Abstract
Background
Studies into the association between hypertensive disorders during pregnancy and end-stage renal disease are limited. We investigated the risk of end-stage renal disease after delivery among women with hypertensive disorders during pregnancy.Methods
We used insurance claims data from 1998 to 2009 to identify 26,651 women aged 19-40 years old who experienced hypertensive disorders during pregnancy; these women had no history of hypertension, diabetes, kidney disease or lupus. We also randomly selected 213,397 women without hypertensive disorders during pregnancy as a comparison cohort; the frequency was matched by age and index year of pregnancy. We compared the incidence of end-stage renal disease in the 2 cohorts. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) after controlling for demographic and clinical factors.Results
Women with hypertensive disorders during pregnancy had a greater risk of chronic kidney disease and end-stage renal disease, with adjusted HRs of 9.38 (95% CI 7.09-12.4) and 12.4 (95% CI 8.54-18.0), respectively, after controlling for urban status, coronary artery disease, congestive heart failure, hyperlipidemia and abruption. The HR for end-stage renal disease was 2.72 (95% CI 1.76-4.22) after we also controlled for hypertension and diabetes. Women with preeclampsia or eclampsia had a higher risk of end-stage renal disease (adjusted HR 14.0, 95% CI 9.43-20.7) than women who had gestational hypertension only (adjusted HR 9.03, 95% CI 5.20-15.7).Interpretation
Women with hypertensive disorders during pregnancy were at a high risk of end-stage renal disease. The risk was much greater for women who had preeclampsia or eclampsia than those who had gestational hypertension only.Free full text

Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study
Abstract
Background:
Studies into the association between hypertensive disorders during pregnancy and end-stage renal disease are limited. We investigated the risk of end-stage renal disease after delivery among women with hypertensive disorders during pregnancy.
Methods:
We used insurance claims data from 1998 to 2009 to identify 26 651 women aged 19–40 years old who experienced hypertensive disorders during pregnancy; these women had no history of hypertension, diabetes, kidney disease or lupus. We also randomly selected 213 397 women without hypertensive disorders during pregnancy as a comparison cohort; the frequency was matched by age and index year of pregnancy. We compared the incidence of end-stage renal disease in the 2 cohorts. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) after controlling for demographic and clinical factors.
Results:
Women with hypertensive disorders during pregnancy had a greater risk of chronic kidney disease and end-stage renal disease, with adjusted HRs of 9.38 (95% CI 7.09–12.4) and 12.4 (95% CI 8.54–18.0), respectively, after controlling for urban status, coronary artery disease, congestive heart failure, hyperlipidemia and abruption. The HR for end-stage renal disease was 2.72 (95% CI 1.76–4.22) after we also controlled for hypertension and diabetes. Women with preeclampsia or eclampsia had a higher risk of end-stage renal disease (adjusted HR 14.0, 95% CI 9.43–20.7) than women who had gestational hypertension only (adjusted HR 9.03, 95% CI 5.20–15.7).
Interpretation:
Women with hypertensive disorders during pregnancy were at a high risk of end-stage renal disease. The risk was much greater for women who had preeclampsia or eclampsia than those who had gestational hypertension only.
Hypertensive disorders during pregnancy are major causes of maternal and fetal morbidity and mortality, affecting 5%–10% of pregnancies.1,2 Hypertensive disorders during pregnancy include gestational hypertension and preeclampsia.3 Gestational hypertension is referred to as new-onset hypertension (blood pressure > 140/90 mm Hg) without proteinuria after 20-weeks’ gestation.3 Preeclampsia is characterized by new-onset hypertension (blood pressure > 140/90 mm Hg) with proteinuria of at least 300 mg in a 24-hour urine sample after 20-weeks’ gestation.3 Gestational hypertension progresses to preeclampsia in 10%–20% of pregnant women.4 The risk factors associated with preeclampsia include family history of preeclampsia, first pregnancy, multiple gestation, advanced maternal age, obesity, pre-existing hypertension, renal disease and diabetes mellitus.5 Women with a history of hypertensive disorders during pregnancy are at higher risk of hypertension, diabetes mellitus and cardiovascular disease in later life. Hypertensive disorders during pregnancy and cardiovascular disease share several common risk factors, such as obesity, pre-existing hypertension, renal disease and insulin resistance.6–14 Hypertensive disorders during pregnancy also increase the risk of cardiovascular disease because of long-term metabolic and vascular changes.15
Hypertensive disorders during pregnancy affect the function and morphology of the kidney.16 Previous studies have reported an increased prevalence of microalbuminuria after pregnancy in women who had a hypertensive disorder during pregnancy.17,18 In a case–control study, there was an association between biopsy-proven renal disease and a history of preeclampsia.19 However, studies about whether hypertensive disorders during pregnancy are associated with end-stage renal disease in later life are limited.20 Only 1 study, performed using birth and renal registries from Norway, has reported that women with preeclampsia during their first pregnancy had a 3.2-fold higher risk of end-stage renal disease.20 In the present study, we investigated the risk of end-stage renal disease among Taiwanese women who had a hypertensive disorder during pregnancy.
Methods
Data source
We obtained a data set consisting of health care reimbursement claims for the 1996–2009 period from the National Health Research Institutes for Taiwan’s National Health Insurance Program. The Taiwan National Health Insurance Program had a coverage rate of 93.1% for the 23 million residents in 1996; the coverage rate was 99% in 2009. Patient identification numbers that were used for linking files were scrambled before the dataset was released to ensure patient privacy.
This study was approved by the research ethics committee of China Medical University Hospital.
Participants
We identified 28 724 women with hypertensive disorders in pregnancy (International Classification of Disease 9th revision, clinical modification [ICD-9-CM] codes 642.3, 642.4–642.6, 642.9) in their first pregnancy during the study period and who were 19–40 years of age between 1998 and 2009. This was defined as the index pregnancy. The date on which a hypertensive disorder was diagnosed was used as the index date for estimating follow-up time. We excluded women who had a history of hypertension (n = 447), diabetes (n = 1194), renal disease (n = 396) and systemic lupus erythematous (n = 36) before the index date. We included the remaining 26 651 women who had a hypertensive disorder during pregnancy as the study cohort.
For each woman with a hypertensive disorder during pregnancy, we randomly selected 8 women without hypertensive disorder during pregnancy (comparison cohort, n = 229 792); these women had a normal first pregnancy and were frequency matched by age and index year. In addition to the exclusion criteria used to select the cohort of pregnant women, we also excluded from the comparison cohort women who had a hypertensive disorder during pregnancy before the development of end-stage renal disease or who were ineligible by the end of 2009 (n = 16 395).
All participants were followed until Dec. 31, 2009, to identify incident chronic kidney disease (ICD-9 code 582, 585, 587, 403 and 404) and end-stage renal disease. In Taiwan’s insurance system, patients with end-stage renal disease are registered in the Catastrophc Illness Patients Database using ICD codes similar to those for chronic kiddney disease (ICD-9 codes 582, 585, 587, 403 and 404). Data for participants who died or withdrew from the National Health Insurance Program were censored. Patients with end-stage renal disease were identified from the Catastrophic Illness Patients Database, which includes those who require long-term renal replacement therapy, such as dialysis or kidney transplant.
Covariables
We recorded demographic data including age at index pregnancy (19–24, 25–29, 30–34 and 35–40 yr) and urban status level. Baseline disorders, including coronary artery disease, congestive heart failure and hyperlipidemia were identified before the index date. Postpartum coronary artery disease, congestive heart failure, hypertension, hyperlipidemia and diabetes were also identified before the outcome or Dec. 31, 2009. Preterm delivery and abruption were identified during the index pregnancy. These disorders were considered to be comorbidities that were potentially associated with the development of end-stage renal disease. The National Health Research Institutes has divided Taiwan into 7 levels based on urban status (level 1 = highest urban areas; level 7 = lowest urban areas). We grouped levels 6 and 7 with level 5 because of the low population in those areas. We classified the subtypes of hypertensive disorders during pregnancy as gestational hypertension only and preeclampsia.
Statistical analysis
We compared the demographic data and comorbidities between cohorts with and without hypertensive disorders. We used the χ2 test for categorical variables and t test for continuous variables. We calculated the incidence of end-stage renal disease in both cohorts. We used Cox proportional hazards regression analysis to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of chronic kidney disease and end-stage renal disease for the cohort with hypertensive disorders during pregnancy relative to the cohort without these disorders. We examined the Cox proportional hazards assumption using the likelihood test. The assumption was not violated (p = 0.2).
We used 4 models to estimate crude HRs and adjusted HRs for evaluating the risk of end-stage renal disease. We used univariate analysis for model 1. Model 2 controlled for urban status, coronary artery disease, congestive heart failure, hyperlipidemia and abruption. In addition to these factors, we also adjusted for hypertension and diabetes in model 3. Model 4 was a time-dependent Cox proportional hazards regression model with hypertension and diabetes as time-dependent variables; this model was also adjusted for variables included in model 3.
We also examined how postpartum hypertension and diabetes altered the risk of chronic kidney disease and end-stage renal disease. We used Kaplan–Meier analysis to assess the proportion of participants without end-stage renal disease in each cohort.
Results
Compared with the cohort without hypertensive disorders during pregnancy (n = 213 397), those with hypertensive disorders during pregnancy (n = 26 651) had fewer urban residents but had higher rates of congestive heart failure and hyperlipidemia before the index date and more comorbidities after the index date (Table 1).
Table 1:
Demographic characteristics and comorbidities among women with and without hypertensive disorders in pregnancy
| Characteristic | Hypertensive disorder during pregnancy, no. (%)* | p value | |
|---|---|---|---|
| No n = 213 397 | Yes n = 26 651 | ||
| Age, yr mean ± SD | 29.5 ± 4.75 | 29.6 ± 4.80 | |
| Age, yr | 0.9 | ||
 19–24 19–24 | 28 164 (13.2) | 3 515 (13.2) | |
 25–29 25–29 | 68 695 (32.2) | 8 580 (32.2) | |
 30–34 30–34 | 74 148 (34.8) | 9 259 (34.7) | |
 35–40 35–40 | 42 390 (19.9) | 5 297 (19.9) | |
| Urban status† | 213 359 | 26 648 | < 0.001 |
 Level 1 Level 1 | 68 310 (32.0) | 8 412 (31.6) | |
 Level 2 Level 2 | 69 253 (32.5) | 8 493 (31.9) | |
 Level 3 Level 3 | 36 961 (17.3) | 4 586 (17.2) | |
 Level 4 Level 4 | 25 023 (11.7) | 3 215 (12.1) | |
 Levels 5–7 Levels 5–7 | 13 812 (6.5) | 1 942 (7.3) | |
| Comorbidities at baseline | |||
 Coronary artery disease Coronary artery disease | 88 (0.04) | 11 (0.04) | 0.9 |
 Congestive heart failure Congestive heart failure | 45 (0.02) | 12 (0.05) | 0.02 |
 Hyperlipidemia Hyperlipidemia | 119 (0.06) | 37 (0.14) | < 0.001 |
| Comorbidities after index pregnancy | |||
 Coronary artery disease Coronary artery disease | 148 (0.07) | 97 (0.36) | < 0.001 |
 Congestive heart failure Congestive heart failure | 131 (0.06) | 130 (0.49) | < 0.001 |
 Hypertension Hypertension | 549 (0.26) | 973 (3.65) | < 0.001 |
 Preterm delivery Preterm delivery | 14 091 (6.60) | 6 551 (24.6) | < 0.001 |
 Abruption Abruption | 1 264 (0.59) | 632 (2.37) | < 0.001 |
 Diabetes Diabetes | 2 719 (1.27) | 1 181 (4.43) | < 0.001 |
Note: SD = standard deviation.
The crude HR for chronic kidney disease was 10.8 (95% CI 8.2–14.2); the adjusted HR was 9.38 (95% CI 7.09–12.4) (Table 2). Among women without hypertension during follow-up, women who had experienced hypertensive disorders during pregnancy had a higher risk of chronic kidney disease (adjusted HR 4.37, 95% CI 2.71–7.07).
Table 2:
Crude and adjusted hazard ratios for chronic kidney disease among women with and without postpartum hypertension and diabetes during follow-up
| Group | Chronic kidney disease | Incidence rate per 10 000 person-years | Crude HR (95% CI) | Adjusted HR* (95% CI) |
|---|---|---|---|---|
| No hypertensive disorder in pregnancy | 90 | 0.67 | 1.00 | 1.00 |
| Hypertensive disorder in pregnancy | 121 | 7.24 | 10.8 (8.20–14.2) | 9.38 (7.09–12.4) |
| Hypertension during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 43 | 86.6 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 94 | 121 | 1.39 (0.97–2.00) | 1.35 (0.94–1.94) |
| No hypertension during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 47 | 0.35 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 27 | 1.69 | 4.81 (3.00–7.72) | 4.37 (2.71–7.07) |
| Diabetes during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 5 | 2.12 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 19 | 19.4 | 9.20 (3.43–24.6) | 7.62 (2.80–20.8) |
| No diabetes during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 85 | 0.64 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 102 | 6.47 | 10.0 (7.53–13.4) | 8.92 (6.65–12.0) |
Note: CI = confidence interval, HR = hazard ratio.
The incidence of end-stage renal disease was 14.1-fold higher among women who had hypertensive disorders during pregnancy than among those without (crude HR 14.1, 95% CI 9.76–10.30) (Table 3). The HR decreased slightly after we controlled for urban status, coronary disease, congestive heart failure, hyperlipidemia and abruption (model 2), and decreased further after we adjusted for hypertension and diabetes (model 3). In the time-dependent (model 4), the adjusted HR was 1.91 (95% CI 1.20–3.07). With a mean follow-up time of about 6.3 years for both cohorts, our Kaplan–Meier analysis showed that the overall proportion of participants without end-stage renal disease was lower for the cohort with hypertensive disorders during pregnancy compared with the cohort without such conditions (Figure 1).
Table 3:
Multivariable Cox proportional hazards regression analysis of the risk of end-stage renal disease among women with and without hypertensive disorders in pregnancy
| Variable, n of women | End-stage renal disease | Incidence rate per 10 000 person-years | Hazard ratio (95% confidence interval) | |||
|---|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |||
| No hypertensive disorders in pregnancy, n = 213 397 | 45 | 0.34 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hypertensive disorders in pregnancy, n = 26 651 | 79 | 4.72 | 14.1 (9.76–10.3) | 12.4 (8.54–18.0) | 2.72 (1.76–4.22) | 1.91 (1.20–3.07) |
 Gestational hypertension, n = 8 653 Gestational hypertension, n = 8 653 | 18 | 3.40 | 10.2 (5.89–17.6) | 9.03 (5.20–15.7) | 1.81 (0.99–3.30) | 1.38 (0.74–2.57) |
 Preeclampsia/eclampsia, n = 17 998 Preeclampsia/eclampsia, n = 17 998 | 61 | 5.33 | 15.9 (10.8–23.3) | 14. 0 (9.43–20.7) | 3.19 (2.02–5.02) | 2.17 (1.33–3.54) |
| p for trend | < 0.001¶ | < 0.001** | < 0.001** | < 0.001** | 0.001 | |
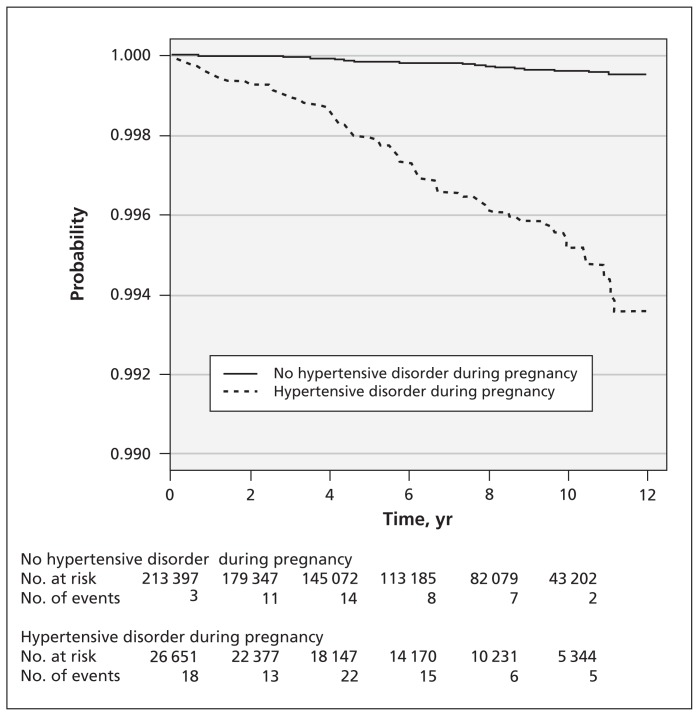
Estimated proportion of women without end-stage renal disease among those with and without hypertensive disorders during pregnancy. Log-rank test, p < 0.001.
Women with preeclampsia or eclampsia were at higher risk of end-stage renal disease than those who had only gestational hypertension (Table 3). To reduce the immediate effect of pregnancy complications on the risk of adverse outcomes, we performed an additional analysis that excluded events in the first year after delivery. The HRs for end-stage renal disease were 12.7 (95% CI 8.65–18.6), 11.2 (95% CI 7.56–16.5), 2.11 (95% CI 1.34–3.30) and 1.44 (95% CI 0.90–2.32) for models 1 to 4, respectively. The interaction between preterm delivery and hypertensive disorders in pregnancy was not significant (p = 0.8).
The incidence of end-stage renal disease was greater for women with postpartum hypertension than for those with postpartum diabetes (Table 4). Among women without postpartum hypertension or diabetes, women with hypertensive disorders during pregnancy had higher HRs for end-stage renal disease than women without hypertensive disorders in pregnancy.
Table 4:
Crude and adjusted hazard ratios for end-stage renal disease for women with and without hypertension and diabetes during postpartum follow-up
| Group | End-stage renal disease | Incidence rate per 10 000 person-years | Crude HR (95% CI) | Adjusted* HR (95% CI) |
|---|---|---|---|---|
| Hypertension during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 25 | 49.7 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 61 | 76.8 | 1.56 (0.98–2.48) | 1.51 (0.95–2.41) |
| No hypertension during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 20 | 0.15 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 18 | 1.13 | 7.56 (4.00–14.3) | 6.67 (3.48–12.8) |
| Diabetes during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 2 | 0.85 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 9 | 9.14 | 11.3 (2.44–52.4) | 10.6 (2.31–51.0) |
| No diabetes during follow-up | ||||
 No hypertensive disorders in pregnancy No hypertensive disorders in pregnancy | 43 | 0.33 | 1.00 | 1.00 |
 Hypertensive disorders in pregnancy Hypertensive disorders in pregnancy | 70 | 4.44 | 13.6 (9.33–19.9) | 11.9 (8.09–17.6) |
Note: CI = confidence interval, HR = hazard ratio.
Interpretation
We found an increased risk of subsequent end-stage renal disease among Taiwanese women who had experienced a hypertensive disorder during pregnancy, including preeclampsia or eclampsia and gestational hypertension. We also found that women with preeclampsia or eclampsia were at higher risk of end-stage renal disease than those who had gestational hypertension only.
The pathogenesis of hypertensive disorders during pregnancy includes uteroplacental hypoperfusion and fetal ischemia.21 The hypoperfused placenta leads to antiangiogenic factors moving into the maternal circulation, causing systemic endothelial cell dysfunction, hypertension and proteinuria.22 Karumanchi and colleagues reported that glomerular endothelial swelling characterizes glomerular lesions in women with hypertensive disorders during pregnancy.5 Depletion of the VEGF protein by antiangiogenic factors may be responsible for glomerular endotheliosis,23 which decreases glomerular filtration rate and may contribute to proteinuria. Glomerular endotheliosis usually disappears within 8 weeks after delivery, consistent with the resolutions of proteinuria and hypertension.5 However, hypertensive disorders during pregnancy may damage the kidneys, with incomplete healing or scarring.24
Among women with hypertensive disorders during pregnancy, the increased presence of microalbuminuria lasts 5–7 years after delivery.17,25 A systematic meta-analysis reported that women with preeclampsia have a 4-fold higher risk of subsequent microalbuminuria,18 which increases the risk of both cardiovascular disease and end-stage renal disease.26,27 Vikse and colleagues analyzed registry data from Norway and found that women with preeclampsia during their first pregnancy were at risk of having a kidney biopsy.28 They also reported that women with preeclampsia during one pregnancy had a 3.2-fold higher adjusted relative risk of end-stage renal disease.20 The risk was even higher for women with recurrent preeclampsia. In addition, women with preeclampsia and a low–birth weight infant or preterm delivery had further increased risk. These conditions suggest a more severe type of preeclampsia. In contrast, serum creatinine levels and estimated glomerular filtration rates during the follow-up period were not significantly different between women with and without a history of preeclampsia.18
Women with preeclampsia also have a higher risk of end-stage renal disease than women with gestational hypertension only. Using consistent definitions of end-stage renal disease as Vikse and colleagues,20 we found that women with hypertensive disorders during pregnancy had a nearly 14-fold higher incidence of end-stage renal disease than women without hypertensive disorders during pregnancy; women with pre-existing hypertension, diabetes, renal disease or lupus were excluded from our analysis. The adjusted HR was considerably lower after we adjusted for hypertension and diabetes, indicating that these 2 factors account for a large portion of the risk attributed to hypertensive disorders during pregnancy and that these may be intermediate steps in the development of end-stage renal disease.
We found no statistically significant interaction between hypertensive disorders during pregnancy and preterm delivery. We also found that women with only gestational hypertension had a 10-fold higher incidence of end-stage renal disease than women without hypertensive disorders during pregnancy (3.40 v. 0.34 per 10 000 person-years).
Diabetes and hypertension are also major risk factors for chronic kidney diseases.29 During the follow-up period, women may develop postpartum hypertension and postpartum diabetes. Among women with hypertension or diabetes in later life, those with hypertensive disorders during pregnancy had a much higher incidence of end-stage renal disease than those without. However, we found that the risk was much greater among women with postpartum diabetes than among those with postpartum hypertension (Table 4). Among women with postpartum hypertension, the adjusted risk of end-stage renal disease among women who had a hypertensive disorder during pregnancy was not significant after we controlled for covariates. Therefore, hypertension that develops after pregnancy may mediate the association between hypertensive disorders during pregnancy and end-stage renal disease.
Limitations
The incidence and prevalence of end-stage renal disease in Taiwan is among the highest in the world.29 Although we performed a large national cohort study, our study has several limitations. Information on marital status, educational level, smoking habits, body mass index and laboratory measures of glomerular filtration rate and proteinuria were unavailable from the claims files. Therefore, we could not adjust for these variables in our data analysis. However, the prevalence of smoking among women in the general population of Taiwan is low, and less than 2.5% of pregnant women smoke.30
We excluded women with pre-existing renal disease, hypertension and diabetes. Mild kidney disease may have been unreported or undetected in some women. A Norwegian cohort study had a similar limitation.20
Our study included an Asian population, and it is possible that other populations would have different risks. The estimated risk of end-stage renal disease in our population of women with hypertensive disorders during pregnancy was higher than among Norwegian women with preeclampsia.20
The follow-up period after pregnancy in our study was relatively short for the measure of lifetime end-stage renal disease and comorbidities. Whether pre-existing renal disease causes hypertensive disorders during pregnancy and is followed by the deterioration of renal function is unclear.
We identified hypertensive disorders during pregnancy and other comorbidities using ICD-9-CM codes. In Taiwan, prenatal care has been very well performed since the implementation of the National Health Insurance Program.31 Auditors of the insurance system randomly review and validate claims to ensure accuracy of the claims. Although we could not calculate the validity of diagnostic codes for hypertensive disorders in pregnancy, the high validity of the diagnostic codes of the National Health Research Institutes database has been reported.32,33
Finally, we used the first pregnancy during the study period as the index pregnancy. This might have led to misclassification for a small number of women, whose second pregnancy may have been classified as the index pregnancy.
Conclusion
We found that women with hypertensive disorders during pregnancy were at higher risk of end-stage renal disease than women without complicated pregnancies. Postpartum hypertension and diabetes further increased this risk. The risk of end-stage renal disease is likely higher in this Taiwanese population than in Western populations. Close surveillance for microalbuminuria, blood pressure and diabetes should be considered for women with a history of hypertensive disorders during pregnancy. Preventive strategies, such as pharmacologic or lifestyle interventions, should also be considered for women at high risk of end-stage renal disease. Further prospective studies are needed to investigate the relation between hypertensive disorders during pregnancy and subsequent end-stage renal disease.
Acknowledgement
The authors thank the Taiwan National Health Research Institute for providing the National Health Insurance database.
Notes
See related commentary by Spaan and Brown on page 199 and at www.cmaj.ca/lookup/doi/10.1503/cmaj.130007
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Funding: This study was supported by the National Sciences Council, Executive Yuan (grant nos. NSC 97–2625-M-039–003, NSC 100–2621-M-039–001); China Medical University Hospital (grant no. 1MS1); Department of Health, Taiwan, Clinical Trial and Research Center for Excellence (grant no. DOH101-TD-B-111–004); and Cancer Research Center of Excellence (grant no. DOH101-TD-C-111–005).
Contributors: I-Kuan Wang, Yi-Chih Chang, Chiz-Tzung Chang, Chih-Chia Liang, Shih-Yi Lin, Tzung-Hai Yen, Feng-Rong Chuang, Chiu-Ching Huang, Chi-Pang Wen, Fung-Chang Sung and Donald E. Morisky designed the study. Chih-Hsin Muo and Pei-Chun Chen carried out the statistical analysis. I-Kuan Wang, Chih-Hsin Muo, Yi-Chih Chang, Chih-Chia Liang, Chiz-Tzung Chang, Shih-Yi Lin, Tzung-Hai Yen and Feng-Rong Chuang drafted the manuscript. Pei-Chun Chen, Chiu-Ching Huang, Chi-Pang Wen, Fung-Chang Sung and Donald E. Morisky revised the manuscript. All authors approved the final version submitted for publication.
References
Articles from CMAJ : Canadian Medical Association Journal are provided here courtesy of Canadian Medical Association
Full text links
Read article at publisher's site: https://doi.org/10.1503/cmaj.120230
Read article for free, from open access legal sources, via Unpaywall:
http://www.cmaj.ca/content/cmaj/185/3/207.full.pdf
Citations & impact
Impact metrics
Article citations
Pregnancy-Induced Hypertension Pathophysiology and Contemporary Management Strategies: A Narrative Review.
Cureus, 16(7):e63961, 06 Jul 2024
Cited by: 1 article | PMID: 39105037 | PMCID: PMC11299475
Review Free full text in Europe PMC
Trophoblast Differentiation: Mechanisms and Implications for Pregnancy Complications.
Nutrients, 15(16):3564, 12 Aug 2023
Cited by: 5 articles | PMID: 37630754 | PMCID: PMC10459728
Review Free full text in Europe PMC
The effect of thromboembolic prophylaxis after cesarean section in patients with hypertensive disorders.
North Clin Istanb, 10(2):222-227, 26 Apr 2023
Cited by: 0 articles | PMID: 37181057 | PMCID: PMC10170378
Comparisons of urine protein-to-creatinine ratios and their dynamic change patterns during labor at term between normal pregnant women and women with pregnancy induced hypertension.
Int J Med Sci, 19(9):1473-1481, 15 Aug 2022
Cited by: 0 articles | PMID: 36035364 | PMCID: PMC9413555
Hypertensive Disorders of Pregnancy and Cardiovascular Disease Risk Across Races and Ethnicities: A Review.
Front Cardiovasc Med, 9:933822, 28 Jun 2022
Cited by: 12 articles | PMID: 35837605 | PMCID: PMC9273843
Review Free full text in Europe PMC
Go to all (75) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Hypertensive disorders of pregnancy and the risk of chronic kidney disease: A Swedish registry-based cohort study.
PLoS Med, 17(8):e1003255, 14 Aug 2020
Cited by: 19 articles | PMID: 32797043 | PMCID: PMC7428061
Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization.
Am J Obstet Gynecol, 220(6):582.e1-582.e11, 08 Feb 2019
Cited by: 18 articles | PMID: 30742823
Perinatal outcomes in women with elevated blood pressure and stage 1 hypertension.
Am J Obstet Gynecol, 224(5):521.e1-521.e11, 04 Nov 2020
Cited by: 16 articles | PMID: 33157064
Five-year risk of all-cause death and cardiovascular events in women with gestational diabetes and hypertensive disorders of pregnancy.
Curr Probl Cardiol, 49(9):102698, 13 Jun 2024
Cited by: 1 article | PMID: 38876163
Review





