Abstract
Free full text

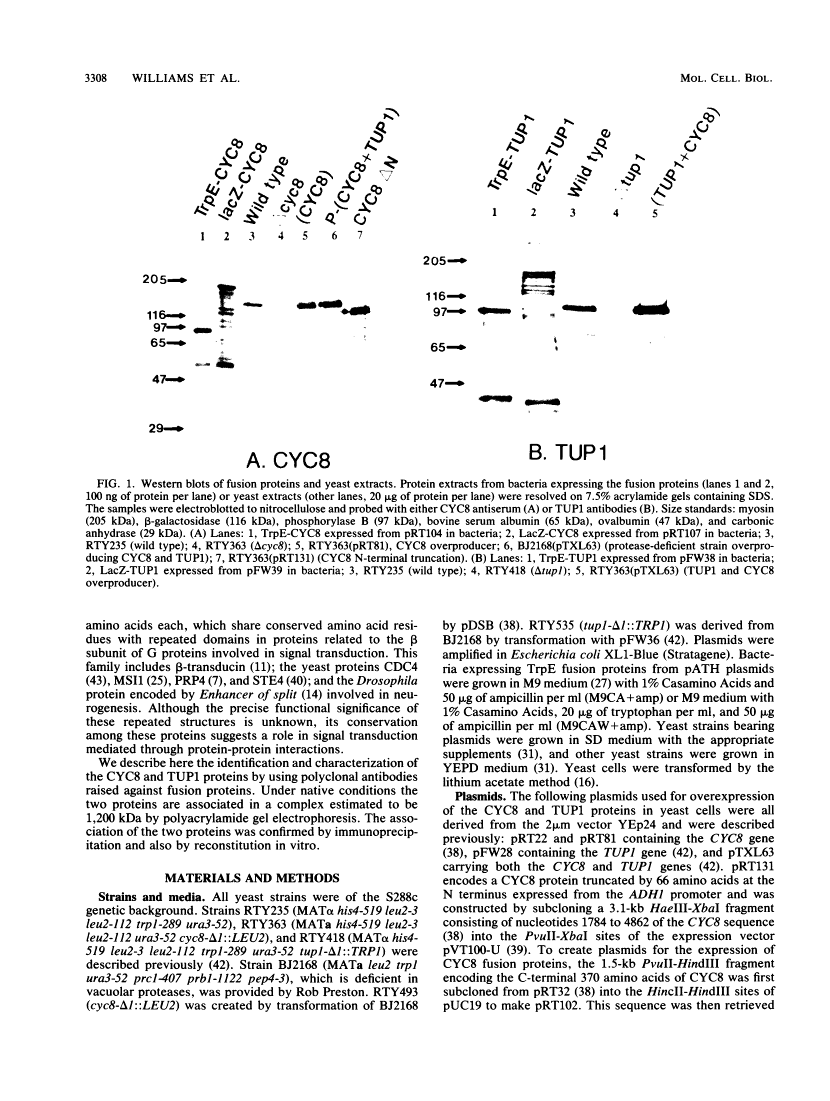
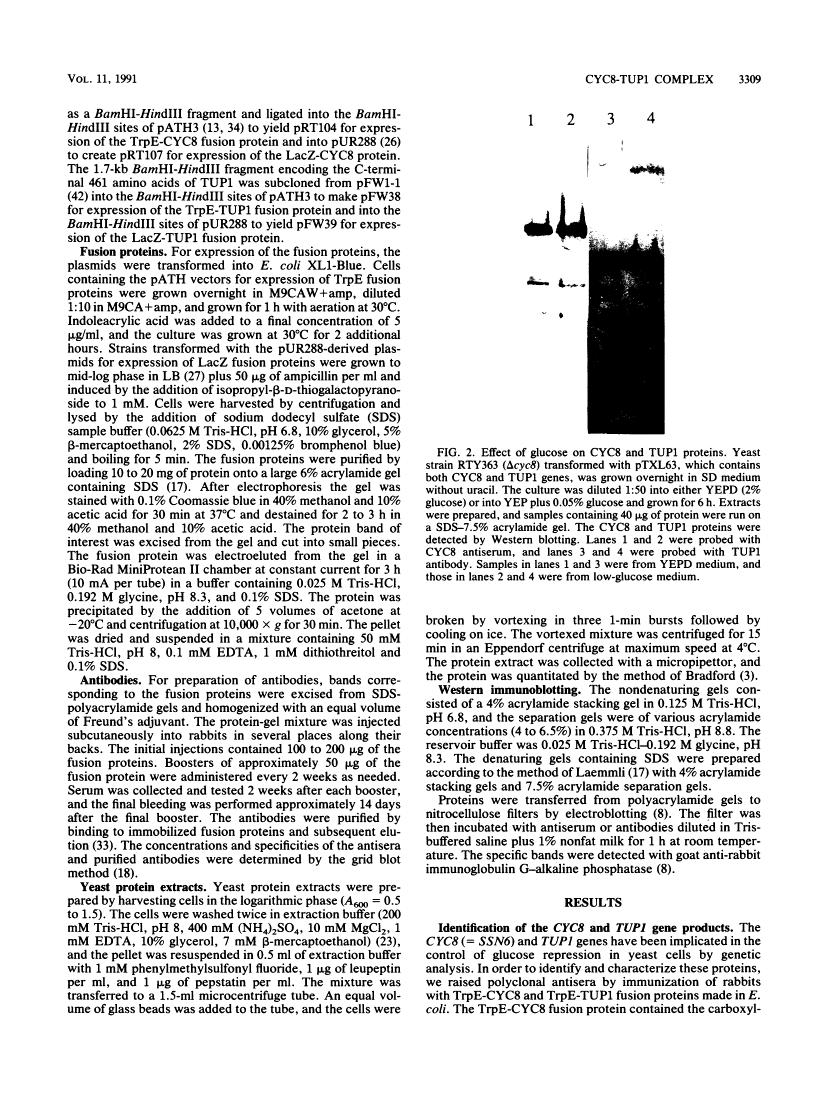
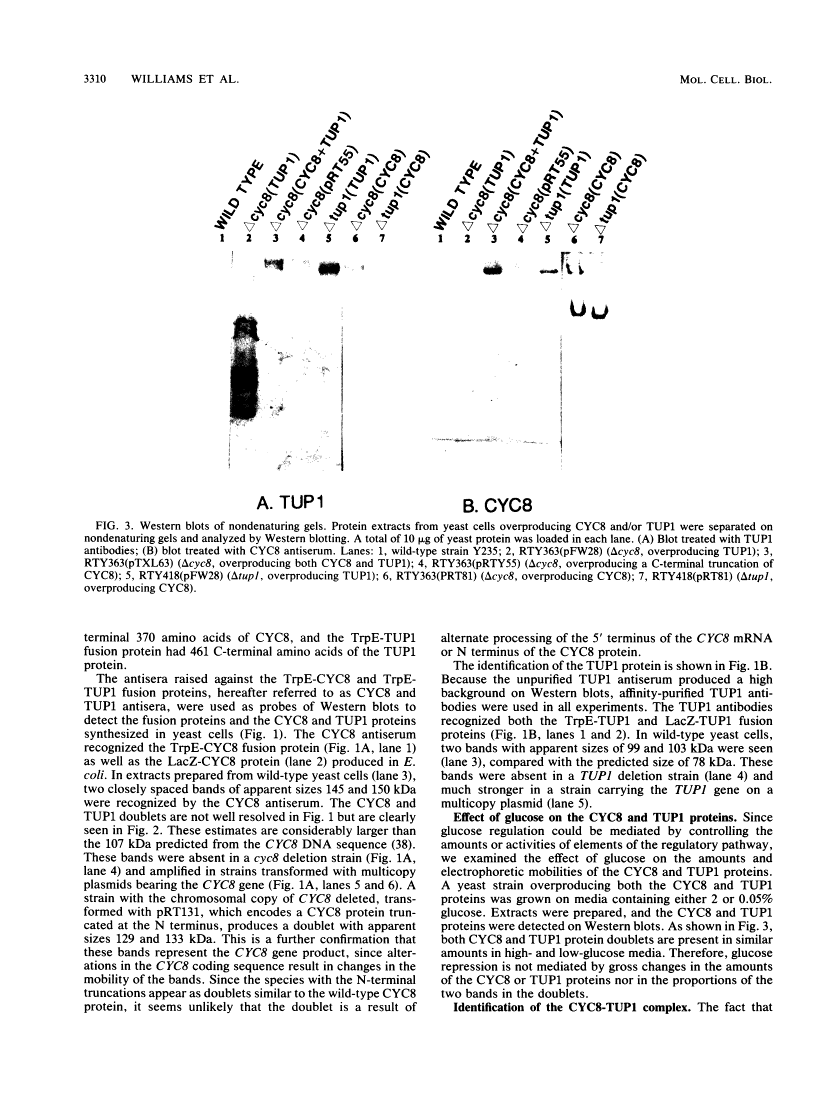
The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex.
Abstract
Mutations of yeast CYC8 or TUP1 genes greatly reduce the degree of glucose repression of many genes and affect other regulatory pathways, including mating type. The predicted CYC8 protein contains 10 copies of the 34-amino-acid tetratricopeptide repeat unit, and the predicted TUP1 protein has six repeated regions found in the beta subunit of heterotrimeric G proteins. The absence of DNA-binding motifs and the presence of these repeated domains suggest that the CYC8 and TUP1 proteins function via protein-protein interaction with transcriptional regulatory proteins. We raised polyclonal antibodies against TrpE-CYC8 and TrpE-TUP1 fusion proteins expressed in Escherichia coli. The CYC8 and TUP1 proteins from yeast cells were detected as closely spaced doublets on Western immunoblots of sodium dodecyl sulfate-polyacrylamide gels. Western blots of nondenaturing gels revealed that both proteins are associated in a high-molecular-weight complex with an apparent size of 1,200 kDa. In extracts from delta cyc8 strains, the size of the complex is reduced to 830 kDa. The CYC8 and TUP1 proteins were coprecipitated by either antiserum, further supporting the conclusion that they are associated with each other. The complex could be reconstituted in vitro by mixing extracts from strains with complementary mutations in the CYC8 and TUP1 genes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati BB, Gasser SM. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell. 1988 Sep 23;54(7):967–978. [Abstract] [Google Scholar]
- Andersson L-O, Borg H, Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972 Feb 1;20(2):199–202. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Carlson M, Osmond BC, Neigeborn L, Botstein D. A suppressor of SNF1 mutations causes constitutive high-level invertase synthesis in yeast. Genetics. 1984 May;107(1):19–32. [Europe PMC free article] [Abstract] [Google Scholar]
- Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. [Abstract] [Google Scholar]
- Clos J, Westwood JT, Becker PB, Wilson S, Lambert K, Wu C. Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell. 1990 Nov 30;63(5):1085–1097. [Abstract] [Google Scholar]
- Dalrymple MA, Petersen-Bjorn S, Friesen JD, Beggs JD. The product of the PRP4 gene of S. cerevisiae shows homology to beta subunits of G proteins. Cell. 1989 Sep 8;58(5):811–812. [Abstract] [Google Scholar]
- Ey PL, Ashman LK. The use of alkaline phosphatase-conjugated anti-immunoglobulin with immunoblots for determining the specificity of monoclonal antibodies to protein mixtures. Methods Enzymol. 1986;121:497–509. [Abstract] [Google Scholar]
- Ferguson B, Krippl B, Andrisani O, Jones N, Westphal H, Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. [Europe PMC free article] [Abstract] [Google Scholar]
- Firestone GL, Winguth SD. Immunoprecipitation of proteins. Methods Enzymol. 1990;182:688–700. [Abstract] [Google Scholar]
- Fong HK, Hurley JB, Hopkins RS, Miake-Lye R, Johnson MS, Doolittle RF, Simon MI. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. [Europe PMC free article] [Abstract] [Google Scholar]
- Gasser SM, Laemmli UK. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. [Abstract] [Google Scholar]
- Hardy WR, Strauss JH. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. [Europe PMC free article] [Abstract] [Google Scholar]
- Hartley DA, Preiss A, Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell. 1988 Dec 2;55(5):785–795. [Abstract] [Google Scholar]
- Hirano T, Kinoshita N, Morikawa K, Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. [Abstract] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lemontt JF. Pathways of ultraviolet mutability in Saccharomyces cerevisiae. III. Genetic analysis and properties of mutants resitant to ultraviolet-induced forward mutation. Mutat Res. 1977 May;43(2):179–204. [Abstract] [Google Scholar]
- Lemontt JF, Fugit DR, Mackay VL. Pleiotropic Mutations at the TUP1 Locus That Affect the Expression of Mating-Type-Dependent Functions in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Apr;94(4):899–920. [Europe PMC free article] [Abstract] [Google Scholar]
- MacConnell WP, Verma IM. Expression of FBJ-MSV oncogene (fos) product in bacteria. Virology. 1983 Dec;131(2):367–374. [Abstract] [Google Scholar]
- Neigeborn L, Carlson M. Mutations causing constitutive invertase synthesis in yeast: genetic interactions with snf mutations. Genetics. 1987 Feb;115(2):247–253. [Europe PMC free article] [Abstract] [Google Scholar]
- Olesen J, Hahn S, Guarente L. Yeast HAP2 and HAP3 activators both bind to the CYC1 upstream activation site, UAS2, in an interdependent manner. Cell. 1987 Dec 24;51(6):953–961. [Abstract] [Google Scholar]
- Rothstein RJ, Sherman F. Genes affecting the expression of cytochrome c in yeast: genetic mapping and genetic interactions. Genetics. 1980 Apr;94(4):871–889. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruggieri R, Tanaka K, Nakafuku M, Kaziro Y, Toh-e A, Matsumoto K. MSI1, a negative regulator of the RAS-cAMP pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8778–8782. [Europe PMC free article] [Abstract] [Google Scholar]
- Rüther U, Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. [Europe PMC free article] [Abstract] [Google Scholar]
- Schamhart DH, Ten Berge AM, Van De Poll KW. Isolation of a catabolite repression mutant of yeast as a revertant of a strain that is maltose negative in the respiratory-deficient state. J Bacteriol. 1975 Mar;121(3):747–752. [Europe PMC free article] [Abstract] [Google Scholar]
- Schultz J, Carlson M. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3637–3645. [Europe PMC free article] [Abstract] [Google Scholar]
- Schultz J, Marshall-Carlson L, Carlson M. The N-terminal TPR region is the functional domain of SSN6, a nuclear phosphoprotein of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4744–4756. [Europe PMC free article] [Abstract] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. [Abstract] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. [Europe PMC free article] [Abstract] [Google Scholar]
- Spindler KR, Rosser DS, Berk AJ. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. [Europe PMC free article] [Abstract] [Google Scholar]
- Stark HC, Fugit D, Mowshowitz DB. Pleiotropic properties of a yeast mutant insensitive to catabolite repression. Genetics. 1980 Apr;94(4):921–928. [Europe PMC free article] [Abstract] [Google Scholar]
- Thrash-Bingham C, Fangman WL. A yeast mutation that stabilizes a plasmid bearing a mutated ARS1 element. Mol Cell Biol. 1989 Feb;9(2):809–816. [Europe PMC free article] [Abstract] [Google Scholar]
- Trumbly RJ. Isolation of Saccharomyces cerevisiae mutants constitutive for invertase synthesis. J Bacteriol. 1986 Jun;166(3):1123–1127. [Europe PMC free article] [Abstract] [Google Scholar]
- Trumbly RJ. Cloning and characterization of the CYC8 gene mediating glucose repression in yeast. Gene. 1988 Dec 15;73(1):97–111. [Abstract] [Google Scholar]
- Vernet T, Dignard D, Thomas DY. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. [Abstract] [Google Scholar]
- Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, Grant FJ, O'Hara P, MacKay VL. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. [Abstract] [Google Scholar]
- Wickner RB. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. [Europe PMC free article] [Abstract] [Google Scholar]
- Williams FE, Trumbly RJ. Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6500–6511. [Europe PMC free article] [Abstract] [Google Scholar]
- Yochem J, Byers B. Structural comparison of the yeast cell division cycle gene CDC4 and a related pseudogene. J Mol Biol. 1987 May 20;195(2):233–245. [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.11.6.3307-3316.1991
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/11/6/3307
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/11/6/3307
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Regulation of blue infertile flower pigmentation by WD40 transcription factor HmWDR68 in Hydrangea macrophylla 'forever summer'.
Mol Biol Rep, 51(1):328, 23 Feb 2024
Cited by: 0 articles | PMID: 38393428
Systematic analysis of tup1 and cyc8 mutants reveals distinct roles for TUP1 and CYC8 and offers new insight into the regulation of gene transcription by the yeast Tup1-Cyc8 complex.
PLoS Genet, 19(8):e1010876, 11 Aug 2023
Cited by: 2 articles | PMID: 37566621 | PMCID: PMC10446238
Tup1 is critical for transcriptional repression in Quiescence in S. cerevisiae.
PLoS Genet, 18(12):e1010559, 21 Dec 2022
Cited by: 4 articles | PMID: 36542663 | PMCID: PMC9815585
Tup1 Paralog CgTUP11 Is a Stronger Repressor of Transcription than CgTUP1 in Candida glabrata.
mSphere, 7(2):e0076521, 28 Mar 2022
Cited by: 1 article | PMID: 35341317 | PMCID: PMC9044973
Genetic analysis argues for a coactivator function for the Saccharomyces cerevisiae Tup1 corepressor.
Genetics, 219(2):iyab120, 01 Oct 2021
Cited by: 3 articles | PMID: 34849878 | PMCID: PMC8633128
Go to all (150) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits.
Mol Cell Biol, 16(12):6707-6714, 01 Dec 1996
Cited by: 89 articles | PMID: 8943325 | PMCID: PMC231673
Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae.
Mol Cell Biol, 10(12):6500-6511, 01 Dec 1990
Cited by: 142 articles | PMID: 2247069 | PMCID: PMC362927
Functional dissection of the yeast Cyc8-Tup1 transcriptional co-repressor complex.
Nature, 369(6483):758-761, 01 Jun 1994
Cited by: 227 articles | PMID: 8008070
Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes.
Trends Biochem Sci, 25(7):325-330, 01 Jul 2000
Cited by: 216 articles | PMID: 10871883
Review