Abstract
Free full text

Basal Forebrain Activation Enhances Cortical Coding of Natural Scenes
Abstract
The nucleus basalis (NB) of the basal forebrain is an essential component of the neuromodulatory system controlling the behavioral state of an animal, and it is thought to play key roles in regulating arousal and attention. However, the effect of NB activation on sensory processing remains poorly understood. Using polytrode recording in rat visual cortex, we show that NB stimulation causes prominent decorrelation between neurons and marked improvement in the reliability of neuronal responses to natural scenes. The decorrelation depends on local activation of cortical muscarinic acetylcholine receptors, while the increased reliability involves distributed neural circuits, as evidenced by NB-induced changes in thalamic responses. Further analysis showed that the decorrelation and increased reliability improve cortical representation of natural stimuli in a complementary manner. Thus, the basal forebrain neuromodulatory circuit, which is known to be activated during aroused and attentive states, acts through both local and distributed mechanisms to improve sensory coding.
Neuromodulatory circuits are essential for regulating the behavioral state of an animal1–4. In particular, the basal forebrain cholinergic system originating from the nucleus basalis (NB) projects diffusely throughout the neocortex5, and it is implicated in vital brain functions such as arousal, attention6–8, and experience-dependent cortical plasticity9–11. The cholinergic neurons in NB are active during waking and REM sleep but not during slow wave sleep12,13, and recent studies in awake animals have shown that NB activity varies in a task-dependent manner14,15. Since sensory perception can be markedly enhanced by arousal and attention, the state-dependent NB activation suggests that the basal forebrain circuit may play a significant role in dynamic modulation of sensory processing. However, the causal relationship between NB activation and enhanced sensory processing has not yet been demonstrated.
Sensory processing in the cortex is known to be affected by the intrinsic dynamics of the cortical network16–20. Since neuromodulators such as acetylcholine (ACh) can alter neuronal excitability21–23 and synaptic efficacy24–27, they may rapidly regulate circuit dynamics21,28 and sensory processing29. Indeed, local iontophoretic application of ACh has been shown to affect the contrast gain30, orientation and direction selectivity31-34, and attentional modulation35 of visual cortical neurons. However, these local changes may not fully reflect the effects of NB activation, as the NB projection to the cortex is highly diffuse, and its activation is known to affect the global pattern of brain activity21,36. Thus, to understand the role of the basal forebrain neuromodulatory circuit in sensory perception, it is important to test the effect of NB activation on the sensory responses of a population of cortical neurons.
In this study, we used multielectrode recording in rat visual cortex to demonstrate two prominent effects of NB activation on cortical responses to natural stimuli. Brief NB stimulation caused a strong decorrelation between cortical neurons and a marked increase in the reliability of visually evoked responses. Furthermore, we showed that these two effects are mediated by distinct mechanisms and that they contribute to improved visual coding in a complementary manner. These findings provide a direct demonstration that a neuromodulatory circuit can dynamically regulate cortical coding of sensory inputs.
Results
We stimulated the NB of urethane-anesthetized rats with a bipolar electrode while recording from ipsilateral V1 with a silicon polytrode spanning the cortical depth37 (Fig. 1a). The NB location was identified during the experiment by measuring the change in power spectrum of the interhemispheric electroencephalogram (EEG, Supplementary Fig. 1 online)21,36 induced by a stimulus train (500 ms, 100 Hz) and confirmed by acetylcholinesterase staining38 in a subset of experiments (Fig. 1b,c).
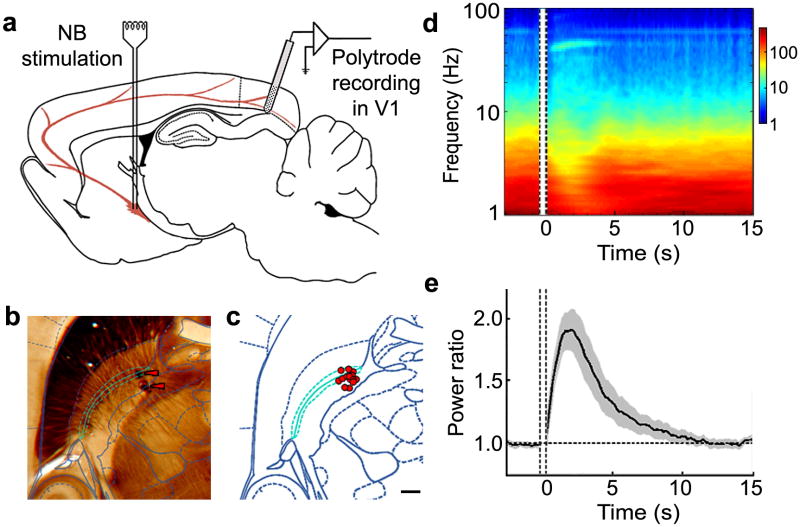
Effects of NB stimulation on local field activity in V1. (a) Schematic illustration of experimental setup, adapted from Paxinos and Watson rat brain atlas38. (b) An example of stimulation electrode localization using acetylcholinesterase histochemistry. NB is shown in cyan in overlay diagram (from Paxinos and Watson atlas38). Red arrowheads, bipolar electrode tracts. (c) Summary of electrode positions relative to NB (cyan) for 15 experiments. Red circle, centroid of the pair of electrode tracts in each experiment, which was within 300 μm of NB for all 15 experiments. Scale bar, 500 μm. (d) Time-frequency analysis of LFP before and after NB stimulation from an example experiment, averaged over 30 trials. Amplitude is color coded (scale bar on the right, arbitrary unit). Vertical lines, period of NB stimulation (500 ms). No visual stimuli were presented. (e) Power ratio (LFP power at 10 to 100 Hz divided by LFP power at 1 to 10 Hz) averaged across 27 experiments. In each experiment the ratio was normalized by its mean value before NB stimulation. Black line, mean; shaded area, ± s.e.m.
In the absence of visual input, each NB stimulus train caused a marked change in the power spectrum of V1 local field potential (LFP), with an increase in power at 10 to 100 Hz (particularly in the gamma band, 30 to 50 Hz) and a decrease at low frequencies (<10 Hz) (Fig. 1d). As quantified by the power ratio (LFP power at 10 to 100 Hz divided by that at 1 to 10 Hz), this effect on spontaneous cortical activity lasted for5 to 10 s (Fig. 1e). To characterize the effect of NB stimulation on visually driven responses, we presented each natural movie to the contralateral eye both before (control) and immediately after NB stimulation (5 s per movie, 30 repeats per condition) and recorded multiunit activity from 27 channels of the polytrode (see Online Methods). In the control condition, the multiunit activity was highly correlated among the channels throughout the cortical column, but poorly time-locked to the stimulus (Fig. 2). However, following NB stimulation, the activity was less correlated among channels but appeared more time-locked to the movie.

Effect of NB stimulation on multiunit activity throughout the depth of cortex. (a) Multiunit spike rate (color coded, scale bar on the right) recorded by the polytrode plotted against cortical depth and time during one repeat of a natural movie (only 18 of the 27 polytrode channels are shown). (b) Multiunit responses to the first 10 repeats (trials) of the movie, before (control) and 0 to 5 s after NB stimulation, both plotted on the color scale shown in (a).
NB stimulation reduces correlation, increases reliability
We then examined these effects at the level of single units, each sorted from the signals recorded at 2 to 4 nearby channels (see Online Methods). As shown by the spike trains of multiple simultaneously recorded single units in three example experiments (Fig. 3a; each neuron indicated by a different color), NB stimulation caused a conspicuous decorrelation between cells in response to natural movies. To quantify the decorrelation for each cell, we computed the average Pearson correlation coefficient (CC) between its response and the responses of all other cells in the same recording. We found that NB stimulation caused a strong reduction of between-cell CC across 166 neurons from 19 experiments (Fig. 3b; P < 10−28, Wilcoxon signed-rank test).
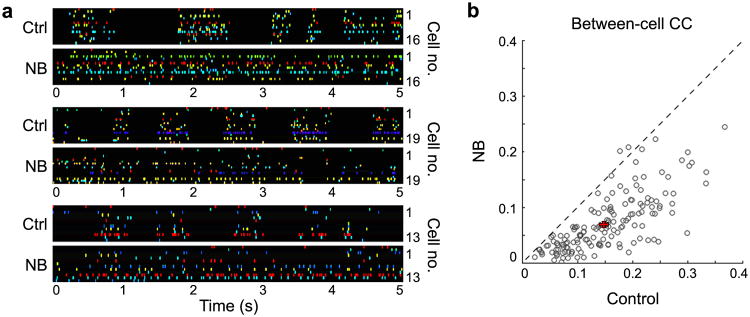
NB stimulation decreases correlation between cortical neurons during visual stimulation. (a) Three example experiments illustrating changes in between-cell correlation before (Ctrl) and after (NB) basal forebrain activation. Each panel shows the responses of multiple single units recorded simultaneously during a single movie repeat. Different neurons are indicated by different colors and ordered by cortical depth. (b) Summary of between-cell CC before and after NB stimulation (n = 166 cells from 19 experiments). Each circle represents the average CC between a single neuron and all other neurons in the same recording. Error bars, ± s.e.m.
We also examined the effect of NB stimulation on the response reliability of each neuron by analyzing the single unit responses to 30 repeats of a natural movie (Fig. 4a; each neuron indicated by a different color). In the control condition, there was considerable variability across trials, but NB stimulation markedly improved the reliability of the responses. To quantify the reliability for each cell, we computed the Pearson CC of its responses between each pair of repeats and averaged the CCs over all pairwise combinations. Across the 166 neurons recorded in 19 experiments, NB stimulation consistently increased the response reliability (Fig. 4b; P < 10–28, Wilcoxon signed-rank test). Note that in the experiments in which electrical stimulation of NB did not elicit significant desynchronization of cortical EEG (n = 4, excluded from the above analyses), neither decreased correlation nor increased reliability was observed (Supplementary Table 1 online), suggesting that these effects rely on activation of the same set of neurons that are also responsible for EEG desynchronization.
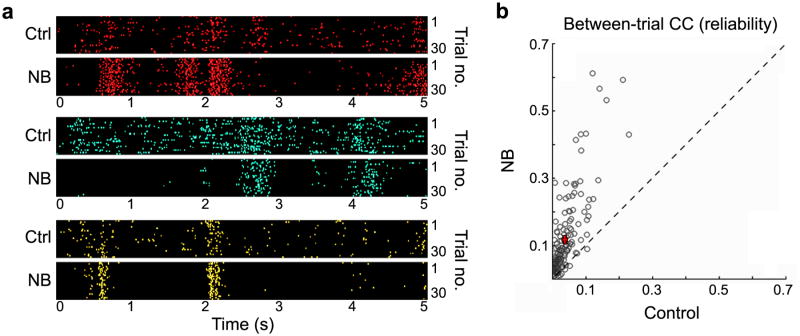
NB stimulation increases reliability of individual neurons in response to natural scenes. (a) Three example neurons (indicated by different colors) illustrating changes in response reliability before (Ctrl) and after (NB) basal forebrain activation. Each panel shows the responses of a single neuron to 30 repeats (trials) of a movie. (b) Summary of response reliability measured by between-trial CC before and after NB stimulation (166 cells from 19 experiments). Each circle represents data from one neuron, averaged over CCs for all pairwise combinations of the 30 trials. Error bars, ± s.e.m.
When the above analyses were performed for each cortical layer separately, we observed significant decreases in between-cell correlation and increases in response reliability in all layers (Supplementary Fig. 2a,b online). In contrast, the effect of NB stimulation on cortical firing rate varied across layers: it caused modest increases in layers 4, 5, and 6, but a decrease in layer 2/3 (Supplementary Fig. 2c online). To ensure that the changes in between-cell correlation and response reliability were not due to NB-induced changes in cortical firing rate, we equalized the firing rates of each neuron before and after NB stimulation by randomly deleting spikes from the spike train with higher rate (Supplementary Fig. 3a,c online). Both effects remained highly significant after spike rate equalization (Supplementary Fig. 3b,d online; P < 10−22, Wilcoxon signed-rank test, n = 166 neurons from 19 experiments). Furthermore, these changes could not be accounted for by the effect of repeated visual stimulus presentation, since the blocks of control and NB trials were interleaved in the experiment (see Online Methods), and the differences in reliability and between-cell CC across blocks were much smaller than those between the control and NB conditions (Supplementary Fig. 4 online).
We also tested whether the NB-induced changes in the cortical responses to natural scenes were accompanied by changes in the receptive field properties. Both ON and OFF receptive fields of each neuron were measured with sparse noise stimuli before and after NB stimulation (Supplementary Fig. 5a,b online). We found no significant change in receptive field size [P = 0.07 (ON) and P = 0.41 (OFF), n = 38 from 5 experiments, Supplementary Fig. 5c online], but a significant increase in the ratio of the peak amplitude to baseline noise [P < 10−4 (ON) and P < 10−4 (OFF), n = 38 from 5 experiments, Supplementary Fig. 5d online]. This is likely to reflect NB-induced improvement in response reliability that is also observed with natural stimuli. Previous studies showed that cortical iontophoresis of ACh could induce changes in orientation tuning and direction selectivity, although the observed effects were variable between cells and across studies31–34. Using drifting gratings to measure the orientation tuning of cortical neurons before and after NB stimulation (Supplementary Fig. 6a,b online), we found no significant change in orientation tuning width (P = 0.78, n = 35 from 10 experiments, Supplementary Fig. 6c online) or direction selectivity (P = 0.20, n = 35 from 10 experiments, Supplementary Fig. 6d online) at the population level. Thus, instead of systematic alteration of receptive field properties, the most consistent effect of NB stimulation across cortical neurons was the improvement of visual response reliability.
NB-induced decorrelation depends on cortical mAChRs
Previous studies showed that the change in EEG pattern induced by NB activation depends on cortical muscarinic acetylcholine receptors (mAChRs)21. We thus tested the role of mAChRs in NB-induced changes in cortical responses by applying atropine sulfate, a selective mAChR antagonist, to the recording area via either intracortical injection with a micropipette (100 μM) or local application to the cortical surface (1 mM; see Online Methods). As shown in an example multiunit recording (Fig. 5a), atropine application greatly reduced the degree of decorrelation induced by NB stimulation. At the single unit level, although the between-cell CC was still reduced by NB stimulation, the magnitude of decorrelation was much smaller than that in the absence of atropine (P < 10−28, Wilcoxon rank-sum test; n = 79 neurons from 12 experiments; Fig. 5b). Notably, atropine application was completely ineffective in blocking the NB-induced increase in response reliability. As quantified by the between-trial CC (Fig. 5c), NB stimulation induced a highly significant increase in reliability in the presence of atropine (P < 10−13, Wilcoxon signed-rank test; n = 79 cells from 12 experiments). In fact, compared to the recordings in the absence of atropine (Fig. 4b), the NB-induced increase in reliability was slightly larger in the presence of atropine (Fig. 5c), although the difference was not statistically significant (P = 0.08, Wilcoxon rank-sum test).
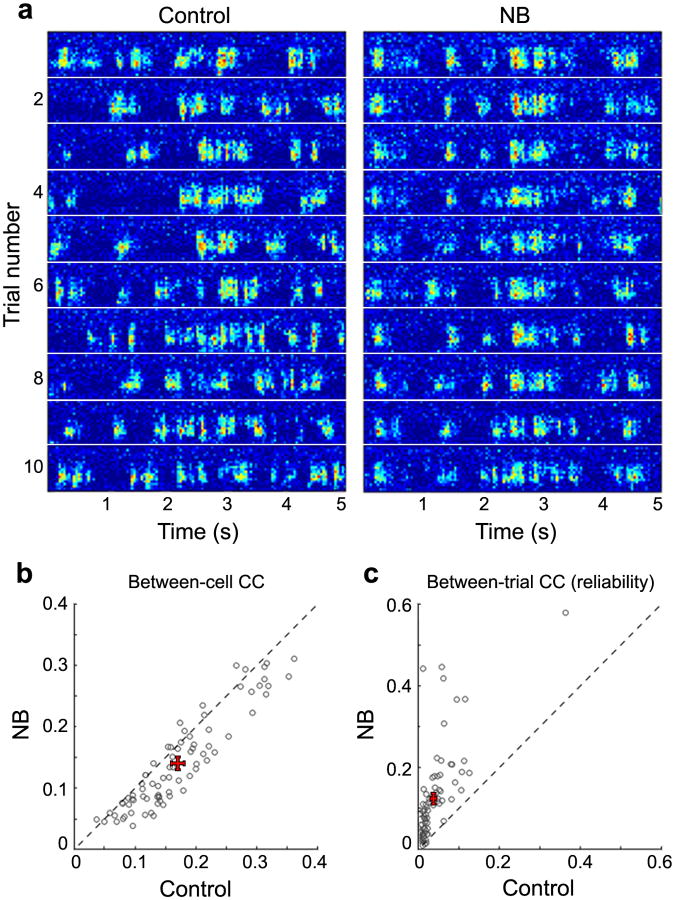
Application of mAChR antagonist diminishes NB-induced decorrelation but does not affect increases in response reliability. (a) An example of multiunit responses to the first 10 repeats of a movie before (Control) and after (NB) basal forebrain activation in the presence of atropine (100 μM, intracortical injection). Note that the correlation between channels was strong in both Control and NB conditions, but the response was much more time-locked to the movie after NB stimulation. (b) Summary of between-cell CC before (Control) and after NB stimulation in the presence of atropine (n = 79 single units from 12 experiments). (c) Summary of between-trial CC before (Control) and after NB stimulation in the presence of atropine (n = 79 single units from 12 experiments).
Increased reliability involves distributed neural circuits
We also tested whether activation of nicotinic acetylcholine receptors (nAChRs) could underlie the increased response reliability, as they are known to enhance thalamocortical synaptic efficacy25 and boost the visual responses of cortical neurons30. However, a marked NB-induced increase in response reliability was still present after cortical application of the selective nAChR antagonist mecamylamine [1 mM to 10 mM (surface application) or 100 μM to 1 mM (intracortical injection); P < 10−12, Wilcoxon signed-rank test, n = 70 cells from 9 experiments] or Dihydro-β-erythroidine hydrobromide [DHβE, 100 μM (intracortical injection); P < 0.001, n = 16 cells from 3 experiments]. In the presence of these nAChR antagonists, the magnitude of the NB-induced increase was not reduced from that observed in the absence of the antagonists (P = 0.97, Wilcoxon rank-sum test). This suggests that NB stimulation may have altered the visual signals before they reached V1. To test this possibility, we made polytrode recordings in the lateral geniculate nucleus (LGN) of the thalamus (Fig. 6a). Indeed, single units in the LGN showed significant increases in response reliability following NB stimulation (Fig. 6b,c; P < 10−12, Wilcoxon signed-rank test; n = 124 cells from 9 experiments), an effect that also remained highly significant after the spike rate equalization procedure (P < 10−5, Wilcoxon signed-rank test). This may explain why cortical AChR antagonist application was ineffective in blocking the NB-induced increase in response reliability. Furthermore, NB stimulation caused a significant reduction of the ratio between burst and tonic spikes in LGN activity (P < 10−10, Wilcoxon signed-rank test; Fig. 6d). This shift from burst to tonic firing mode has been shown to increase the linearity39 and change the temporal response properties40 of LGN neurons, which may further shape the input to the cortex. Thus, the NB-induced increase in response reliability is not a local effect in the cortex, but involves distributed neural circuits. Application of atropine (100 μM) and mecamylamine (100 μM) into the LGN (see Online Methods) did not block the NB-induced increase in cortical response reliability (P < 10−7, n = 42 cells from 4 experiments, Supplementary Fig. 7 online), indicating that this effect is not mediated by cholinergic input to the LGN.

NB stimulation increases response reliability and shifts firing mode in the LGN. (a) Schematic illustration of experimental setup. (b) Responses of two example LGN single units before (Ctrl) and after NB stimulation. Each panel shows the responses to 30 repeats of a movie. (c) Between-trial CC before (Control) and after NB stimulation (n = 124 cells from 9 experiments). (d) Burst-tonic ratio of LGN neurons before (Control) and after NB stimulation in the absence of visual stimulus. Each circle represents the burst-tonic ratio of one LGN neuron (n = 105 cells from 3 experiments). Error bars, ± s.e.m.
Decorrelation and increased repeatability improve coding
In principle, increased reliability and decreased correlation may improve visual coding by enhancing the coding capacity of each neuron and by reducing the redundancy between neurons, respectively41. To test this idea, we performed a simple discrimination analysis based on the V1 population responses to natural movies and compared the results before and after NB stimulation. For each pair of movie segments (100 ms per segment), we measured the discriminability of their responses by classifying the single-trial responses based on the similarity to each of the two template responses (mean responses across trials) (Fig. 7a; see Online Methods). We found that while the discrimination performance increased monotonically with the number of cells in the population (N), NB stimulation improved the performance at all N values tested (P < 0.01, Wilcoxon signed-rank test, with Bonferroni correction; 12 experiments; Fig. 7b).
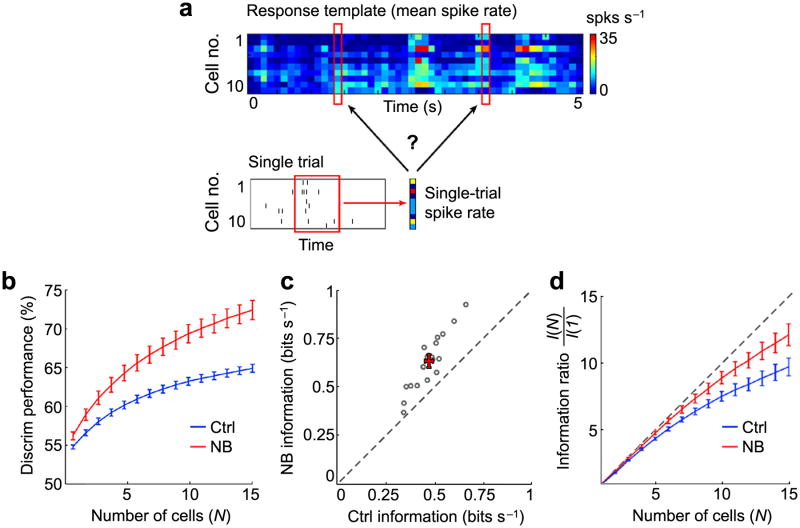
Increased reliability and decreased correlation both contribute to improved coding of natural stimuli. (a) Schematic illustration of the discrimination analysis. The population response during a given stimulus segment (100 ms, red box) in each trial was classified as one of two categories based on its Euclidean distances from the two templates (population responses averaged across trials). (b) Mean discrimination performance (% of correct classifications) as a function cell number (N) included in the population analysis for control and NB conditions (12 experiments). (c) Single-neuron information [I(1)] before and after NB stimulation. Each point represents I(1) averaged across all cells in each experiment (19 experiments). (d) Information ratio [I(N)/I(1)] as a function of cell number (N) before and after NB stimulation (12 experiments). Diagonal line indicates linear summation of information (no redundancy between cells). Error bars, ± s.e.m.
To further investigate the effect of NB stimulation on visual coding, we evaluated the discrimination performance using an information theoretic measure (see Online Methods). Using single-neuron information, a measure unaffected by between-cell correlations, we found increased information after NB stimulation in all 19 experiments (P < 0.001, Wilcoxon signed-rank test; Fig. 7c). This is likely to reflect the contribution of increased response reliability of individual neurons. To test the effect of decorrelation on neural coding, we calculated the information ratio, defined as the total information from N cells [I(N), 1 ≤ N ≤ 15, averaged across all combinations of N cells in each recording] divided by the single-neuron information [I(1), averaged across all single neurons in each recording]. For this measure (Fig. 7d), the ratio I(N)/I(1) should be equal to N (diagonal line) if there is no redundancy between neurons. We found that in both control and NB conditions, the ratio was lower than N, reflecting redundancy between cells. However, the redundancy was significantly reduced by NB activation (P < 0.05 for N = 7 to 15 cells, Wilcoxon signed-rank test, with Bonferroni correction; 12 experiments). Thus, whereas the NB-induced improvement in response reliability increased the information coded by individual neurons (Fig. 7c), the decorrelation was associated with decreased redundancy among a population of neurons (Fig. 7d).
Discussion
Previous studies have shown that local application of ACh or AChR agonists can affect the firing rate and receptive field properties of visual cortical neurons31–34,42, although the observed changes were diverse across neurons and sometimes inconsistent between studies. In this study, by directly activating the source of cholinergic projections to the entire cortex, we have demonstrated two robust effects of NB activation on visual coding, mediated by distinct mechanisms. Furthermore, we found that both the decorrelation and increased reliability contribute to improved discriminability of the neuronal responses to different natural stimuli, a hallmark of enhanced visual coding.
In rat neocortex, nAChRs are expressed primarily in layer 4 (and to a lesser extent in layer 5) in both cortical neurons and thalamic terminals, whereas mAChRs (M1–M4) are expressed throughout the cortex, with different subtypes preferentially expressed in different layers43. Thalamocortical input is shown to be enhanced by nAChR activation, while excitatory intracortical synaptic activity can be suppressed by mAChRs24,25. The mAChR-mediated hyperpolarization of fast-spiking interneurons may also suppress thalamocortical feedforward inhibition23,26. Based on these effects at the cellular level, a common view is that ACh enhances thalamocortical inputs but suppresses intracortical interactions24–27,44. Our finding that NB-induced decorrelation between neurons requires local activation of mAChRs is consistent with this notion. However, while previous studies showed that activation of cortical AChRs increases the amplitude of thalamocortical input30,45, we found that the improved response reliability was not reduced by local block of either mAChRs or nAChRs in the cortex (Fig. 5). This indicates that the NB-induced improvement in response reliability is not due primarily to the increased amplitude of thalamocortical input, but involves changes in the sensory signals earlier in the processing pathway. Our observation is also consistent with a recent finding that iontophoretic application of ACh in the visual cortex does not reduce the trial-to-trial variability of the neuronal responses34.
Our experiments showed that NB stimulation increases the response reliability and decreases the burst-tonic ratio of LGN neurons (Fig. 6). Since NB does not project directly to the LGN, these effects must be mediated by indirect pathway(s) (through either cholinergic or GABAergic projections from the NB46,47), such as NB → cortex → LGN, NB → thalamic reticular nucleus → LGN22,46, or NB → brainstem reticular formation → LGN22,46 (although the lack of effect of atropine and mecamylamine injected into the LGN argues against the involvement of cholinergic projection from the reticular formation). These pathways could depolarize LGN neurons29 and inactivate T-type Ca2+ channels22,39, thereby increasing the response reliability and reducing the burst-tonic ratio. The changes in the LGN activity are likely to improve the fidelity of the sensory input to the cortex and thus explain the NB-induced increase in cortical response reliability (Fig. 4). Such distributed improvement of neuronal responses along the sensory pathway could contribute to NB-induced enhancement of sensory cortical plasticity9,11, in addition to the local cholinergic effects in the cortex10. An intrinsic limitation of the extracellular stimulation technique is that it is difficult to distinguish the contributions of cholinergic and GABAergic NB neurons and to rule out the potential activation of axons originating from other brain regions passing through the NB. Future studies using optogenetic techniques48 to activate specific cell types may help to determine the role of each cell type in regulating sensory coding along the thalamocortical pathway.
The activity of cholinergic neurons in the basal forebrain undergoes striking changes from the sleep to awake states12,13,15. Recent studies showed that even in the awake state NB activity varies in a task-dependent manner14,15, and it may be involved in regulating sensory processing under different forms of uncertainty49. Since NB receives input from both subcortical regions and prefrontal cortex7, it may act as a way station for both bottom-up and top-down signals to regulate sensory coding in a behaviorally-relevant manner. Our finding that NB activation can rapidly improve visual representation in the cortex offers a mechanism by which perceptual abilities are enhanced during wakefulness, arousal, and attention.
Methods
Surgery
All experimental procedures were approved by the Animal Care and Use Committee at the University of California, Berkeley. Adult male Long-Evans rats (250–350 g) were anesthetized with urethane (i.p., 1.45 g kg−1). Animals were restrained in a stereotaxic apparatus (David Kopf Instruments); body temperature was maintained at 37.5° C via a heating pad. Bipolar stimulating electrodes were stereotaxically implanted in the left nucleus basalis (NB), and NB was stimulated with trains of 50 pulses (0.1 ms pulse−1) at 100 Hz. A craniotomy (diameter ~1 mm) was made either above the monocular region of left V1 or above the left LGN. A small portion of the dura was removed to allow insertion of a silicon polytrode (27 active channels separated by 50 μm, NeuroNexus Technologies). Signals were recorded with the Cheetah 32-channel acquisition system (Neuralynx) at 30 kHz. The right eye was fixed with a metal ring to prevent eye movement and irrigated with sterile saline. Following the experiment, animals were euthanized with an overdose of isoflurane. A total of 49 rats were used in this study.
Histochemistry
For histochemistry experiments, the animal was deeply anesthetized with urethane and immediately perfused with chilled 4% paraformaldehyde (PFA) solution in 0.1 M PBS. The brain was removed and fixed in 4% PFA/PBS solution overnight at 4° C. After fixation, the brain was sectioned into 150 μm horizontal slices using a vibratome (Serial 1000 Tissue Sectioning System, Ted Pella Inc). For acetylcholinesterase histochemistry, slices were incubated in 4 mM acetylcholine iodide, 4 mM copper sulphate, and 16 mM glycine in a 50 mM sodium acetate solution (pH 5.0) for 15 hr at 23° C and developed in 1% sodium sulphide solution (pH 7.0) for 10 min at 23° C. This procedure was performed in 15 of the 49 experiments included in this study.
Pharmacology
For topical drug application, a microwell was made by gluing a plastic ring to the skull surrounding the craniotomy. During application, antagonists to mAChRs (atropine, 1 mM) or nAChR (mecamylamine, 1–10 mM) were loaded into the microwell 15 min prior to recording. For intracortical injection, a glass micropipette (tip size ~10 μm) was fixed to the front of the polytrode such that the tip was close to the center of the polytrode recording sites (layer 4 or 5). Pharmacological antagonists to mAChRs (atropine, 100 μM) or nAChRs (mecamylamine, 100 μM – 1 mM; DHβE, 100 μM) were injected at a rate of 15 nl min−1 using a Hamilton syringe and a syringe pump, starting 15 min prior to and continuing throughout the recording period (total volume ~500 nl). Topical and intracortical pharmacology experiments were combined, since results were very similar for both methods.
To block LGN nAChRs and mAChRs (Supplementary Fig. 7 online), a mixture of 100 μM atropine and 100 μM mecamylamine was injected at 15 nl min−1 starting 15 min prior to and continuing throughout the recording. The volume of injection (~500 nl) was determined in pilot experiments using dye injections to achieve coverage of most of the LGN without going much beyond its borders. To further ensure that the LGN neurons projecting to the recorded region of V1 were well exposed to the antagonists, we bonded a tungsten electrode to the injection pipette and mapped the LGN multiunit receptive field as closely as possible to the V1 multiunit receptive field.
Visual stimuli
Visual stimuli were generated with a PC computer containing a NVIDIA GeForce 6600 graphics board and presented with a XENARC 700V LCD monitor (19.7 cm × 12.1 cm, 960 × 600 pixels, 75 Hz refresh rate, 300 cd m−2 maximum luminance, gamma corrected with custom software) located 14 cm from the right eye, positioned such that the receptive fields of the recorded neurons were at the center of the monitor. For natural stimuli, three 5 s clips were selected from the van Hateren natural movie database. Each image (64 × 64 pixels, 36° × 36°, mean contrast 43%) was updated every 3 refresh frames, corresponding to an effective frame rate of 25 Hz. To avoid onset and offset transients, the first frame was displayed for 1 s prior to the movie and the last frame was displayed for 1 s following the movie.
Each experiment consisted of 6 blocks, and each block consisted of 5 repeats of the three movies under control and 5 repeats under NB conditions (Supplementary Fig. 4a online). Under the control condition, the movies were repeated 5 times with 2 s of static image before each movie. Under the NB condition, the movies were repeated 5 times as in the control condition, and NB stimulation was administered from 1000 ms to 500 ms prior to the start of each movie. To ensure that the effect of NB stimulation had diminished before the start of control trials, a blank frame was shown for 30 s following each block. Each experiment thus consisted of 30 movie repeats under control and 30 repeats under NB conditions. In blocks 1 to 3, the control repeats preceded the NB repeats; in blocks 4 to 6, the sequence was reversed to further eliminate the potential effect of stimulus history on cortical responses.
For measuring receptive fields (Supplementary Fig. 5 online), sparse noise consisting of random flashes of white and black pixels (100% contrast, 4.5° pixel−1, 100 ms per flash) on a gray background was used. For measuring orientation tuning and direction selectivity (Supplementary Fig. 6 online), drifting gratings (100% contrast, 2 Hz, 0.04 cycles deg−1)were used.
Analyses
Local field potential analysis was carried out using Gabor/Morlet wavelet decomposition (http://dxjones.com/matlab/timefreq/). For single unit isolation, polytrode contact sites (channels) were separated into groups (2–4 channels per group) and spike waveforms were sorted using NeuroScope (http://neuroscope.sourceforge.net), NDManager (http://ndmanager.sourceforge.net), and Klusters (http://klusters.sourceforge.net)50. In some instances, a single neuron was picked up by more than one electrode group. To ensure that duplicate neurons were not included in the subsequent analyses, pairwise between-neuron correlation coefficients (CC; binned at 1000 Hz) were calculated following clustering; for any pair with CC > 0.1, the cell with lower firing rate was discarded. For the remaining single units, only those with firing rates > 0.5 spikes s−1 were included in further analyses, performed in MATLAB (The Mathworks).
For calculation of between-cell and between-trial CC (Figs. 2 and and3),3), firing rates were binned at 10 Hz (although results were similar for binning from 5 Hz to 25 Hz). To determine whether a given cell was visually driven, we compared the average between-trial CC within movies and between movies. Only cells that had significantly higher within-movie CC (threshold α = 0.01, Wilcoxon signed-rank test) were included in further analyses. The burst-tonic ratio was calculated by measuring the number of burst spikes (two or more spikes occurring with ISI < 4 ms following an absence of spiking for > 100 ms) relative to the number of tonic spikes (all spikes not meeting the burst criteria).
To quantify the ON and OFF receptive fields measured with sparse noise (Supplementary Fig. 5 online), each receptive field was fitted with a two-dimensional Gaussian function:
where R(x,y) is the response at pixel position (x,y), a0 is the DC component, a is the receptive field peak amplitude, (x0,y0) is the receptive field center, and σM and σm are the standard deviations along the major and minor axes. The receptive field size is measured by πσMσm, and the amplitude/baseline ratio is measured by a/a0.
To measure orientation tuning and direction selectivity (Supplementary Fig. 6 online), firing rate as a function of orientation was fitted as the sum of two Gaussians with peaks 180° apart:
where R(θ) is the response at orientation θ, a0 is the DC component, a1 and a2 are the amplitudes of the two Gaussians, θ0 is the preferred orientation, and σ is the standard deviation. Tuning width is measured by σ, and direction selectivity is measured by [R(θ0)−R(θ0+180°)]/[R(θ0)+R(θ0+180°)].
For the discrimination analysis (Fig. 7), the responses were also binned at 10 Hz. For each discrimination, the single-trial response in a given bin (Ai, where i is the trial number) was compared to the mean responses (averaged across trials) in the same bin (<A>) and in a different bin (<B>) based on the Euclidian distances d(Ai, <A>) and d(Ai, <B>). The classification was considered correct if d(Ai, <A>) < d(Ai, <B>), and incorrect if d(Ai, <A>) > d(Ai, <B>). Discrimination performance was assessed by the percentage of correct classifications for all the trials. Since the discrimination performance is expected to increase nonlinearly with the number of neurons, it is difficult to measure the redundancy between neurons. We thus converted the discrimination performance into a measure of information as I = 1 + (p)log2(p) + (1−p)log2(1−p)), where p is the discrimination performance (note that when discrimination is at chance level, p = 50%, I = 0). This definition of I represents the mutual information between the actual stimulus and the stimulus decoded from the neuronal response by the ‘ideal observer’ in the discrimination analysis. I(N) is computed as the average information across all combinations of N simultaneously recorded cells in each experiment, and it should increase linearly with N if there is no redundancy between neurons. Downward deviation from the diagonal line in Fig. 7d thus reflects the degree of redundancy. For population analyses (Fig. 7b,d), only experiments with ≥ 15 simultaneously recorded single units were included (12/19 experiments).
To test statistical significance, Wilcoxon signed-rank test was used for paired samples and Wilcoxon rank-sum test was used for unpaired samples; multiple comparisons were corrected with the Bonferroni method.
Acknowledgments
This work was supported by grants from the US National Institutes of Health to Y.D., and a Ruth L. Kirschstein National Research Service Award to M.G. (Award Number F31NS059258 from the US National Institute of Neurological Disorders and Stroke). We thank T. Blanche, D. Feldman, R. Froemke, D. Jones, D. Kleinfeld, C. Niell, and A. Vahidnia for technical help and useful discussions.
Footnotes
Author Contributions: M.G. conducted all experiments; M.G. and Y.D. designed the experiments and wrote the manuscript.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nn.2402
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3576925?pdf=render
Citations & impact
Impact metrics
Article citations
Vagus nerve stimulation recruits the central cholinergic system to enhance perceptual learning.
Nat Neurosci, 27(11):2152-2166, 16 Sep 2024
Cited by: 0 articles | PMID: 39284963
Vasoactive intestinal peptide-expressing interneurons modulate the effect of behavioral state on cortical activity.
Front Cell Neurosci, 18:1465836, 12 Sep 2024
Cited by: 0 articles | PMID: 39329085 | PMCID: PMC11424404
Simultaneous interneuron labeling reveals cell type-specific, population-level interactions in cortex.
iScience, 27(9):110736, 21 Aug 2024
Cited by: 1 article | PMID: 39280622 | PMCID: PMC11399611
Differential stability of task variable representations in retrosplenial cortex.
Nat Commun, 15(1):6872, 11 Aug 2024
Cited by: 0 articles | PMID: 39127731 | PMCID: PMC11316801
Whole brain alignment of spatial transcriptomics between humans and mice with BrainAlign.
Nat Commun, 15(1):6302, 30 Jul 2024
Cited by: 0 articles | PMID: 39080277 | PMCID: PMC11289418
Go to all (366) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Control of cerebral cortical blood flow by stimulation of basal forebrain cholinergic areas in mice.
J Physiol Sci, 61(3):201-209, 20 Mar 2011
Cited by: 16 articles | PMID: 21424590 | PMCID: PMC10717047
Integrated contributions of basal forebrain and thalamus to neocortical activation elicited by pedunculopontine tegmental stimulation in urethane-anesthetized rats.
Neuroscience, 119(3):839-853, 01 Jan 2003
Cited by: 58 articles | PMID: 12809705
Intermittent stimulation in the nucleus basalis of meynert improves sustained attention in rhesus monkeys.
Neuropharmacology, 137:202-210, 27 Apr 2018
Cited by: 11 articles | PMID: 29704983 | PMCID: PMC6553880
Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention.
Brain Res, 1314:112-123, 21 Aug 2009
Cited by: 59 articles | PMID: 19699722 | PMCID: PMC2819645
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NEI NIH HHS (1)
Grant ID: R01 EY018861
NINDS NIH HHS (2)
Grant ID: F31 NS059258
Grant ID: F31NS059258





