Abstract
Objective
Transverse sinus stenosis (TSS) is common in idiopathic intracranial hypertension (IIH), but its effect on the course and outcome of IIH is unknown. We evaluated differences in TSS characteristics between patients with IIH with "good" vs. "poor" clinical courses.Methods
All patients with IIH seen in our institution after September 2009 who underwent a high-quality standardized brain magnetic resonance venogram (MRV) were included. Patients were categorized as having a good or poor clinical course based on medical record review. The location and percent of each TSS were determined for each patient, and were correlated to the clinical outcome.Results
We included 51 patients. Forty-six patients had bilateral TSS. The median average percent stenosis was 56%. Seventy-one percent of patients had stenoses >50%. Thirty-five of the 51 patients (69%) had no final visual field loss. Eight patients (16%) had a clinical course classified as poor. There was no difference in the average percent stenosis between those with good clinical courses vs those with poor courses (62% vs. 56%, p = 0.44). There was no difference in the percent stenosis based on the visual field grade (p = 0.38). CSF opening pressure was not associated with either location or degree of TSS.Conclusion
TSS is common, if not universal, among patients with IIH, and is almost always bilateral. There is no correlation between the degree of TSS and the clinical course, including visual field loss, among patients with IIH, suggesting that clinical features, not the degree of TSS, should be used to determine management in IIH.Free full text

Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis
Abstract
Objective:
Transverse sinus stenosis (TSS) is common in idiopathic intracranial hypertension (IIH), but its effect on the course and outcome of IIH is unknown. We evaluated differences in TSS characteristics between patients with IIH with “good” vs “poor” clinical courses.
Methods:
All patients with IIH seen in our institution after September 2009 who underwent a high-quality standardized brain magnetic resonance venogram (MRV) were included. Patients were categorized as having a good or poor clinical course based on medical record review. The location and percent of each TSS were determined for each patient, and were correlated to the clinical outcome.
Results:
We included 51 patients. Forty-six patients had bilateral TSS. The median average percent stenosis was 56%. Seventy-one percent of patients had stenoses >50%. Thirty-five of the 51 patients (69%) had no final visual field loss. Eight patients (16%) had a clinical course classified as poor. There was no difference in the average percent stenosis between those with good clinical courses vs those with poor courses (62% vs 56%, p = 0.44). There was no difference in the percent stenosis based on the visual field grade (p = 0.38). CSF opening pressure was not associated with either location or degree of TSS.
Conclusion:
TSS is common, if not universal, among patients with IIH, and is almost always bilateral. There is no correlation between the degree of TSS and the clinical course, including visual field loss, among patients with IIH, suggesting that clinical features, not the degree of TSS, should be used to determine management in IIH.
Idiopathic intracranial hypertension (IIH) is a syndrome of isolated elevated intracranial pressure (ICP) of unknown etiology, responsible for visual loss from papilledema in about 25% of cases.1 Transverse sinus stenosis (TSS) is commonly described in patients with IIH, and likely contributes to intracranial hypertension via venous hypertension when the stenosis is bilateral and severe enough to compromise cerebral venous outflow.2–4 Previous reports5–7 have described improvement in these stenoses after CSF removal, suggesting that some stenoses may be secondary to increased ICP rather than causal. However, it remains unclear what role TSS plays in the pathogenesis of IIH, its visual prognosis, and response to treatment. Over the past decade, endovascular stenting of TSS has been proposed as a possible treatment for refractory IIH.8,9 Some clinicians believe that the presence of obvious TSS should alter the management of patients with IIH, whereas others do not take the presence or degree of TSS into account when considering management strategies.9–11
Our aim was to determine the characteristics of TSS within an IIH cohort and to determine if the degree of anatomical TSS influences the clinical course or visual outcome of patients with IIH.
METHODS
Standard protocol approvals, registrations, and patient consents
This retrospective study was granted a waiver of informed consent by our institutional review board.
Study conduct
Patients with definite IIH according to the most recent diagnostic criteria12 were identified using our database, and their medical records were reviewed to determine eligibility. Patients were included if they had a high-quality contrast-enhanced magnetic resonance venography (MRV) study performed at our institution since September 2009, when an improved technology for MRV was implemented. Patients were excluded if the MRV was deemed to be of inadequate quality by our neuroradiologist (A.M.S.). All included patients were evaluated in a standardized fashion by experienced neuro-ophthalmologists (B.B.B., V.B., N.J.N.), including documentation of body habitus, detailed neuro-ophthalmic examination with formal visual field (VF) testing (static perimetry using a Humphrey automated perimeter [HVF] or kinetic perimetry using a Goldmann perimeter [GVF]), review of lumbar puncture (LP) data (including CSF opening pressure), and review of neuroimaging tests. Management strategies were similar for all patients included in this study, including diagnostic LP, discontinuation of any medications possibly associated with the development of raised ICP, the use of acetazolamide with total daily dosages between 500 and 1,500 mg, and recommendation for weight loss. Surgery (either CSF-shunting procedure or optic nerve sheath fenestration) was performed in fulminant forms,13 in patients with severe visual loss at presentation, when the visual function was abnormal at baseline and did not improve within a few weeks, when visual function deteriorated despite maximal medical therapy, or for intractable headaches clearly related to elevated ICP. None of our patients underwent endovascular venous stenting.
In order to compare HVF and GVF tests, we used a 3-point scale to evaluate the severity of VF loss in our patient population. The degree of final VF loss was graded on a 0 to 2 point scale: 0) normal or enlarged blind spot, 1) mild or moderate VF deficit, 2) severe VF deficit by consensus review of 2 neuro-ophthalmologists (B.D.R. and B.B.B.). HVF mean deviations were recorded when available.
Because surgical procedures are rarely needed in the management of IIH and usually reflect a more severe form of IIH (despite an often good outcome if the procedure was performed at an appropriate time), we chose to describe the overall clinical course in addition to the final VF outcome. Charts of eligible patients were reviewed to determine the clinical course. We classified all eligible patients into 2 groups: those with “good” and those with “poor” clinical courses based on review of the entire clinical course. The group considered to have a poor clinical course included patients who had fulminant disease,13 had progressive VF defects, or required a surgical procedure for IIH management. Patients with a good clinical course had none of the poor clinical features.
MRI was performed using a standardized protocol at either 3.0 T (Siemens Trio, Erlangen, Germany) or 1.5 T (Siemens Avanto or GE Signa, Milwaukee, WI) using a standard head coil. All patients underwent standardized contrast-enhanced MRI along with contrast-enhanced MRV. The MRI/MRV protocol included routine precontrast axial diffusion-weighted, T1-weighted, T2-weighted gradient-recalled echo (GRE) and sagittal T1-weighted images. An axial precontrast MRV mask was obtained (repetition time 4–6 msec, echo time 1–2 msec, flip angle of 22–30 degrees with slice thickness of 0.8–1.4 mm). A standard dose (0.1 mmol/kg) IV gadolinium-based contrast agent (Multihance, Bracco Diagnostics Inc.) was administered at 2.0 cc/s, and the axial MRV sequence was repeated 60 seconds following contrast administration. Postcontrast axial T2-weighted and T1-weighted and sagittal volumetric T1-weighted GRE images of the brain were then acquired. The precontrast MRV dataset was subtracted from the postcontrast dataset, and multiple oblique maximum intensity projections (MIP) were generated from this subtracted dataset with rotation around the craniocaudal axis (“spin”) or the transverse axis (“nod”) at 6-degree increments.
Curved reformats of each transverse sinus (TS) (figure 1) were generated using the source images for each patient. The area of greatest stenosis of each TS was identified and measured. Appropriately accounting for the workstation field of view, the length and width of the stenosis and the distance from the torcular to the midpoint of the stenosis were measured. The apparent width of the TS at the point of the greatest stenosis was measured based on the adjacent nonstenosed sinus. The percent of stenosis was calculated by dividing the width of the stenosis by the apparent normal width of the sinus. The average of the right and left TSs of each patient was calculated to provide a single measure of average percent stenosis for each patient. An average distance from the torcular was calculated in a similar fashion. Any sinus without a discernable stenosis was excluded from the calculation of the average distance from the torcular. Any sinus with multiple areas of stenosis was measured at the point of greatest stenosis. To incorporate the length of stenosis, a length-weighted percent stenosis was calculated by a weighted average of the 2 sides' percent stenosis by the respective length of stenosis. To provide an estimate of total venous outflow capacity, a residual area was measured as the sum of the squares of the difference of sinus width and stenosis width bilaterally. All MRV reformatting and measurements were performed by a single investigator (B.D.R.) blinded to the clinical outcome and the measurements were confirmed to be accurate by a neuroradiologist (A.M.S.). Asymmetry between the 2 TSs was noted and right or left TS dominance was documented. Codominance was defined as less than 5% difference between the 2 sinuses. Hypoplastic or aplastic TSs were also recorded.
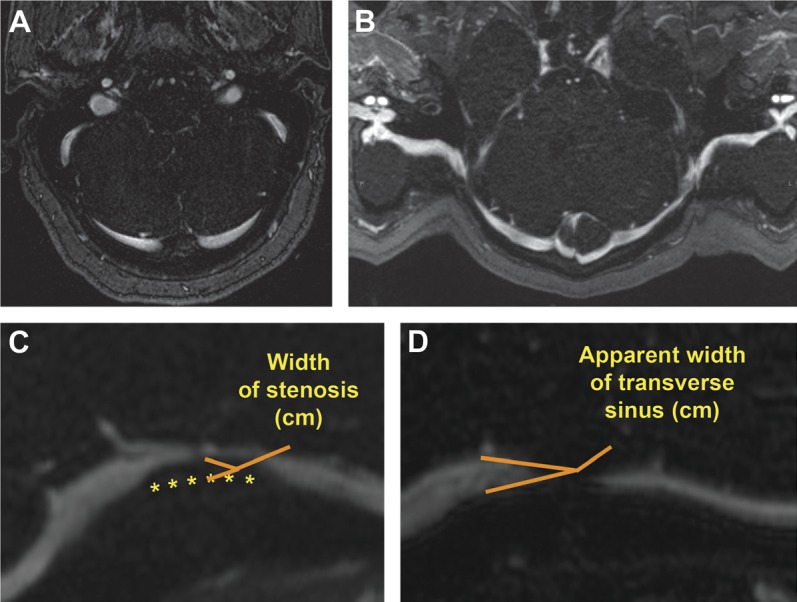
The entire course of the transverse sinus in a single axial magnetic resonance venography image (A) is difficult to visualize, but with curved reformatting (B), the entire course of the transverse sinus is apparent. The percent stenosis was calculated by dividing the width of stenosis (C) by the width of the adjacent normal-appearing transverse sinus (D), yielding the percent stenosis.
Statistical analysis
Statistical analysis was performed with R: A language and environment for statistical computing (R Foundation for Statistical Computing, http://www.R-project.org). Medians and interquartile ranges (IQR) were reported for continuous data; percentages were reported for categorical data. Distributions of continuous variables by categorical variables were compared using the Wilcoxon Mann-Whitney rank sum or Kruskal-Wallis tests depending on the number of subgroups. Proportions were compared using Fisher exact tests. Linear regression analysis was performed between continuous variables. Two-tailed p values <0.05 were considered statistically significant.
RESULTS
Seventy-one patients with definite IIH had a contrast-enhanced MRI/MRV at our institution during the study enrollment period and were eligible for inclusion. Twenty patients were excluded due to either nonavailability of the data or a poor contrast bolus, leading to inadequate imaging for accurate stenosis measurement.
The 51 included IIH patients were mostly women (92%), with median age 29 years and median body mass index 39 kg/m2. Black patients constituted 53% of our cohort, consistent with the patient population seen at our institution. All patients had a LP showing an elevated ICP, and the median CSF opening pressure was 35 cm H2O (IQR 29–43 cm H2O). Three patients had LPs performed prior to MRV, but all of them had persistent bilateral TSS on MRV. Forty-three patients had good clinical courses, with 35 of these having no persistent VF loss. Eight patients (16%) had clinical courses classified as poor. Two of these patients had no VF loss (VF score of 0), but had poor clinical courses due to intractable headache secondary to raised ICP relieved only by ventriculoperitoneal shunting.
By our methodology, all 51 patients had some degree of unilateral or bilateral TSS. Twenty-four patients (47%) were right TS dominant, 19 (37%) were left TS dominant, and 8 (16%) were codominant. Five patients (9.8%) had unilateral TSS, but 4 of these 5 patients (80%) had a contralateral hypoplastic sinus. The range of the average percent stenosis was 19%–96%; the median average percent stenosis of the distal TSS was 56% (IQR 49%–65%) (figure 2), and the median average length weighted percent stenosis was 60% (IQR 51%–70%). Seventy-one percent of patients had 50% or greater average percent stenosis. The median residual area was 16.8 mm2 (range 0.49–67.3 mm2).
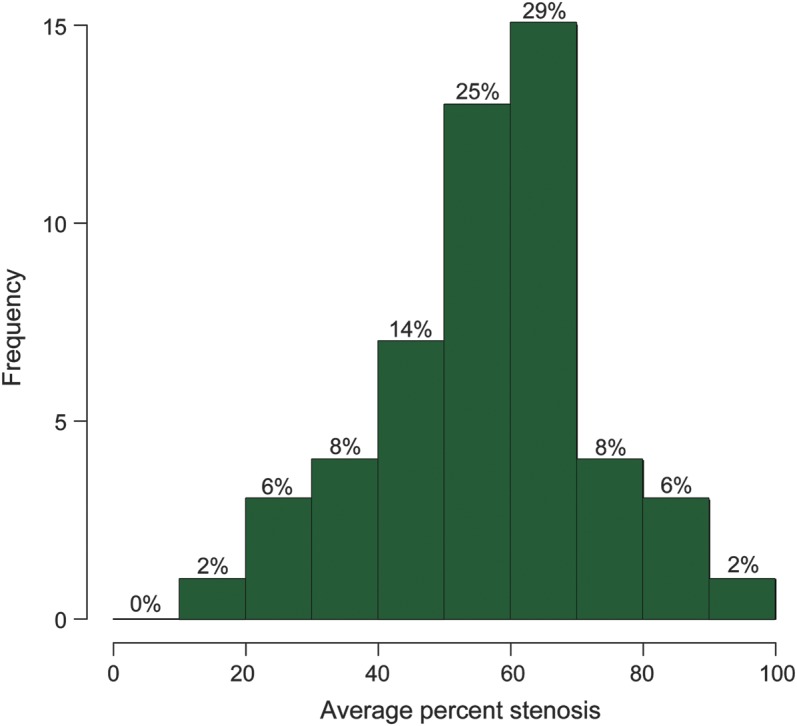
The average percent stenosis ranged from 19% to 96% with a median of 56% (interquartile range 49%–65%). Seventy-one percent of patients had 50% or greater average percent stenosis.
The degree of stenosis of the TS was not associated with the location of the stenosis along the TS, defined as the distance from the torcular to the midpoint of the stenosis (−0.001 cm from torcular/percent stenosis, p = 0.84). The CSF opening pressure was not associated with either location along the TS (+0.02 cm from torcular/cm H2O, p = 0.11), the degree of TSS (figure 3, −0.0027% stenosis/cm H2O, p = 0.28), or the residual area (0.0311 mm2/cm H2O, p = 0.78). Of note, the patient with the highest opening pressure (60 cm) had the lowest average percent stenosis (19%).
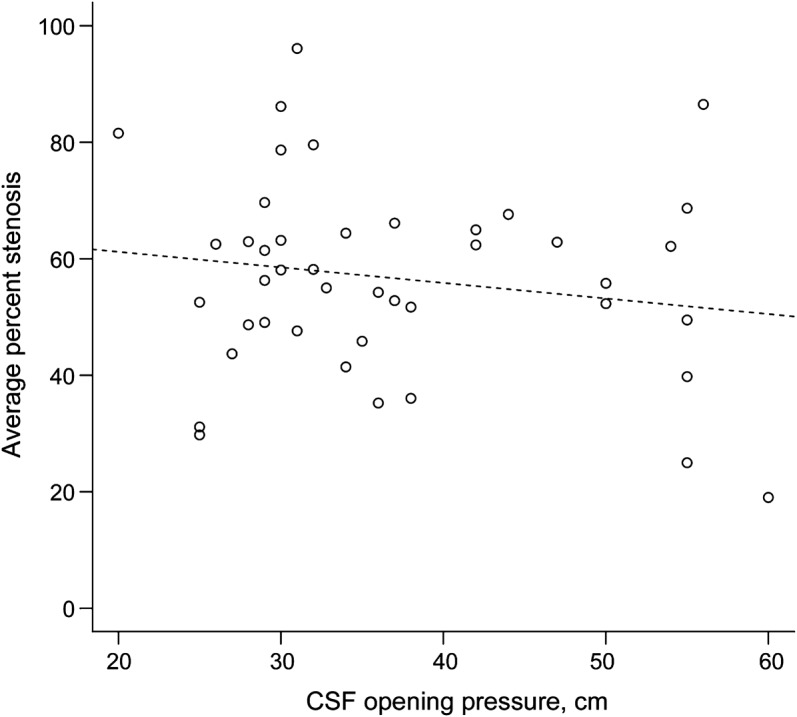
There was no relationship between the two (slope: −0.0027 percent stenosis/cm H2O, p = 0.28).
Comparing those patients with good clinical courses vs those with poor courses (figure 4), there was no difference in the average percent stenosis (62% vs 56%, p = 0.44), the average length weighted percent stenosis (64% vs 57%, p = 0.23), or the residual area (17 vs 14, p = 0.27). The final VF deficit (figure 5) was not associated with either the average percent stenosis (VF grade 0: 58.1%, VF grade 1: 54.0%, VF grade 2: 57.6%; p = 0.38) or the residual area (VF grade 0: 17 mm2, VF grade 1: 15 mm2, VF grade 2: 18 mm2; p = 0.81). Likewise, for the 47 patients who had HVFs, there was no correlation between the mean deviation (average of both eyes or worst eye) and either the average percent stenosis or the residual area. Having unilateral TSS did not correlate with the clinical course (p = 0.58) nor with the presence or absence of a VF defect (p = 1.0). However, none of the 5 patients with unilateral TSS had a poor clinical course and only 1 had some nasal VF loss at final evaluation.
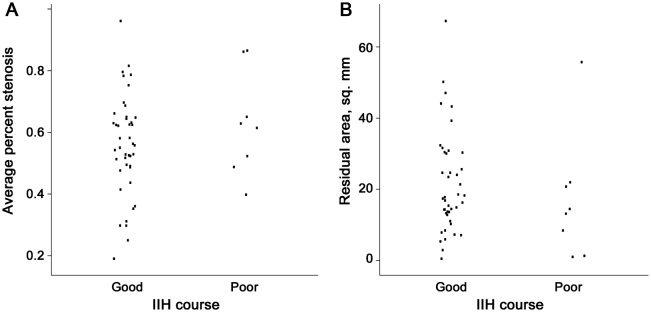
Average percent stenosis (A) and residual area (B). There was no association with either transverse sinus stenosis (p = 0.44) or residual area (p = 0.27). IIH = idiopathic intracranial hypertension.
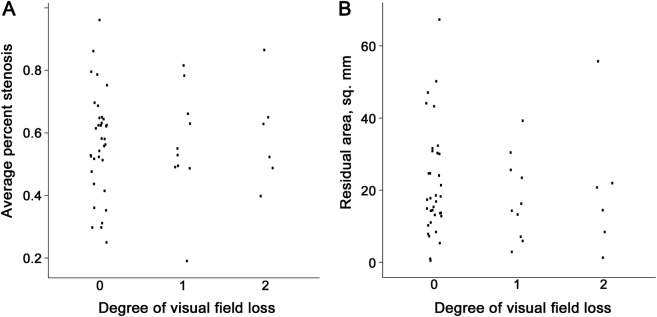
Average percent stenosis (A) and residual area (B). Degree of visual field loss: 0 = none, 1 = mild/moderate, 2 = severe. There was no association with either transverse sinus stenosis (p = 0.98) or residual area (p = 0.81).
There was no association between the location of the stenosis along the course of the TS and the clinical course (5.8 vs 6.2, p = 0.61) or VF loss (VF grade 0: 5.8, VF grade 1: 5.9, VF grade 2: 6.5; p = 0.58). There were trends toward higher CSF opening pressure predicting both a poor clinical course (odds 1.9 times higher per 10 cm increase in CSF opening pressure, 95% confidence interval 0.9–4.0, p = 0.10) and greater VF loss (odds of one level increase in VF degree 1.8 times per 10 cm increase in CSF opening pressure, 95% confidence interval 1.0–3.3, p = 0.054).
DISCUSSION
All of our patients with IIH had some degree of TSS, but we found no association between the degree of TSS in these patients and their clinical course, including the severity of their VF loss. While all of our patients with unilateral TSS had a good clinical course, and all but one had a normal VF grade, this finding did not reach significance. Even if patients with lower degrees of stenosis tend to have better outcomes, our study shows that higher degrees of stenosis do not necessarily portend a poor outcome. Indeed, the patient with the most severe stenosis had a good clinical course and normal VFs. This was surprising given the prevailing assumption that TSS is on a pathologic feedback loop that would be expected to increase ICP and thus lead to poorer outcomes.3,14
Prior studies have attempted to describe the stenoses seen in patients with IIH, in some cases semiquantitatively.15–18 Varying frequencies of TSS in patients with IIH have been reported, with generally a very high prevalence (65%–100%).15–17,19 These differences can be explained by differences in the imaging techniques used to observe the stenoses (i.e., digital subtraction angiography, time-of-flight MRV, measurement of pressure gradients). Prior to the development of more recent MRV techniques, interpretation of MRVs and quantification of TSS was plagued by “flow-gaps” that are likely artifactual in nature, related to turbulent flow.20 With recent advances in MRV technology and gadolinium-enhanced studies, these artifacts are minimized and TSS can be better defined.21
Another major difficulty in determining the true prevalence of TSS stems from the varying methodologies used in assessing stenosis. Because there is no accepted definition of what constitutes clinically relevant venous stenosis,22,23 we used direct measurements of the stenoses rather than determining only the presence or absence of stenosis or broadly categorizing the degree of stenosis. From our absolute measurements, we derived 2 quantitative global measures of venous stenosis: average percent stenosis and residual area. If stenosis is defined as any narrowing of the TS compared to adjacent “normal” TS, we found that stenosis occurs almost universally in IIH.
In a previous study of TSS, 3 neuroradiologists blinded to the diagnosis of IIH reviewed contrast-enhanced images acquired using the auto-triggered elliptic-centric-ordered (ATECO) gadolinium-enhanced MRV technique.21 They devised a semiquantitative scale to measure the degree of TSS. Based on a cutoff that they derived by comparison to controls, TSS was present in over 90% of patients with IIH but in only 7% of controls, leading them to conclude that TSS is an expected finding in IIH. This concurs with our findings, although we did not include control patients to address the prevalence of TSS in comparison to normal patients. Our MRI technique is similar to the ATECO technique used in their study,17 but we believe our technique has several advantages over the ATECO technique, which is proprietary to the other investigators' institution. Our technique has a shorter acquisition time (3 minutes vs 4.5 minutes), requires minimal postprocessing, uses a predetermined time delay rather than being triggered when contrast reaches a given location (resulting in no mistimed studies in our cases), and is performed using commercially available technology.
In agreement with prior investigators,17 we found no association between CSF opening pressure and the degree of TSS. This lack of association may be explained by the development of collateral flow, thereby maintaining overall venous resistance. Blood flow through enlarged dural veins in superior sagittal sinus occlusion has been demonstrated, and may represent one collateral path.24 Two additional paths include the vein of Labbe25 (a cortical vein that drains the lateral surface of the hemispheres) and the occipital sinus26 (draining directly from the torcular). Both of these structures can, albeit with variation, drain into the sigmoid sinus, bypassing the distal TS where the majority of stenoses in patients with IIH occur.
Given recent literature27–30 reporting improvement in papilledema, headache, and CSF opening pressure with endovascular stenting of TSS, this procedure has become a potential treatment strategy for IIH. Some investigators have suggested performing systematic catheter venography with measurement of pressure gradients to determine if the stenosis is hemodynamically significant; if so, stenting is recommended.27 Our study suggests that more severe degrees of anatomic TSS are not a reliable indicator of poor prognosis in IIH, perhaps because the effects of these gradients are mitigated by collateral flow. Thus, we believe that presence and severity of anatomic TSS should not be used alone to guide the aggressiveness of treatment and should not necessarily prompt invasive venography for manometry and pressure gradient measurement. However, if further studies demonstrate long-term safety of TS stenting, this procedure may prove to be a reasonable option for patients with IIH in whom the clinical course is poor and the usual therapeutic options are exhausted.
One limitation of our study is the lack of a comparison group, limiting our ability to assess the degree of stenosis typical of normal individuals. In addition, while our quantitative measurements have advantages over qualitative and semiquantitative measures, our technique only assesses the point of maximal stenosis instead of the overall contour of the venous sinus or magnetic resonance flow-related changes, either of which could provide additional information regarding the severity of stenosis. However, the simplicity and noninvasive nature of our techniques for measuring the anatomic TSS will allow for more widespread application in clinical practice. Finally, currently available noninvasive techniques do not allow the easy and reliable measurement of the venous pressure gradient across a TSS. Thus, it remains possible that pressure gradient measurements across a TSS could predict the clinical course or outcome of patients with IIH. While we are unable to make any conclusions based on our data regarding the relationship between venous pressures and IIH, it is likely that the anatomic presence of TSS on MRV is not helpful for making treatment decisions for patients with IIH.
We present the only study, to our knowledge, which examines the relationship between clinical course and TSS among patients with IIH. Our quantitative measures of the TSs were not predictive of the clinical course of patients with IIH, suggesting that other factors such as those previously associated with poor outcomes in IIH14,31,32 (i.e., black race, male sex, anemia, recent weight gain, and morbid obesity) are more relevant than the anatomic degree of TSS. Most importantly, our study does not support the notion that patients with IIH with high-grade stenosis on MRV require TS stenting or need different management than patients with IIH without severe TSS in order to prevent poor outcomes.
GLOSSARY
| ATECO | auto-triggered elliptic-centric-ordered |
| GRE | gradient-recalled echo |
| GVF | Goldmann visual field |
| HVF | Humphrey visual field |
| ICP | intracranial pressure |
| IIH | idiopathic intracranial hypertension |
| IQR | interquartile range |
| LP | lumbar puncture |
| MIP | maximum intensity projections |
| MRV | magnetic resonance venography |
| TS | transverse sinus |
| TSS | transverse sinus stenosis |
| VF | visual field |
AUTHOR CONTRIBUTIONS
Bryan Riggeal, MD: study design, acquisition/analysis/interpretation of data, drafting/revising manuscript for content. Beau Bruce, MD, MS: study design, analysis/interpretation of data, revising manuscript for content. Amit Saindane, MD: study design, interpretation of data, revising manuscript for content. Maysa Ridha, MD: interpretation of data. Linda Kelly, MD: interpretation of data. Nancy J. Newman, MD: study design, interpretation of data, revising manuscript for content. Valérie Biousse, MD: study design, interpretation of data, revising manuscript for content.
STUDY FUNDING
Supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, NY, and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology).
DISCLOSURE
B. Riggeal reports no disclosures. B. Bruce received research support from Teva Pharmaceuticals via an Emory University–approved research agreement; received the American Academy of Neurology Practice Research Fellowship; and receives research support from the NIH (K23-EY019341) and from Pfizer via an Emory University–approved research agreement. A. Saindane, M. Ridha, and L. Kelly report no disclosures. N. Newman received the Research to Prevent Blindness and a Lew R. Wasserman Merit Award. V. Biousse received research support from the NIH (PHS Grant UL1-RR025008). Go to Neurology.org for full disclosures.
REFERENCES
Articles from Neurology are provided here courtesy of American Academy of Neurology
Full text links
Read article at publisher's site: https://doi.org/10.1212/wnl.0b013e31827debd6
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3589184?pdf=render
Citations & impact
Impact metrics
Article citations
Exploring the utility of retinal optical coherence tomography as a biomarker for idiopathic intracranial hypertension: a systematic review.
J Neurol, 271(8):4769-4793, 10 Jun 2024
Cited by: 0 articles | PMID: 38856724 | PMCID: PMC11319609
Review Free full text in Europe PMC
CSF hyperdynamics in rats mimicking the obesity and androgen excess characteristic of patients with idiopathic intracranial hypertension.
Fluids Barriers CNS, 21(1):10, 25 Jan 2024
Cited by: 1 article | PMID: 38273331 | PMCID: PMC10810013
Severe bilateral papilledema after sigmoid sinus constriction surgery: a case report.
BMC Ophthalmol, 23(1):500, 08 Dec 2023
Cited by: 1 article | PMID: 38066460 | PMCID: PMC10704719
Modelling idiopathic intracranial hypertension in rats: contributions of high fat diet and testosterone to intracranial pressure and cerebrospinal fluid production.
Fluids Barriers CNS, 20(1):44, 16 Jun 2023
Cited by: 6 articles | PMID: 37328884 | PMCID: PMC10276479
Evaluation of Magnetic Resonance Findings of Circumventricular Organs in Idiopathic Intracranial Hypertension Patients.
Cureus, 14(11):e31795, 22 Nov 2022
Cited by: 1 article | PMID: 36425047 | PMCID: PMC9681503
Go to all (80) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure?
Clin Neurol Neurosurg, 115(8):1215-1219, 05 Dec 2012
Cited by: 22 articles | PMID: 23219404 | PMCID: PMC3610812
Reversible transverse sinus collapse in a patient with idiopathic intracranial hypertension.
BMJ Case Rep, 2015:bcr2014011606, 15 May 2015
Cited by: 10 articles | PMID: 25979956 | PMCID: PMC4434394
Role of contrast-enhanced magnetic resonance high-resolution variable flip angle turbo-spin-echo (T1 SPACE) technique in diagnosis of transverse sinus stenosis.
Eur J Radiol, 120:108644, 10 Sep 2019
Cited by: 4 articles | PMID: 31539793
[Idiopathic intracranial hypertension: stent or not].
Rev Neurol (Paris), 168(10):685-690, 13 Sep 2012
Cited by: 3 articles | PMID: 22981295
Review
Funding
Funders who supported this work.
NEI NIH HHS (4)
Grant ID: K23 EY019341
Grant ID: L30 EY019180
Grant ID: P30-EY06360
Grant ID: P30 EY006360






