Abstract
Free full text

Colony-stimulating factor 1 expression is down-regulated during the adipocyte differentiation of H-1/A marrow stromal cells and induced by cachectin/tumor necrosis factor.
Abstract
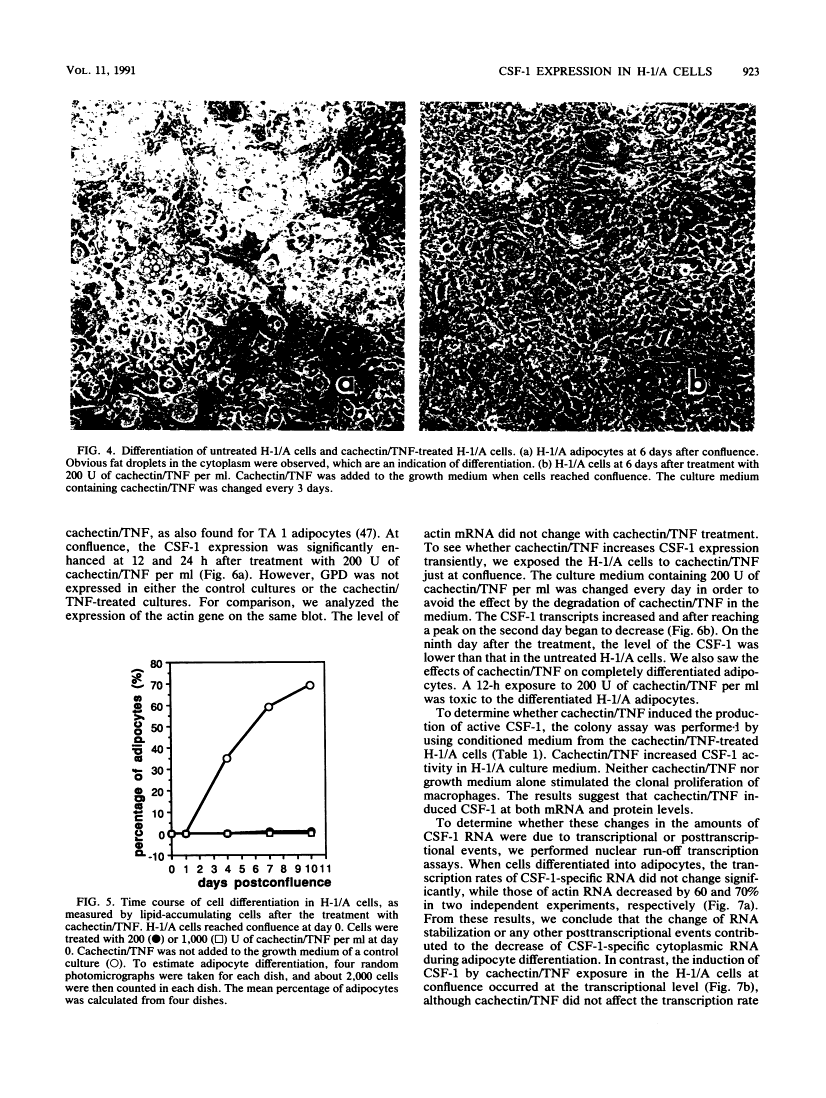
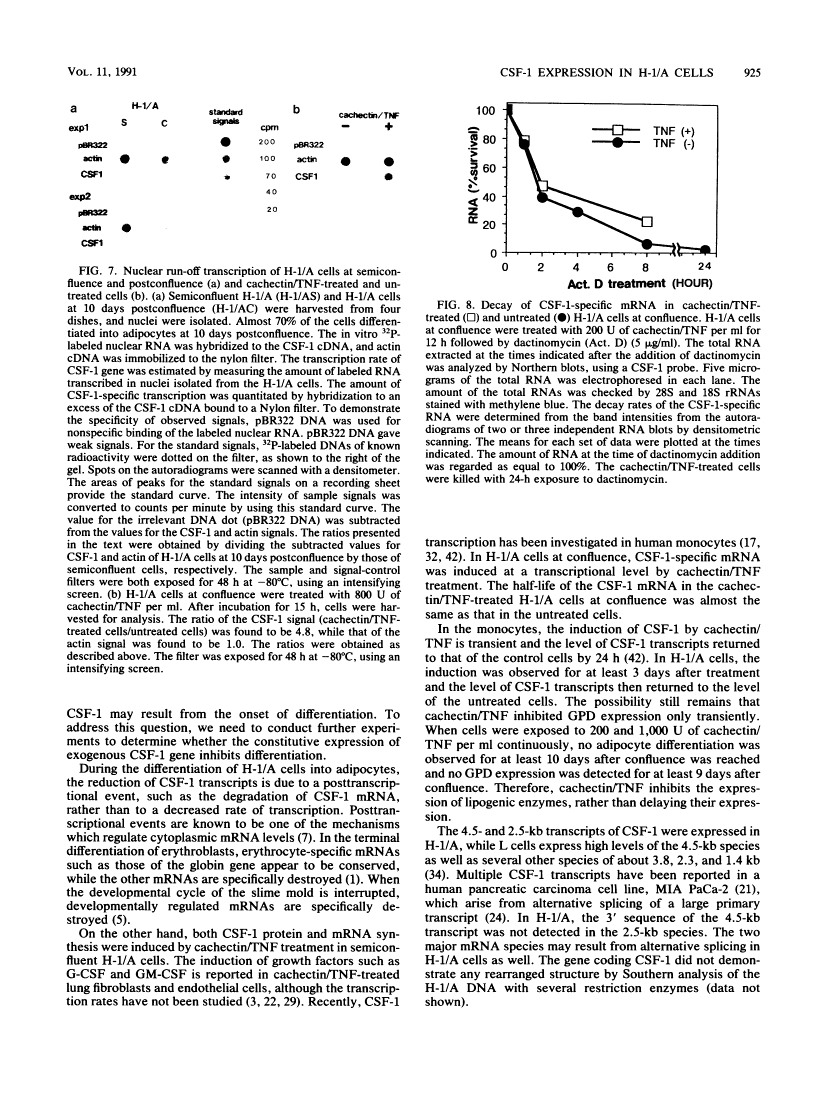
We isolated clonal sublines of the established mouse marrow stromal cell line, H-1. These clonal sublines underwent differentiation into adipocytes in various degrees. One subline, H-1/A, underwent adipocyte differentiation after confluence, while another subline, H-1/D, did not differentiate. In H-1/A cells, the 4.5- and 2.5-kb major mRNA species of colony-stimulating factor 1 (CSF-1) were expressed before differentiation and were down-regulated at a posttranscriptional level during the differentiation of H-1/A cells. The down-regulation of the CSF-1 gene was not a result of arrested cellular growth, because no down-regulation was detected in the nondifferentiating sister line, H-1/D. This down-regulation appeared to be an early event in differentiation. Cachectin/tumor necrosis factor transiently induced the expression of CSF-1 and inhibited the differentiation of H-1/A cells into adipocytes. This induced expression of CSF-1 was due to an increased rate of transcription.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastos RN, Volloch Z, Aviv H. Messenger RNA population analysis during erythroid differentiation: a kinetical approach. J Mol Biol. 1977 Feb 25;110(2):191–203. [Abstract] [Google Scholar]
- Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989 Dec 22;59(6):959–968. [Abstract] [Google Scholar]
- Broudy VC, Kaushansky K, Segal GM, Harlan JM, Adamson JW. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. [Europe PMC free article] [Abstract] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. [Abstract] [Google Scholar]
- Chung S, Landfear SM, Blumberg DD, Cohen NS, Lodish HF. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. [Abstract] [Google Scholar]
- Coppola JA, Cole MD. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. [Abstract] [Google Scholar]
- Darnell JE., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- Fung MC, Hapel AJ, Ymer S, Cohen DR, Johnson RM, Campbell HD, Young IG. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984 Jan 19;307(5948):233–237. [Abstract] [Google Scholar]
- Gimble JM, Dorheim MA, Cheng Q, Medina K, Wang CS, Jones R, Koren E, Pietrangeli C, Kincade PW. Adipogenesis in a murine bone marrow stromal cell line capable of supporting B lineage lymphocyte growth and proliferation: biochemical and molecular characterization. Eur J Immunol. 1990 Feb;20(2):379–387. [Abstract] [Google Scholar]
- Groudine M, Peretz M, Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. [Europe PMC free article] [Abstract] [Google Scholar]
- Gough NM, Gough J, Metcalf D, Kelso A, Grail D, Nicola NA, Burgess AW, Dunn AR. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. Nature. 309(5971):763–767. [Abstract] [Google Scholar]
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. [Abstract] [Google Scholar]
- Guilbert LJ, Stanley ER. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. [Europe PMC free article] [Abstract] [Google Scholar]
- Harigaya K, Cronkite EP, Miller ME, Shadduck RK. Murine bone marrow cell line producing colony-stimulating factor. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6963–6966. [Europe PMC free article] [Abstract] [Google Scholar]
- Harigaya K, Todoroki T, Nakamura M, Watanabe Y. Conversion to adipocytes of a clonal bone marrow preadipocyte line (H-1/A) and fatty acid composition of the resultant adipocytes. Proc Soc Exp Biol Med. 1986 Apr;181(4):523–528. [Abstract] [Google Scholar]
- Horiguchi J, Sariban E, Kufe D. Transcriptional and posttranscriptional regulation of CSF-1 gene expression in human monocytes. Mol Cell Biol. 1988 Sep;8(9):3951–3954. [Europe PMC free article] [Abstract] [Google Scholar]
- Horiguchi J, Warren MK, Ralph P, Kufe D. Expression of the macrophage specific colony-stimulating factor (CSF-1) during human monocytic differentiation. Biochem Biophys Res Commun. 1986 Dec 30;141(3):924–930. [Abstract] [Google Scholar]
- Hu MC, Sharp SB, Davidson N. The complete sequence of the mouse skeletal alpha-actin gene reveals several conserved and inverted repeat sequences outside of the protein-coding region. Mol Cell Biol. 1986 Jan;6(1):15–25. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawasaki ES, Ladner MB, Wang AM, Van Arsdell J, Warren MK, Coyne MY, Schweickart VL, Lee MT, Wilson KJ, Boosman A, et al. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. [Abstract] [Google Scholar]
- Koeffler HP, Gasson J, Ranyard J, Souza L, Shepard M, Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987 Jul;70(1):55–59. [Abstract] [Google Scholar]
- Lachman HM, Skoultchi AI. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. [Abstract] [Google Scholar]
- Ladner MB, Martin GA, Noble JA, Nikoloff DM, Tal R, Kawasaki ES, White TJ. Human CSF-1: gene structure and alternative splicing of mRNA precursors. EMBO J. 1987 Sep;6(9):2693–2698. [Europe PMC free article] [Abstract] [Google Scholar]
- Ladner MB, Martin GA, Noble JA, Wittman VP, Warren MK, McGrogan M, Stanley ER. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6706–6710. [Europe PMC free article] [Abstract] [Google Scholar]
- Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 312(5993):458–462. [Abstract] [Google Scholar]
- Li CY, Lam KW, Yam LT. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. [Abstract] [Google Scholar]
- Morrison DK, Browning PJ, White MF, Roberts TM. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. [Europe PMC free article] [Abstract] [Google Scholar]
- Munker R, Gasson J, Ogawa M, Koeffler HP. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. [Abstract] [Google Scholar]
- Nakamura M, Harigaya K, Watanabe Y. Correlation between production of colony-stimulating activity (CSA) and adipose conversion in a murine marrow-derived preadipocyte line (H-1/A). Proc Soc Exp Biol Med. 1985 Jul;179(3):283–287. [Abstract] [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. [Abstract] [Google Scholar]
- Oster W, Lindemann A, Horn S, Mertelsmann R, Herrmann F. Tumor necrosis factor (TNF)-alpha but not TNF-beta induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood. 1987 Nov;70(5):1700–1703. [Abstract] [Google Scholar]
- Pike BL, Robinson WA. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. [Abstract] [Google Scholar]
- Rajavashisth TB, Eng R, Shadduck RK, Waheed A, Ben-Avram CM, Shively JE, Lusis AJ. Cloning and tissue-specific expression of mouse macrophage colony-stimulating factor mRNA. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1157–1161. [Europe PMC free article] [Abstract] [Google Scholar]
- Rambaldi A, Wakamiya N, Vellenga E, Horiguchi J, Warren MK, Kufe D, Griffin JD. Expression of the macrophage colony-stimulating factor and c-fms genes in human acute myeloblastic leukemia cells. J Clin Invest. 1988 Apr;81(4):1030–1035. [Europe PMC free article] [Abstract] [Google Scholar]
- Rambaldi A, Young DC, Griffin JD. Expression of the M-CSF (CSF-1) gene by human monocytes. Blood. 1987 May;69(5):1409–1413. [Abstract] [Google Scholar]
- Rettenmier CW, Roussel MF, Ashmun RA, Ralph P, Price K, Sherr CJ. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. [Europe PMC free article] [Abstract] [Google Scholar]
- Roussel MF, Dull TJ, Rettenmier CW, Ralph P, Ullrich A, Sherr CJ. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. [Abstract] [Google Scholar]
- Roussel MF, Rettenmier CW, Look AT, Sherr CJ. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. [Europe PMC free article] [Abstract] [Google Scholar]
- Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. [Abstract] [Google Scholar]
- Sherman ML, Weber BL, Datta R, Kufe DW. Transcriptional and posttranscriptional regulation of macrophage-specific colony stimulating factor gene expression by tumor necrosis factor. Involvement of arachidonic acid metabolites. J Clin Invest. 1990 Feb;85(2):442–447. [Europe PMC free article] [Abstract] [Google Scholar]
- Sherry B, Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988 Oct;107(4):1269–1277. [Europe PMC free article] [Abstract] [Google Scholar]
- Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. [Abstract] [Google Scholar]
- Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978 Jul 13;274(5667):168–170. [Abstract] [Google Scholar]
- Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. [Abstract] [Google Scholar]
- Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. [Abstract] [Google Scholar]
- Tsuchiya M, Asano S, Kaziro Y, Nagata S. Isolation and characterization of the cDNA for murine granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7633–7637. [Europe PMC free article] [Abstract] [Google Scholar]
- Umezawa A, Harigaya K, Abe H, Watanabe Y. Gap-junctional communication of bone marrow stromal cells is resistant to irradiation in vitro. Exp Hematol. 1990 Oct;18(9):1002–1007. [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.11.2.920-927.1991
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc359750?pdf=render
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/11/2/920
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/11/2/920
Citations & impact
Impact metrics
Citations of article over time
Article citations
Abnormal lipid metabolism in skeletal muscle tissue of patients with muscular dystrophy: In vitro, high-resolution NMR spectroscopy based observation in early phase of the disease.
Magn Reson Imaging, 38:163-173, 07 Jan 2017
Cited by: 27 articles | PMID: 28069416
Exogenous Nkx2.5- or GATA-4-transfected rabbit bone marrow mesenchymal stem cells and myocardial cell co-culture on the treatment of myocardial infarction in rabbits.
Mol Med Rep, 12(2):2607-2621, 12 May 2015
Cited by: 8 articles | PMID: 25975979 | PMCID: PMC4464300
The neural plasticity of early-passage human bone marrow-derived mesenchymal stem cells and their modulation with chromatin-modifying agents.
J Tissue Eng Regen Med, 8(5):407-413, 04 Jun 2012
Cited by: 5 articles | PMID: 22674835
Mesenchymal stem cell transplantation to the mouse cochlea as a treatment for childhood sensorineural hearing loss.
Int J Pediatr Otorhinolaryngol, 77(6):936-942, 03 Apr 2013
Cited by: 19 articles | PMID: 23561635
Go to all (34) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transcriptional and posttranscriptional modulation of myeloid colony-stimulating factor expression by tumor necrosis factor and other agents.
Mol Cell Biol, 8(8):3432-3438, 01 Aug 1988
Cited by: 64 articles | PMID: 2463477 | PMCID: PMC363580
Expression of gap-junctional protein (connexin 43 or alpha 1 gap junction) is down-regulated at the transcriptional level during adipocyte differentiation of H-1/A marrow stromal cells.
Cell Struct Funct, 17(3):177-184, 01 Jun 1992
Cited by: 16 articles | PMID: 1322801
Peptide growth factors stimulate macrophage colony-stimulating factor in murine stromal cells.
Blood, 78(1):103-109, 01 Jul 1991
Cited by: 29 articles | PMID: 2070045