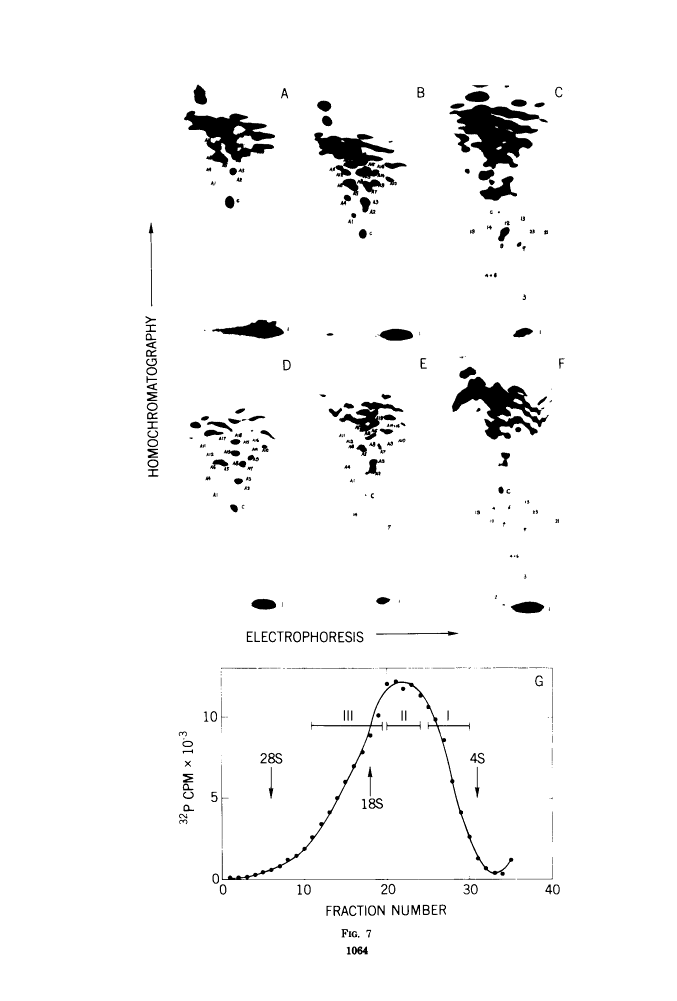
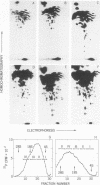
The large RNase T1-resistant oligonucleotides of the nondefective (nd) Rous sarcoma virus (RSV): Prague RSV of subgroup B (PR-B), PR-C and B77 of subgroup C; of their transformation-defective (td0 deletion mutants: td PR-B, td PR-C, and td B77; and of replication-defective (rd) RSV(-) were completely or partially mapped on the 30 to 40S viral RNAs. The location of a given oligonucleotide relative to the poly(A) terminus of the viral RNAs was directly deduced from the smallest size of the poly(A)-tagged RNA fragment from which it could be isolated. Identification of distinct oligonucleotides was based on their location in the electrophoretic/chromatographic fingerprint pattern and on analysis of their RNase A-resistant fragments. The following results were obtained. (i) The number of large oligonucleotides per poly(A)-tagged ffagment increased with increasing size of the fragment. This implies that the genetic map is linear and that a given RNase T1-resistant oligonucleotides has, relative to the poly(A) end, the same location on all 30 to 40S RNA subunits of a given 60 to 70S viral RNA complex, (ii) Three sarcoma-specific oligonucleotides were identified in the RNAs of Pr-B, PR-C and B77 by comparison with the RNAs of the corresponding td viruses...
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. [Abstract] [Google Scholar]
- Beemon K, Duesberg P, Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. [Europe PMC free article] [Abstract] [Google Scholar]
- Billeter MA, Parsons JT, Coffin JM. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. [Europe PMC free article] [Abstract] [Google Scholar]
- Brownlee GG, Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. [Abstract] [Google Scholar]
- Delius H, Duesberg PH, Mangel WF. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. [Abstract] [Google Scholar]
- Duesberg PH, Vogt PK. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. [Europe PMC free article] [Abstract] [Google Scholar]
- Duesberg PH, Vogt PK. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. [Abstract] [Google Scholar]
- Duesberg PH, Vogt PK. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. [Europe PMC free article] [Abstract] [Google Scholar]
- Duesberg P, Vogt PK, Beemon K, Lai M. Avian RNA tumor viruses: mechanism of recombination and complexity of the genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):847–857. [Abstract] [Google Scholar]
- Duesberg PH, Kawai S, Wang LH, Vogt PK, Murphy HM, Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. [Europe PMC free article] [Abstract] [Google Scholar]
- Duff RG, Vogt PK. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. [Abstract] [Google Scholar]
- Kang CY, Temin HM. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawai S, Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. [Abstract] [Google Scholar]
- Kawai S, Yamamoto T. Isolation of different kinds of non-virus producing chick cells transformed by Schmidt-Ruppin strain (subgroup A) of Rous sarcoma virus. Jpn J Exp Med. 1970 Aug;40(4):243–256. [Abstract] [Google Scholar]
- Kung HJ, Bailey JM, Davidson N, Vogt PK, Nicolson MO, McAllister RM. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. [Abstract] [Google Scholar]
- Lai MM, Duesberg PH. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. [Abstract] [Google Scholar]
- Lai MM, Duesberg PH, Horst J, Vogt PK. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. [Europe PMC free article] [Abstract] [Google Scholar]
- Mangel WF, Delius H, Duesberg PH. Structure and molecular weight of the 60-70S RNA and the 30-40S RNA of the Rous sarcoma virus. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4541–4545. [Europe PMC free article] [Abstract] [Google Scholar]
- Martin GS, Duesberg PH. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. [Abstract] [Google Scholar]
- Neiman PE, Wright SE, McMillin C, MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. [Europe PMC free article] [Abstract] [Google Scholar]
- Quade K, Smith RE, Nichols JL. Evidence for common nucleotide sequences in the RNA subunits comprising Rous sarcoma virus 70 S RNA. Virology. 1974 Sep;61(1):287–291. [Abstract] [Google Scholar]
- Quade K, Smith RE, Nichols JL. Poly(riboadenylic acid) and adjacent nucleotides in Rous sarcoma virus RNA. Virology. 1974 Nov;62(1):60–70. [Abstract] [Google Scholar]
- Shoyab M, Markham PD, Baluda MA. Host induced alteration of avian sarcoma virus B-77 genome. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1031–1035. [Europe PMC free article] [Abstract] [Google Scholar]
- Vogt PK. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. [Abstract] [Google Scholar]
- Wang LH, Duesberg PH. DNA polymerase of murine sarcoma-leukemia virus: lack of detectable RNase H and low activity with viral RNA and natural DNA templates. J Virol. 1973 Dec;12(6):1512–1521. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang LH, Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. [Europe PMC free article] [Abstract] [Google Scholar]
- Weissmann C, Parsons JT, Coffin JW, Rymo L, Billeter MA, Hofstetter H. Studies on the structure and synthesis of Rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1043–1056. [Abstract] [Google Scholar]
- Wright SE, Neiman PE. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.16.4.1051-1070.1975
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/16/4/1051.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/16/4/1051
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/16/4/1051
Citations & impact
Impact metrics
Citations of article over time
Article citations
Discovery of oncogenes: The advent of molecular cancer research.
Proc Natl Acad Sci U S A, 112(50):15259-15260, 07 Dec 2015
Cited by: 17 articles | PMID: 26644573 | PMCID: PMC4687565
Oncogenes and the revolution in cancer research: homage to hidesaburo hanafusa (1929-2009).
Genes Cancer, 1(1):6-11, 01 Jan 2010
Cited by: 6 articles | PMID: 21779424 | PMCID: PMC3092171
Transformation-defective mutants with 5' deletions of the src gene are frequently generated during replication of Rous sarcoma virus in established quail fibroblasts.
Virology, 177(2):505-514, 01 Aug 1990
Cited by: 3 articles | PMID: 2164723
Sequences from myeloblastosis-associated virus MAV-2(O) and UR2AV involved in the formation of plaques and the induction of osteopetrosis, anemia, and ataxia.
J Virol, 65(1):23-30, 01 Jan 1991
Cited by: 3 articles | PMID: 1845886 | PMCID: PMC240485
Isolation of the REC1 gene controlling recombination in Ustilago maydis.
Gene, 85(2):335-341, 01 Dec 1989
Cited by: 20 articles | PMID: 2628171
Go to all (171) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization.
Proc Natl Acad Sci U S A, 70(8):2266-2270, 01 Aug 1973
Cited by: 42 articles | PMID: 4365699 | PMCID: PMC433715
RNA of replication-defective strains of Rous sarcoma virus.
Proc Natl Acad Sci U S A, 72(4):1569-1573, 01 Apr 1975
Cited by: 29 articles | PMID: 165514 | PMCID: PMC432579
Properties and location of poly(A) in Rous sarcoma virus RNA.
J Virol, 14(6):1515-1529, 01 Dec 1974
Cited by: 70 articles | PMID: 4372409 | PMCID: PMC355682
Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus.
Proc Natl Acad Sci U S A, 73(2):447-451, 01 Feb 1976
Cited by: 64 articles | PMID: 174108 | PMCID: PMC335926