Abstract
Purpose
Insomnia is increasingly recognized as a major symptom outcome in breast cancer; however, little is known about its prevalence and risk factors among women receiving aromatase inhibitors (AIs), a standard treatment to increase disease-free survival among breast cancer patients.Methods
A cross-sectional survey study was conducted among postmenopausal women with stage 0-III breast cancer receiving adjuvant AI therapy at an outpatient breast oncology clinic of a large university hospital. The insomnia severity index (ISI) was used as the primary outcome. Multivariate logistic regression analyses were performed to evaluate risk factors.Results
Among 413 participants, 130 (31.5 %) had subthreshold insomnia on the ISI, and 77 (18.64 %) exceeded the threshold for clinically significant insomnia. In a multivariate logistic regression model, clinically significant insomnia was independently associated with severe joint pain (adjusted odds ratio (AOR) 4.84, 95 % confidence interval (CI) 1.71-13.69, P = 0.003), mild/moderate hot flashes (AOR 2.28, 95 % CI 1.13-4.60, P = 0.02), severe hot flashes (AOR 2.29, 95 % CI 1.23-6.81, P = 0.015), anxiety (AOR 1.99, 95 % CI 1.08-3.65, P = 0.027), and depression (AOR 3.57, 95 % CI 1.48-8.52, P = 0.004). Age (>65 vs. <55 years; AOR 2.31; 95 % CI 1.11-4.81; P = 0.026) and time since breast cancer diagnosis (<2 vs. 2-5 years; AOR 1.94; 95 % CI 1.02-3.69; P = 0.045) were also found to be significant risk factors. Clinical insomnia was more common among those who used medication for treating insomnia and pain.Conclusions
Insomnia complaints exceed 50 % among AI users. Clinically significant insomnia is highly associated with joint pain, hot flashes, anxiety and depression, age, and time since diagnosis.Free full text

Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors
Abstract
Purpose
Insomnia is increasingly recognized as a major symptom outcome in breast cancer; however, little is known about its prevalence and risk factors among women receiving aromatase inhibitors (AIs), a standard treatment to increase disease free survival among breast cancer patients.
Methods
A cross-sectional survey study was conducted among postmenopausal women with stage 0-III breast cancer receiving adjuvant AI therapy at an outpatient breast oncology clinic of a large university hospital. The Insomnia Severity Index (ISI) was used as the primary outcome. Multivariate logistic regression analyses were performed to evaluate risk factors.
Results
Among 413 participants, 130 (31.5%) had sub-threshold insomnia on the ISI and 77 (18.64%) exceeded the threshold for clinically significant insomnia. In a multivariate logistic regression model, clinically significant insomnia was independently associated with severe joint pain (adjusted odds ratio, 4.84, 95% confidence interval, 1.71–13.69, P=0.003), mild/moderate hot flashes (AOR, 2.28, 95% CI, 1.13–4.60, P=0.02), severe hot flashes (AOR, 2.29, 95% CI, 1.23–6.81, P=0.015), anxiety (AOR, 1.99, 95% CI, 1.08–3.65, P=0.027), and depression (AOR, 3.57, 95% CI, 1.48–8.52, P=0.004). Age (>65 vs. <55 years, AOR, 2.31, 95% CI, 1.11–4.81, p=0.026), and time since breast cancer diagnosis (<2 years vs. 2–5 years, AOR, 1.94, 95% CI, 1.02–3.69, p=0.045) were also found to be significant risk factors. Clinical insomnia was more common among those who used medication for treating insomnia and pain.
Conclusions
Insomnia complaints exceed 50% among AI users. Clinically significant insomnia is highly associated with joint pain, hot flashes, anxiety and depression, age, and time since diagnosis.
INTRODUCTION
Insomnia is one of the most prevalent symptoms experienced by cancer patients [1, 2]. In particular, breast cancer patients report higher prevalence of insomnia (38% to 61%) in comparison to patients with other types of cancer [3–6]. While insomnia in breast cancer patients may be linked to multiple factors, including psychological distress from cancer diagnosis or side effects of cancer treatments, patients receiving adjuvant endocrine therapy may also be at increased risk for insomnia due to the occurrence of menopausal symptoms that this therapy induces [7, 8].
In the past few years, aromatase inhibitors (AIs) have become the standard adjuvant hormonal therapy for postmenopausal women with hormone receptor positive invasive breast cancer [9–12]. It is estimated that more than 100,000 postmenopausal women are diagnosed with estrogen receptor positive breast cancer each year and hence AIs have a wide application in this population [13]. AIs significantly improve survival by blocking the conversion of androgens to estrogens, which inhibits the aromatase enzyme, thereby resulting in significant estrogen depletion [14]. It has a typical treatment course of five years and is taken either after or instead of tamoxifen [15]. However, with the increase in its use, AI-related insomnia has been one of the most troublesome symptoms experienced by women on AIs [16, 17].
AIs have also been associated with various side effects, including bone loss and an increase in vasomotor symptoms such as hot flashes [18–22]. Recent data suggest a high prevalence and impact of arthralgia related to AIs [23, 24]. AI-related symptoms like arthralgia and hot flashes may cause or worsen insomnia in this population since research studies have shown a clear link between these symptoms and insomnia in breast cancer patients [6, 25, 20, 26]. Despite the wide use of AIs and the fact that the side effects caused by their use may increase the risk of insomnia among breast cancer patients, to date; no data have been published to understand the risk factors for insomnia among patients on AIs.
The present study aims to understand the prevalence and risk factors for insomnia in postmenopausal breast cancer patients receiving AIs because sleep may be particularly impacted by the joint symptoms and vasomotor symptoms among these women. This information will help in developing future interventions to manage insomnia in this population. The specific objectives of this study are to: 1) Define the prevalence of insomnia among breast cancer patients who currently receive AIs; 2) Identify demographic, clinical and symptom risk factors for insomnia and 3) Understand the relationship between co-morbid symptoms such as hot flash, joint pain, anxiety and depression and insomnia among women on AIs.
METHODS
Study Design and Patient Population
We conducted a cross-sectional survey study among breast cancer patients receiving care at the Rowan Breast Cancer Center of the Abramson Cancer Center of the University of Pennsylvania (Philadelphia, PA) between April 2008 and August 2009. Potential participants included postmenopausal women with a history of histologically confirmed stage 0 to III, hormone receptor-positive breast cancer who were currently taking a third generation aromatase inhibitor (anastrozole, letrozole, or exemestane), who had completed chemotherapy or radiotherapy at least one month prior to enrollment, and had the ability to understand and provide informed consent in English. Research assistants obtained permission from the treating oncologist, screened medical records and approached potential study subjects for enrollment at their regular follow-up appointments. After informed consent was obtained, each participant was given a self-administered survey. For those participants who could not complete the survey in time, a stamped envelope with return address was provided for them to mail back the survey. The study was approved by the Institutional Review Board of the University of Pennsylvania and the Scientific Review and Monitoring Committee of the Abramson Cancer Center.
Outcome Measurement
Primary outcome measures included patient self-report of insomnia using the Insomnia Severity Index (ISI). The ISI is composed of seven items targeting sleep disturbance severity, sleep related satisfaction, degree of daytime functional impairment, impairment perception, and distress related to sleeping problem. Each item is rated on a five-point Likert scale (0 = “not at all”, 4 = “very much”) and summed to provide a total score ranging from 0 to 28. A score of greater than 14 indicates clinically significant insomnia. A validation study conducted in the general population supported the reliability of the ISI, with an internal consistency of 0.74. The ISI has also been found to be a valid and sensitive measure to detect changes in perceived sleep difficulties following cognitive-behavioral or pharmacologic treatment for insomnia [27]. A study conducted to evaluate the validity of the ISI in a cancer population showed it to be a reliable and valid measure of insomnia in the context of cancer, with an internal consistency of 0.90 [28]. For reporting each item on the ISI, ‘moderate/somewhat’, ‘severe/much’ and ‘very severe/very much’ were combined into one category as ‘having sleep difficulty’ in order to capture the maximum number of patients having sleep issues.
Co-morbid symptoms
Hot flashes and Joint Pain
Hot flashes and joint pain are considered to be major side effects among women on AIs. These symptoms were measured by asking patients: “During the past 4 weeks, how much have you been bothered by the following problems?” Patients rated their response on a five point Likert scale from ‘not at all’ to ‘extremely.’ We grouped the scale into three categories: “Not at all” (No symptom), “slightly/moderately” (mild/moderate symptom) and “quite a bit/extremely” (severe symptom).
Anxiety and Depression
The Hospital Anxiety and Depression score (HADS) was used to measure anxiety and depression. This is a 14-item, self-administered rating scale with two subscales (measuring anxiety and depression), each containing seven items. The individual items are each rated on a four-point scale, scored from 0 to 3, resulting in maximum subscale scores of 21 and an overall distress score ranging from 0 to 42, with higher scores indicating greater levels of distress. The reliability, validity, and factor structure of the HADS has been established in a variety of clinical populations, including cancer patients. [29–31]. Scores of 11 or more on either subscale are considered to be a significant ‘case’ of psychological morbidity (abnormal), while scores of 8–10 represent ‘borderline’ and 0–7 ‘normal’. For the purpose of analysis, we grouped borderline and abnormal together.
Covariates
Participants reported sociodemographic variables, including age, race, ethnicity, education level, and employment status. Clinical and treatment variables including stage of cancer, time since cancer diagnosis, and previous and current cancer treatments (i.e. surgery, chemotherapy, radiation therapy, hormone therapy) were assessed by self report and medical chart abstraction.
Statistical Analysis
Data analysis was performed using STATA 10.0 for Windows (STATA Corporation, College Station, TX). Descriptive statistics were used to report the demographic variables of the study participants. Bivariate analyses using chi-square tests were then conducted to identify the factors associated with insomnia among breast cancer patients on AIs. We then developed a multivariate logistic regression model to determine the relative impact of each variable on the presence of clinically significant insomnia. Variables that were not significant at the 0.1 level in the bivariate analyses were not included in the multivariate analysis. Statistical tests were 2-sided, and p values <0.05 indicated significance.
RESULTS
Participant Characteristics
Of 643 consecutive patients screened, 538 (83.7%) agreed to participate. Among the 105 who declined (16.3%), the main reasons were: lack of time to complete survey (n=26, 4%); did not want to participate in research (n=43; 6.7%); and did not want to have an extra blood draw (n=36; 5.6%). Additionally, one subject withdrew consent and nine subjects (1.4%) were further disqualified because they did not meet eligibility criteria upon further review. Of the 528 subjects (82.1%) who returned data, 510 (79.32%) had an evaluable survey. 70 subjects were further excluded after chart review revealed that 25 (3.89%) had metastatic disease and 45 (7.0%) had stopped AI treatment due to side effects at the time of enrollment. Further, 27 (4.2%) subjects had missing data on ISI score, resulting in a final sample of 413 participants.
Among the 413 study participants, the mean age (range) was 61.7 (33–88). The majority of subjects (82.57%) was non-Hispanic white and a substantial proportion (14.77%) was non-Hispanic black. For the purpose of analysis, we combined the race categories into white and nonwhite. Characteristics of the study population are listed in Table 1.
Table 1
Demographic and Clinical characteristics of participants (N=413)
| N (%) | Clinical Insomnia | No Clinical Insomnia | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Total | 413 | 82 | 18.26 | 367 | 81.74 | ||
| Age, years | |||||||
| <55 | 91 (22.03) | 22 | 24.18 | 69 | 75.82 | 0.083 | |
| 55–65 | 196 (47.46) | 39 | 19.90 | 157 | 80.10 | ||
| >65 | 126 (30.51) | 16 | 12.70 | 110 | 87.30 | ||
| Race/ethnicity | |||||||
| White | 341 (82.57) | 62 | 18.18 | 279 | 81.82 | 0.600 | |
| Non-white* | 72 (17.43) | 15 | 20.83 | 57 | 79.17 | ||
| Educational Level | |||||||
| High school or less | 86 (20.82) | 16 | 18.60 | 70 | 81.40 | 0.031 | |
| College | 181 (43.83) | 43 | 23.76 | 138 | 76.24 | ||
| Graduate or professional school | 146 (35.35) | 18 | 12.33 | 128 | 87.67 | ||
| Employment | |||||||
| Full-time | 164 (40.00) | 27 | 16.46 | 137 | 83.54 | 0.677 | |
| Part-time | 61 (14.88) | 12 | 19.67 | 49 | 80.33 | ||
| Not currently | 185 (45.12) | 37 | 20.00 | 148 | 80.00 | ||
| Stage | |||||||
| I | 167 (40.44) | 34 | 20.36 | 133 | 79.64 | 0.534 | |
| II | 195 (47.22) | 32 | 16.41 | 163 | 83.59 | ||
| III | 51 (12.35) | 11 | 21.57 | 40 | 78.43 | ||
| Chemotherapy | |||||||
| None | 158 (38.26) | 32 | 20.25 | 126 | 79.75 | 0.794 | |
| Chemotherapy, but no Taxane | 105 (25.42) | 19 | 18.1 | 86 | 81.90 | ||
| Chemotherapy included Taxane | 150 (36.32) | 26 | 17.33 | 124 | 82.67 | ||
| Radiation therapy | |||||||
| None | 125 (30.27) | 20 | 16 | 105 | 84.00 | 0.363 | |
| Yes | 288 (69.73) | 57 | 19.79 | 231 | 80.21 | ||
| Prior tamoxifen | |||||||
| None | 273 (66.10 | 53 | 19.41 | 220 | 80.59 | 0.575 | |
| Yes | 140 (33.90) | 24 | 17.14 | 116 | 82.86 | ||
| Hormon therapy (current) | |||||||
| Letrozole (Femara) | 86 (20.82) | 12 | 13.95 | 74 | 86.05 | 0.251 | |
| Anastrozole (Arimidex) | 278 (67.31) | 58 | 20.86 | 220 | 79.14 | ||
| Exemestane (Aromasin) | 49 (11.86) | 7 | 14.29 | 42 | 85.71 | ||
| Time Since Breast Cancer Diagnosis | |||||||
| <2 yrs | 132 (31.96) | 18 | 13.64 | 114 | 86.36 | 0.059 | |
| 2–5 | 132 (31.96) | 33 | 25 | 99 | 75.00 | ||
| 5–10 yrs | 102 (24.70) | 15 | 14.71 | 87 | 85.29 | ||
| >=10 yrs | 47 (11.38) | 11 | 23.40 | 36 | 76.60 | ||
| Duration of AIs | |||||||
| <1 yr | 130 (31.48) | 22 | 16.92 | 108 | 83.08 | 0.662 | |
| 1–3 yrs | 140 (33.90) | 25 | 17.86 | 115 | 82.14 | ||
| >3 yrs | 143 (34.62) | 30 | 20.98 | 113 | 79.02 | ||
Perceived rate and severity of insomnia among women on AIs
Among 413 participants, 206 (49.9%) did not report any insomnia (ISI score <8), 130 (31.5%) had subthreshold insomnia (ISI score 8–14), 67 (16.2%) had moderate insomnia (ISI score 15–21), and 10 (2.4%) had severe insomnia (ISI score 22–28). For the purpose of further analysis we merged these four groups into two groups: clinically-significant insomnia (n=77,18.64 %) and no insomnia (n=336, 81.36 %).
With respect to specific insomnia complaints, 30.3% noted difficulties falling asleep (20.3% moderate, 7.8% severe, and 2.2% very severe), 41.6 % reported having trouble staying asleep at night (28.3% moderate, 10.9% severe, and 2.4% very severe), and 31.3% had problems with waking up too early (18.2% moderate, 9.9% severe, and 3.2% very severe). More than half of the population (59.1%) reported having dissatisfaction with their current sleep pattern, 39.5% said their sleep problem interferes with their daily functioning, 22.8 % felt that their sleep problem was noticeable to others, and 28.6% were worried/distressed about their current sleep problem.
Sociodemographic and clinical factors associated with insomnia among women on AIs
In bivariate analyses, we found statistically significant differences between patients with clinically-significant insomnia and without insomnia by age, education level, and time since breast cancer diagnosis (Table 1). Patients younger than 55 years were more likely to report insomnia than older patients. Patients with a college degree and those diagnosed within two to five years were also more likely to report insomnia (Table 1). In the multivariate logistic regression model women younger than 55 years had a two-fold higher odds of reporting insomnia than women older than 65 years (adjusted OR, 2.31, 95% CI, 1.11–4.81; P=0.026) (Table 2). Also, women who had been diagnosed with breast cancer between two to five years back were significantly more likely to report insomnia than those diagnosed within two years of study entry (adjusted OR, 1.94, 95% CI, 1.02–3.69. P=0.045). No other sociodemographic or clinical variables were related to clinical insomnia (Table 2).
Table 2
Multivariate Logistic Regression Model
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| O.R. (95% C.I.) | P-value | A.O.R. (95% C.I.) | P-value | |
| Demographic and clinical | ||||
| Age, years | ||||
| >65 (Reference) | 1 | 1 | ||
| 55–65 | 1.71 (0.91–3.21) | 0.1 | 1.88 (0.98–3.59) | 0.056 |
| <55 | 2.19 (1.08–4.46) | 0.03 | 2.31 (1.11–4.81) | 0.026 |
| Education | ||||
| Graduate/professional (Reference) | 1 | 1 | ||
| High school or less | 1.36 (0.72–2.59) | 0.34 | 1.21 (0.61–2.33) | 0.57 |
| College | 0.62 (0.29–1.28) | 0.19 | 0.52 (0.24–1.11) | 0.09 |
| Time since breast cancer diagnosis | ||||
| <2 years (Reference) | 1 | 1 | ||
| 2–5 years | 2.11 (1.12–3.98) | 0.021 | 1.94 (1.02–3.69) | 0.045 |
| >5 years | 1.34 (0.70–2.57) | 0.38 | 1.20 (0.62–2.34) | 0.583 |
| Symptom co-morbidity | ||||
| Joint Pain | ||||
| No (Reference) | 1 | 1 | ||
| Mild/Moderate | 2.36 (0.88–6.33) | 0.088 | 1.53 (0.55–4.26) | 0.41 |
| Severe | 10.92 (4.09–29.19) | <0.001 | 4.84 (1.71–13.69)) | 0.003 |
| Hot Flashes | ||||
| No (Reference) | 1 | 1 | ||
| Mild/Moderate | 3.16 (1.65–6.03) | 0.001 | 2.28 (1.13–4.60) | 0.022 |
| Severe | 5.86 (2.76–12.46)) | <0.001 | 2.29 (1.23–6.81) | 0.015 |
| Anxiety | ||||
| No (Reference) | 1 | 1 | ||
| Borderline or clinically abnormal | 4.06 (2.41–6.86) | <0.001 | 1.99 (1.08–3.65) | 0.027 |
| Depression | ||||
| No (Reference) | 1 | 1 | ||
| Borderline or clinically abnormal | 6.80 (3.27–14.16) | <0.001 | 3.57 (1.48–8.52) | 0.004 |
Relation between the co-morbid symptoms and insomnia among women on AIs
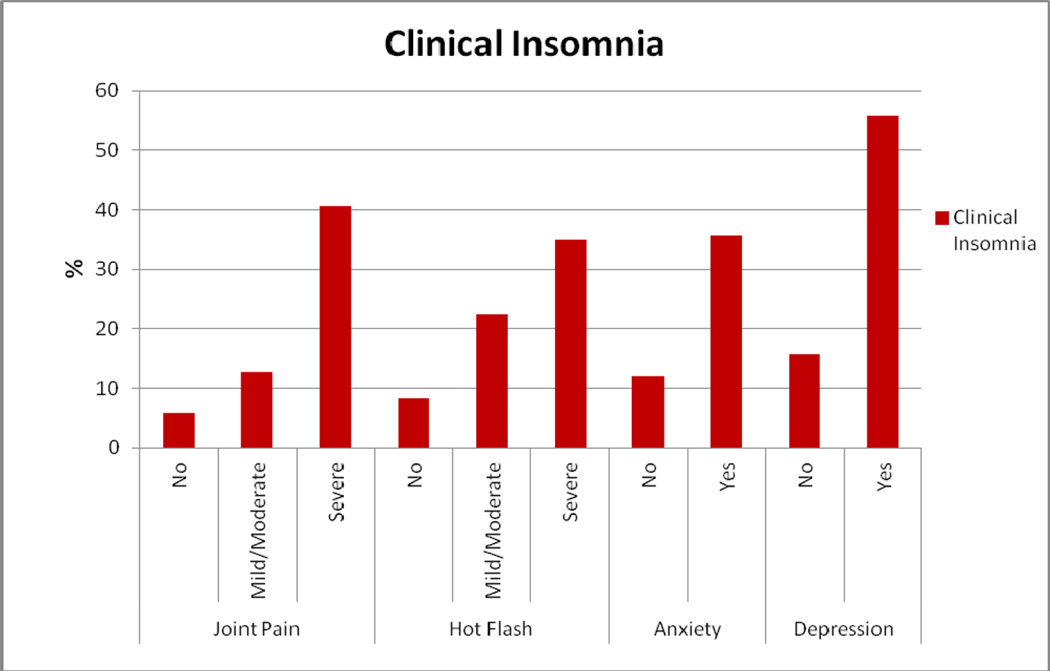
Clinically-significant insomnia was related to severe joint pain, hot flashes, and anxiety and depressive symptoms (Figure 1). In multivariate analysis, women with severe joint pain were significantly more likely to report insomnia than women with no joint pain (adjusted OR, 4.84, 95% CI, 1.71–13.69; P=0.003). Also, when compared to women with no hot flashes, those with mild/moderate hot flashes (OR, 2.28, 95% CI, 1.13–4.60, P = 0.02) and severe hot flashes (OR, 2.29, 95% CI, 1.23–6.81, P=0.015) were more likely to report insomnia. Moreover, women with anxiety (OR, 1.99, 95% CI, 1.08–3.65, P=0.027) and depression (OR, 3.57, 95% CI, 1.48–8.52, P=0.004) were also more likely to report insomnia than women with no anxiety and depression (Table 2).
Relation between medication use and insomnia among women on AIs
Use of insomnia and pain medications was significantly associated with clinical insomnia in bivariate analysis. Among 413 study participants, 31 (7.51%) used insomnia medications. Greater use of insomnia medications was significantly associated with clinical insomnia (use − 58.06% vs. no use − 15.45%, p<0.001). Pain medications were used by 36 (8.72%) study participants. Higher usage of pain medications was also significantly associated with clinical insomnia (use − 38.89% vs. no use − 16.71%, p=0.001). Depression and anxiety medications were used by 92 (22.28%) study participants. However, their use was not associated with clinical insomnia (use −22.83% vs. no use − 17.45%, p=0.243).
DISCUSSION
This study aimed to estimate the prevalence of, and identify risk factors for, insomnia among breast cancer patients on AIs. One in two AI users reported current insomnia complaints and one fifth (18.6 %) exceeded the validated threshold for clinically-significant insomnia based on ISI scores (16.2% moderate and 2.4% severe insomnia). Insomnia was independently associated with current joint pain, hot flashes, depression and anxiety. While age and time since cancer diagnosis were also associated with increased risk for clinical insomnia, no other demographic, clinical or treatment variable was related to clinical insomnia in the multivariate analysis. These findings advance the current understanding of insomnia symptoms as experienced by breast cancer patients on AIs and also highlight the need for effective treatments to manage insomnia in this population.
Across studies of sleep disruption in cancer patients, there is a consistently high prevalence of subjective sleep disturbance in patients with a breast cancer diagnosis. The prevalence estimates of insomnia from prior data among breast cancer patients range from 38% to 61% [3–6]. This estimate is comparable to the prevalence of insomnia complaints among AI users in the present study; however it is higher than the prevalence of clinically-significant insomnia reported in our study. This might be due to the fact that the above mentioned studies used different criteria and instruments to measure insomnia and most did not use validated instruments. The validation study of the ISI scale, used in the present study, found that a cutoff score of 14 is optimal for identifying clinically significant insomnia rather than just identifying symptoms of insomnia of unknown clinical meaning. The use of a more stringent definition of insomnia may have led to the lower prevalence in this population. A prior study conducted to evaluate the prevalence of insomnia among breast cancer patients found 19% of the participants met the diagnostic criteria for an insomnia syndrome [3], an estimate nearly identical to the clinically-significant insomnia (18.6%) reported in the present study.
Some of the risk factors associated with insomnia among breast cancer patients in past studies include chemotherapy [3, 4], radiation therapy [4], stage of cancer [3, 26], physical health [32, 6, 4], fatigue [32, 8], pain [26], depression/psychological distress [26, 32, 8], unemployment, widowhood [3], lower education level, less social support [26], and vasomotor symptoms such as hot flashes [25, 20, 6]. In the present study, insomnia was independently associated with joint pain, hot flashes, and depression and anxiety. With the exception of age and time since breast cancer diagnosis, no other demographic, clinical, or treatment variables were associated with insomnia in this population. These findings are similar to those of Bardwell et al. who found that various cancer-specific, demographic, health behavior, and other patient variables were not significant risk factors in the presence of physical health and psychosocial variables. They found only worse depressive and vasomotor symptoms as meaningful predictors of insomnia [6].
Our study revealed that insomnia was highly associated with joint pain among breast cancer patients on AIs. Pain has been linked with difficulty falling asleep in breast cancer patients and women who report pain also tends to use sleeping pills more frequently [26]. One of the most important side effects of AIs is arthralgia [23, 24]. In the present study, among patients with severe joint pain, 40.6% reported clinical insomnia. Pain from AIs may have been interfering with women being able to fall or remain asleep. Hence it is critical to effectively treat pain in this population in order to manage insomnia.
Our study also revealed that insomnia was independently associated with hot flashes. Hot flashes have been linked with insomnia among breast cancer patients by previous studies. Couzi et al. observed a positive association between the severity of hot flashes and the prevalence of sleep difficulties in breast cancer patients [20]. Polysomnographic data, as reported by Savard et al., suggest that on the nights that patients experienced hot flashes, they had a significantly higher percentage of wake time, a lower percentage of stage 2 sleep, and a longer REM latency compared to those nights without hot flashes [25]. In the present study, 22.4% of patients with mild/moderate hot flashes, and 34.9% of those with severe hot flashes reported clinically-significant insomnia. Since AIs have been associated with increased vasomotor symptoms like hot flashes [18–20], effective management of hot flashes might reduce the risk of insomnia among breast cancer patients on AIs.
Similar to previous studies, our study revealed that the psychological symptoms of depression and anxiety were significant risk factors for insomnia. Since psychological symptoms and insomnia are not necessarily independent of one another and insomnia is a defining symptom of depression and generalized anxiety, it is not surprising to find an association between insomnia and these symptoms. These findings also suggest that treatments for depression and anxiety may help to improve sleep.
Our study also found a significant association between use of medications and insomnia. Higher use of sleep medications was significantly associated with clinical insomnia. This may be due to the fact that patients with more sleep issues take more sleep medications; however, it is also possible that these medications are not helping the patients enough, as they are reporting more clinical insomnia. Higher use of pain medications was also found to have significant relationship with clinical insomnia. As mentioned earlier, pain has been linked with sleep issues among breast cancer patients; our study also found that insomnia was highly associated with joint pain. Patients who take pain medications to manage pain might have reported more clinical insomnia; however one can also question the effectiveness of these medications, and whether they interfere with the normal sleep pattern.
According to current models of insomnia [33, 34], sleep is initially disturbed as a result of one or more precipitating factors including life stress, pain, depression, or the effects of a medication or substance. Over time, perpetuating factors develop that maintain the insomnia even in the absence of the original precipitants. A classic example is when patients with acute insomnia develop sleep-related worry so that while in bed at night their anxiety keeps them from falling asleep, creating a vicious cycle. Taylor et al. provided an elegant demonstration of the transition from acute to chronic insomnia [35]. They found that cancer patients in the acute treatment phase (within 12 weeks of diagnosis) did not show evidence of sleep-related attention bias, a cognitive tendency consistently found in patients with primary insomnia. However, those who continued to have insomnia 12–18 months after diagnosis had developed this cognitive bias over time. In the present study, women who had been diagnosed with breast cancer more than two to five years ago were significantly more likely to report insomnia than those diagnosed less than two years back. Perpetuating factors such as attention bias were not assessed in the present study; it is likely that they might have played a prominent role in this sample given that they were not in the acute phase of cancer treatment. Joint pain and hot flashes were likely acting as precipitating factors in this sample.
As in other studies that have examined this issue, our study was conducted in a practice setting outside a clinical trial, and is subject to selection bias. Our refusal rate was 16.3% and it is possible that those patients who refused to participate were relatively asymptomatic and may have viewed the survey as less relevant. Also, it is possible that the patients might have insomnia due to reasons other than AIs. For example, research suggests that prevalence of sleep apnea, which may promote insomnia, is higher among postmenopausal women [36, 37]. This was not captured in the survey. Another potential limitation might be the fact that our study, like most of the insomnia studies, assessed only the symptoms of insomnia and not insomnia syndrome, a standard for clinically significant insomnia. In addition, because our study relied on self-report, some degree of misclassification bias because of patients’ perception exists; however, for subjective symptoms like insomnia, patient-reported outcome is considered the gold standard. Finally, as the study is cross-sectional in nature, it is challenging to dissect the temporal order in which this set of symptoms occurred. A prospective cohort study may help identify inciting symptoms.
Despite such limitations, the study helped gain a better understanding of insomnia symptoms experienced by breast cancer patients on AIs. Insomnia is a substantial problem in this population and is highly associated with comorbid symptoms like joint pain, hot flashes, anxiety and depression. Under-treating these symptoms might have contributed to high prevalence of insomnia. Thus, effective management of these symptoms may help reduce the risk of insomnia in this population. Since breast cancer patients on AIs report a high degree of symptoms and one symptom may lead to another, future research should focus on understanding the biobehavioral mechanisms and common mediators underlying these symptoms. Such understanding may help develop comprehensive interventions that can help alleviate multiple symptoms, including insomnia, and improve the quality of life for women on AIs.
Acknowledgement
We would like to thank all the breast cancer survivors, physicians, nurse practitioners, and staff for their support. We would like to thank all the work study students for their dedication to the data collection and management process.
Funding Support: Penn Clinical Pharmacogenomic Epidemiology Pilot Grant National Institutes of Health (NIH) 5P20RR020741, Penn Institute of Aging Pilot Grant and NIH AT004695. Dr. Mao is supported by American Cancer Society CCCDA-08-107-01 and NIH K23 AT004112
Reference
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00520-012-1490-z
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3600410
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s00520-012-1490-z
Article citations
Sleep disruption in patients with active and treated endogenous Cushing's syndrome.
Pituitary, 27(5):654-664, 09 Sep 2024
Cited by: 0 articles | PMID: 39251540 | PMCID: PMC11513747
A randomized controlled study of auricular point acupressure to manage chemotherapy-induced neuropathy: Study protocol.
PLoS One, 19(9):e0311135, 26 Sep 2024
Cited by: 0 articles | PMID: 39325795 | PMCID: PMC11426428
Returning to Work after Breast Cancer: A One-Year Mixed-Methods Study.
Int J Environ Res Public Health, 21(8):1057, 13 Aug 2024
Cited by: 1 article | PMID: 39200667 | PMCID: PMC11353812
Letrozole delays acquisition of water maze task in female BALB/c mice: Possible involvement of anxiety.
Horm Behav, 162:105524, 21 Mar 2024
Cited by: 0 articles | PMID: 38513526
The Efficacy of Internet-Based Cognitive Behavioral Therapy for Patients With Breast Cancer: A Systematic Review and Meta-Analysis.
Integr Cancer Ther, 23:15347354241293449, 01 Jan 2024
Cited by: 0 articles | PMID: 39441748 | PMCID: PMC11528811
Review Free full text in Europe PMC
Go to all (62) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Incidence of menopausal symptoms in postmenopausal breast cancer patients treated with aromatase inhibitors.
Oncotarget, 8(25):40558-40567, 01 Jun 2017
Cited by: 6 articles | PMID: 28489562 | PMCID: PMC5522209
Prevalence and risk factors for fatigue among breast cancer survivors on aromatase inhibitors.
Eur J Cancer, 101:47-54, 14 Jul 2018
Cited by: 27 articles | PMID: 30014974 | PMCID: PMC6148367
Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors.
Breast Cancer Res Treat, 124(1):205-211, 25 Feb 2010
Cited by: 26 articles | PMID: 20182796 | PMCID: PMC3670946
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.
Cochrane Database Syst Rev, (1):CD003370, 24 Jan 2007
Cited by: 16 articles | PMID: 17253488
Review
Funding
Funders who supported this work.
NCCIH NIH HHS (3)
Grant ID: K23 AT004112
Grant ID: R21 AT004695
Grant ID: AT004695
NCI NIH HHS (1)
Grant ID: P30 CA016520
NCRR NIH HHS (2)
Grant ID: 5P20RR020741
Grant ID: P20 RR020741