Abstract
Free full text

Klebsiella pneumoniae Antimicrobial Drug Resistance, United States, 1998–2010
Abstract
We studied antimicrobial-resistant Klebsiella pneumoniae for 1998–2010 by using data from The Surveillance Network. Susceptibility results (n = 3,132,354) demonstrated significant increases in resistance to all antimicrobial drugs studied, except tetracycline. Cross-resistance among carbapenem-resistant K. pneumoniae was lower for tetracycline and amikacin.
Klebsiella spp. are among the most common pathogens isolated in intensive care units (ICUs), and K. pneumoniae is the most frequently encountered carbapenemase-producing Enterobacteriaceae (1). Increasing antimicrobial drug resistance, including carbapenem-resistant K. pneumoniae (CRKP), accounts for substantial increases in illness and death (1). Few antimicrobial therapy options exist for infections caused by CRKP (2).
The emergence of K. pneumoniae resistance to carbapenems is well documented (3). However, few studies have analyzed the trends and prevalence of in vitro K. pneumoniae antimicrobial drug resistance since carbapenem resistance emerged in the United States during the late 1990s (4). Furthermore, few investigations have examined antimicrobial drug resistance with regard to specimen source or cross-resistance patterns among CRKP.
We examined the prevalence of K. pneumoniae antimicrobial drug resistance in US inpatients using a large national surveillance system. Our objectives were to analyze K. pneumoniae antimicrobial drug resistance among US inpatients, resistance patterns by specimen source, and cross-resistance among imipenem-resistant K. pneumoniae isolates.
The Study
We examined inpatients’ antimicrobial susceptibility test results from The Surveillance Network (TSN) Database-USA (Eurofins Medinet, Chantilly, VA, USA) for 1998–2010. TSN is a nationally representative repository of antimicrobial susceptibility results from ≈200 community, government, and university health care institutions in the United States and has been used in investigations of trends and prevalences of antimicrobial drug resistance (5). Susceptibility testing of isolates is conducted onsite by using Food and Drug Administration (FDA)–approved testing methods and interpreted by using Clinical Laboratory Standards Institute breakpoint criteria for all agents except tigecycline, for which FDA breakpoints were used. Details of quality control in TSN Database-USA have been described (6). No institutional review board approval was needed for this research because no personal identifying information was collected.
K. pneumoniae antimicrobial susceptibility results were stratified by specimen source (blood, sputum, urine, and wounds). Imipenem-resistant K. pneumoniae isolates from 2010 were examined for cross-resistance to other antimicrobial agents and prevalence in ICU versus non-ICU settings. We used χ2 testing to determine whether changes in K. pneumoniae antimicrobial drug resistance were statistically significant from 2000 to 2010 and whether 2010 antimicrobial drug resistance differed by specimen source. The α level was set at 0.05. Analyses were performed by using R version 2.11.0 (www.r-project.org).
We analyzed a total of 3,132,354 K. pneumoniae antimicrobial susceptibility results for 1998–2010 (Table 1). Statistically significant increases in antimicrobial drug resistance to all agents (p<0.0001) except tetracycline (p = 0.0745) (Figure 1) were observed. Resistance to imipenem first appeared in TSN Database-USA in 2004 and rose gradually to 4.3% by the end of our study period. In 2010, K. pneumoniae resistance to tigecycline was 2.6% (data not shown). The largest increases in antimicrobial drug resistance from 1998 to 2010 were observed for aztreonam (7.7% to 22.2%), ceftazidime (5.5% to 17.2%), and ciprofloxacin (5.5% to 16.8%). Changes in resistance were smaller for tetracycline (14.2% to 16.7%) and amikacin (0.7% to 4.5%).
Table 1
| Antimicrobial drug | No. | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total change† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TET | 80,862 | 14.2 | 14.7 | 14.6 | 13.9 | 14.2 | 13 | 15.6 | 14.8 | 15.6 | 15.2 | 15.3 | 16.4 | 16.7 | 2.5 |
| AMK | 232,933 | 0.7 | 0.9 | 0.8 | 0.9 | 1 | 1.1 | 2.1 | 3.7 | 4.9 | 4.6 | 4.9 | 4.6 | 4.5 | 3.8 |
| IPM | 259,589 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0.6 | 0.6 | 1.5 | 3.3 | 3.8 | 4.3 | 4.3 |
| GEN | 344,597 | 4.9 | 4.6 | 4.6 | 5.2 | 5.5 | 5.6 | 6.9 | 8.3 | 8.4 | 7.8 | 8.7 | 9.1 | 9.2 | 4.3 |
| CPM | 211,134 | 2.1 | 2 | 2.7 | 2.3 | 2.3 | 2.5 | 5.1 | 5.3 | 5.5 | 5.8 | 7.7 | 7.3 | 7.7 | 5.6 |
| TMP/SXT | 344,522 | 10.9 | 11 | 10.9 | 10.4 | 10.4 | 10.9 | 13 | 15 | 16.2 | 16.5 | 19.4 | 18.9 | 19.3 | 8.4 |
| TZP | 250,554 | 4 | 5.5 | 5.4 | 5.3 | 5.3 | 5.6 | 7.7 | 8.8 | 8.4 | 7.8 | 10.5 | 12 | 12.7 | 8.7 |
| TOB | 289,287 | 4.6 | 4.5 | 4.4 | 4.8 | 5.2 | 5.5 | 8 | 9.8 | 11.9 | 10.4 | 13.2 | 13.2 | 13.8 | 9.2 |
| CRO | 287,091 | 1.8 | 2.7 | 3.2 | 2.9 | 2.6 | 3.1 | 5.7 | 6.9 | 8.1 | 8.7 | 11.1 | 11.2 | 12.1 | 10.3 |
| CIP | 306,348 | 5.5 | 6.1 | 6.5 | 7 | 7.6 | 7.9 | 9.9 | 11.7 | 14.4 | 14.6 | 17 | 16.8 | 16.8 | 11.3 |
| CAZ | 248,004 | 5.5 | 6.8 | 7.9 | 7.9 | 7.7 | 7.8 | 11.3 | 13.3 | 12.3 | 12.3 | 15.6 | 15.2 | 17.2 | 11.7 |
| ATM | 184,981 | 7.7 | 7.7 | 7.7 | 7.5 | 8 | 7.3 | 11.2 | 12.9 | 12.8 | 12.8 | 16.6 | 16.1 | 22.2 | 14.5 |
*TET, tetracycline; AMK, amikacin; IPM, imipenem; GEN, gentamicin; CPM, cefepime; TMP/SXT, trimethoprim/sulfamethoxazole; TZP, piperacillin/tazobactam; TOB, tobramycin; CRO, ceftriaxone; CIP, ciprofloxacin; CAZ, ceftazidime; ATM, aztreonam.
†Change from 1998 to 2010. p<0.0001 for all antimicrobial agents except tetracycline (p = 0.0745).
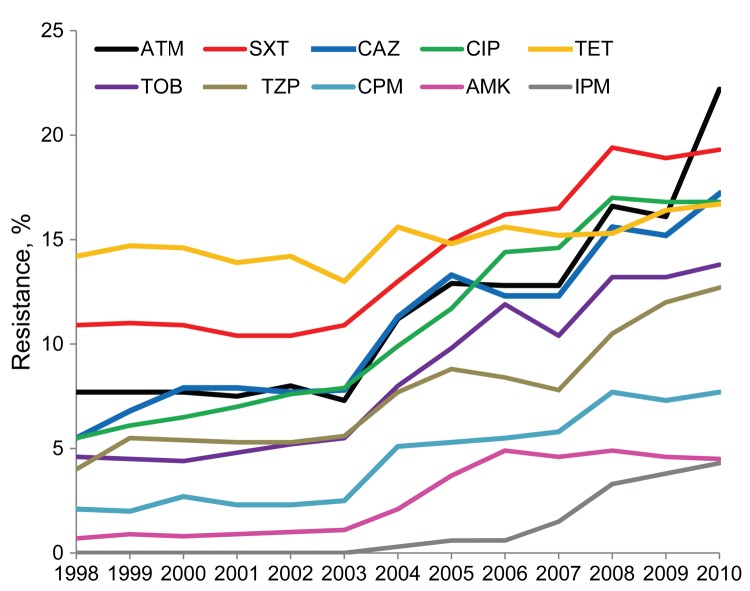
Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998–2010. ATM, aztreonam; SXT, trimethoprim/sulfamethoxazole; CAZ, ceftazidime; CIP, ciprofloxacin; TET, tetracycline; TOB, tobramycin; TZP, piperacillin/tazobactam; CPM, cefepime; AMK, amikacin; IPM, imipenem. Ceftriaxone and gentamicin were not included for better data presentation.
In 2010, isolates from the lower respiratory tract showed higher levels of resistance than did isolates from urine for all antimicrobial agents (p<0.0001) except tetracycline (p = 0.54) (Table 2). CRKP was more prevalent in ICU settings than in non-ICU settings (6.3% vs. 3.8%, respectively) (Technical Appendix).
Table 2
| Source, no. samples | AMK | GEN | TOB | TZP | ATM | IPM | CAZ | CRO | CPM | TMP/SXT | CIP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All, 187,359 | 4.5 | 9.2 | 13.8 | 12.7 | 22.2 | 4.3 | 17.2 | 12.1 | 7.7 | 19.3 | 16.8 | 16.7 |
| Blood, 20,185 | 4.2 | 10.6 | 14.9 | 15 | 23.6 | 5.9 | 19.2 | 13.4 | 7.1 | 21.3 | 18.9 | 17.1 |
| Urine, 112,567 | 4.1 | 7.8 | 12.4 | 10.6 | 21 | 3.8 | 15.1 | 10.9 | 7.1 | 18.3 | 15.1 | 17.3 |
| Wound, 22,225 | 4.5 | 10.1 | 14.4 | 14.7 | 20.9 | 5 | 17.5 | 12.2 | 8.3 | 19.5 | 16.8 | 16.4 |
| Respiratory, 32,382 | 5.8 | 11.1 | 15.8 | 16.4 | 24.5 | 4.7 | 21.3 | 15.5 | 9.9 | 20.2 | 20 | 15.5 |
*AMK, amikacin; GEN, gentamicin; TOB, tobramycin; TZP, piperacillin/tazobactam; ATM, aztreonam; IPM, imipenem; CAZ, ceftazidime; CRO, ceftriaxone; CPM, cefepime; TMP/SXT, trimethoprim/sulfamethoxazole; CIP, ciprofloxacin; TET, tetracycline.
Imipenem-resistant isolates of K. pneumoniae showed the lowest resistance to tetracycline (19.9%) and amikacin (36.8%). High prevalence of cross-resistance was observed for ciprofloxacin (96.4%) (Figure 2).
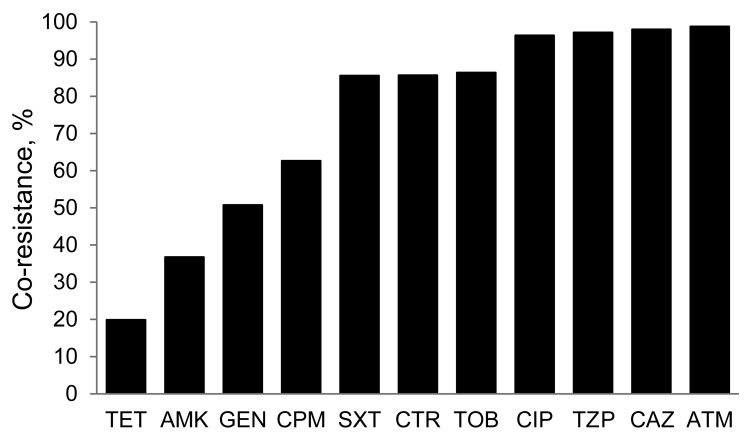
Prevalence of antimicrobial cross-resistance among imipenem-resistant Klebsiella pneumoniae isolates, United States, 2010. TET, tetracycline; AMK, amikacin; GEN, gentamicin; CPM, cefepime; SXT, trimethoprim/sulfamethoxazole; CRO, ceftriaxone; TOB, tobramycin; CIP, ciprofloxacin; TZP, piperacillin/tazobactam; CAZ, ceftazidime; ATM, aztreonam.
Conclusions
In our study, the proportion of K. pneumoniae isolates resistant to carbapenems was lower than those previously reported (7,8). In 2010, we observed a resistance rate of 4.3% for imipenem. The Centers for Disease Control and Prevention (CDC) reported that, among health care–associated infections, 8% of Klebsiella spp. isolates were carbapenem resistant in 2007 compared with <1% in 2000 (9). Most studies of K. pneumoniae antimicrobial drug resistance have focused on patient populations with higher exposures to antimicrobial agents, such as those in critical care and academic hospital settings. In contrast, the lower prevalence of CRKP in our study might have resulted from a wider variety of institution types and inclusion of isolates from hospital patients outside of the critical care setting. Furthermore, within our study, a high percentage of isolates were from urine and showed lower levels of resistance than did isolates from respiratory samples. Interpretive breakpoint criteria for the antimicrobial agents included did not change during the study period.
The low cross-resistance to tetracycline among CRKP and stable resistance rate of K. pneumoniae to this agent during the study period are noteworthy. In our analysis of cross-resistance among imipenem-resistant K. pneumoniae, tetracycline had the greatest antimicrobial activity against CRKP. Although resistance of K. pneumoniae increased for all antimicrobial agents studied, resistance to tetracycline increased only slightly from 1998 to 2010. Later-generation tetracyclines may prove useful in the treatment of CRKP-related infections because of their improved tissue penetration, antimicrobial activity, and decreased propensity to develop antimicrobial drug resistance compared with their older counterparts (10). Tigecycline, a glycylcycline antimicrobial agent that is structurally similar to tetracycline, has been used to treat CRKP-related infections and is often active against carbapenemase–producing K. pneumoniae (11,12). Data for tigecycline that used FDA interpretive breakpoints showed K. pneumoniae antimicrobial drug resistance was 2.6% in 2010. Tigecycline data were included only for 2010 because the drug was not FDA approved until 2005 and an insufficient number of results were available before 2010.
The widespread transmission of carbapenemase-producing K. pneumoniae has become the most common cause of carbapenem resistance among Enterobacteriaceae in the United States (13) and probably accounts for most of the imipenem resistance shown in this study. The spread of carbapenemase-producing organisms threatens to extend carbapenem resistance to the community (14). The increasing antimicrobial drug resistance to K. pneumoniae in our study, a concurrent lack of novel antimicrobial agent development (15), and limited therapeutic options available for treating CRKP-related infections add further urgency to improve prevention efforts and treatment strategies.
Our study data have strengths and limitations. The strengths are the wide variety of antimicrobial agents included, the number of laboratories reporting data, the nationally representative geographic distribution of these institutions, and the large number of isolates. Geography is a critical consideration with surveillance of this organism because distribution of K. pneumoniae antimicrobial drug resistance varies within the United States (13). The limitations of these data include a lack of central laboratory testing and the variety of test methods used. Because of a lack of Clinical Laboratory Standards Institute or FDA interpretive breakpoints for K. pneumoniae and colistin or fosfomycin, these data were not collected by TSN Database-USA and were not included in this study. Resistance to carbapenems might have been underreported at the beginning of our study period because of a lower frequency of susceptibility testing of these agents and the inability of antimicrobial susceptibility test methods to detect low-level carbapenem resistance.
Our study shows that K. pneumoniae antimicrobial drug resistance increased for every antimicrobial class studied except tetracyclines. Cross-resistance among imipenem-resistant K. pneumoniae was high for ciprofloxacin but lower for tetracycline and amikacin. This emerging problem presents a major threat to public health and warrants due diligence in future surveillance efforts.
Klebsiella pneumoniae imipenem resistance among ICU and non-ICU isolates, United States, 1998–2010.
Acknowledgments
We thank Eurofins-Medinet for providing the data and recognize Alexis M. Baird and Jacqueline N. Wilkins for their contributions in editing and formatting this work.
No authors received financial support for doing this research, nor do they have any conflicts of interest to disclose. No organizations have a financial interest in the completion of this work.
Biography
Mr Sanchez is studying medicine and epidemiology as a physician assistant student at The George Washington University. His research interests are infectious disease epidemiology and emerging antimicrobial drug resistance.
Footnotes
Suggested citation for this article: Sanchez GV, Master RN, Clark RB, Fyyaz M, Duvvuri P, Ekta G, et al. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998–2010. Emerg Infect Dis [Internet]. 2013 Jan [date cited]. http://dx.doi.org/10.3201/eid1901.120310
References
Articles from Emerging Infectious Diseases are provided here courtesy of Centers for Disease Control and Prevention
Full text links
Read article at publisher's site: https://doi.org/10.3201/eid1901.120310
Read article for free, from open access legal sources, via Unpaywall:
https://wwwnc.cdc.gov/eid/article/19/1/pdfs/12-0310.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3201/eid1901.120310
Article citations
Antimicrobial resistance and prevalence of extended-spectrum beta-lactamase-producing <i>Klebsiella</i> species in East Tennessee dairy farms.
Microbiol Spectr, 12(10):e0353723, 06 Sep 2024
Cited by: 0 articles | PMID: 39240080 | PMCID: PMC11448431
Bayesian estimation of the prevalence of antimicrobial resistance: a mathematical modelling study.
J Antimicrob Chemother, 79(9):2317-2326, 01 Sep 2024
Cited by: 0 articles | PMID: 39051678 | PMCID: PMC11368424
Prevalence, regional distribution, and trends of antimicrobial resistance among female outpatients with urine Klebsiella spp. isolates: a multicenter evaluation in the United States between 2011 and 2019.
Antimicrob Resist Infect Control, 13(1):21, 14 Feb 2024
Cited by: 2 articles | PMID: 38355621 | PMCID: PMC10865585
Antibiotic resistance spectrum of E. coli strains from different samples and age-grouped patients: a 10-year retrospective study.
BMJ Open, 13(4):e067490, 12 Apr 2023
Cited by: 6 articles | PMID: 37045577 | PMCID: PMC10106033
On the Potential of Relational Databases for the Detection of Clusters of Infection and Antibiotic Resistance Patterns.
Antibiotics (Basel), 12(4):784, 19 Apr 2023
Cited by: 1 article | PMID: 37107146 | PMCID: PMC10135313
Go to all (71) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Trends in Susceptibility Rates and Extended-Spectrum β-Lactamase Production of Klebsiella pneumoniae in Bloodstream Infections Across the United States Veterans Affairs Healthcare System.
Microb Drug Resist, 21(6):590-599, 24 Jul 2015
Cited by: 5 articles | PMID: 26207392
Antimicrobial polymers as therapeutics for treatment of multidrug-resistant Klebsiella pneumoniae lung infection.
Acta Biomater, 78:78-88, 20 Jul 2018
Cited by: 21 articles | PMID: 30031912
Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: ineffectiveness of carbapenems and increasing resistance to colistin.
Chemotherapy, 56(6):448-452, 18 Nov 2010
Cited by: 27 articles | PMID: 21088396
Colistin resistance in Klebsiella pneumoniae.
Int J Antimicrob Agents, 44(1):8-15, 12 Apr 2014
Cited by: 96 articles | PMID: 24794735
Review






