Abstract
Free full text

Promotion of Caspase Activation by Caspase-9-mediated Feedback Amplification of Mitochondrial Damage
Abstract
Mitochondrial disruption during apoptosis results in the activation of caspase-9 and a downstream caspase cascade. Triggering this caspase cascade leads to the cleavage of anti-apoptotic Bcl-2 family proteins, resulting in feedback amplification of mitochondrial disruption. However, whether such a feedback loop plays an important role in the promotion of caspase activation and execution of apoptosis has not been well established. We observed that mutated Bcl-2 or Bcl-xL that are resistant to cleavage by caspases inhibited caspase-9-induced caspase activation in human H9 T cells. The release of Smac after the activation of caspase-9 was also inhibited by cleavage-resistant Bcl-2 or Bcl-xL. Consistently, caspase-9-deficient cells were defective in the release of Smac after induction of apoptosis. Moreover, addition of a Smac mimetic overcame the inhibitory effects of cleavage-resistant Bcl-2/Bcl-xL, and restored caspase-9-mediated cell death. Our data suggest that caspase-9-induced feedback disruption of mitochondria plays an important role in promoting the activation of caspases, while a defect in this process can be overcome by promoting Smac functions.
Introduction
In mitochondrial apoptosis, the disruption of the mitochondrial outer membrane allows the release of cytochrome c from the mitochondria into the cytosol [1,2]. Cytochrome c, Apaf-1 and caspase-9 form the apoptosome for the dimerization and activation of caspase-9 [3,4,5]. Caspase-9 then proteolytically cleaves downstream effector caspases, such as caspase-3, resulting in the activation of these caspases [6,7,8]. Other proteins released from the mitochondria, including apoptosis-inducing factor (AIF), endonuclease G (Endo G), high temperature requirement protein A2 (HtrA2) and second mitochondria-derived activator of caspases (Smac)/direct IAP-binding protein with low pI (DIABLO), also regulate cell death [9,10,11,12,13,14,15,16,17,18]. While AIF, Endo G and HtrA2 may cause cell death independent of caspases [19], Smac promotes caspase activation by releasing caspase-9 and caspase-3 from inhibition by the inhibitor of apoptosis (IAP) proteins [17,18,20,21].
Anti-apoptotic Bcl-2 family proteins, such as Bcl-2 and Bcl-xL, maintain the stability of the mitochondrial outer membrane and inhibit the release of mitochondrial apoptotic proteins [1,2,22,23]. However, Bcl-2 and Bcl-xL are cleavable by caspases in apoptotic cells [24,25,26]. Cleavage-resistant Bcl-2 and Bcl-xL are more effective in inhibiting apoptosis than their wild type counterparts, suggesting that caspase-induced cleavage inactivates the anti-apoptosis function of Bcl-2 and Bcl-xL [24,25,26,27]. Interestingly, loss of mitochondrial membrane potential during cell death is inhibited when caspase-9 or caspases-3 and -7 are deficient [28,29,30,31,32,33], suggesting an important role for caspases in feedback disruption of mitochondria.
Nuclear fragmentation following the activation of the caspase cascade is a hallmark of apoptosis [34]. The degradation of DNA from apoptotic cells may be important for the prevention of autoimmunity [35,36]. Caspase-activated DNase (CAD)/DNA Fragmentation Factor 40 kDa (DFF40) plays a prominent role in mediating nuclear fragmentation [37,38,39,40]. CAD is associated with its inhibitor and chaperone, inhibitor of CAD (ICAD)/DNA Fragmentation Factor 45 kDa (DFF45) [37,38,39]. Processing of ICAD by caspases results in the release and activation of CAD to induce nuclear damage [37,38,39]. Deletion of either CAD or its inhibitor/chaperone ICAD blocks nuclear fragmentation in apoptotic cells, suggesting the importance of CAD in mediating nuclear damages [41,42]. Whether caspase-9-induced feedback disruption of mitochondria is important for promoting CAD activation and inducing nuclear damage is not known.
To study the signaling events downstream of caspase-9, we have expressed caspase-9 that can be dimerized for the direct activation of caspase-9 in intact cells [6,24]. Dimerization of caspase-9 induces the activation of a downstream caspase cascade through caspase-3 [6], and triggers a feedback loop for mitochondrial disruption through cleavage of anti-apoptotic Bcl-2 family proteins [24]. Here we show that cleavage-resistant Bcl-2 or Bcl-xL suppresses the release of Smac and cytochrome c after dimerization of caspase-9 in H9 T cells, leading to inhibition of both endogenous caspase activation and nuclear damage. A Smac mimetic overcame the inhibitory effects of cleavage-resistant Bcl-2 or Bcl-xL to promote caspase activation and apoptosis, supporting the importance for using Smac mimetics in cancer therapy.
Materials and Methods
Cell lines
5' HA-tagged inducible caspase-9 (iCasp9) was created by fusing a mutated FK506-binding protein with the protease domain of caspase-9 [43]. Human H9 T cell lines that stably express iCasp9 (H9-iCasp9 cells) were established as described [6,24]. AP20187 (ARIAD Pharmaceuticals, Cambridge, MA), a dimeric FK506 analog, was used as a chemical-inducer of dimerization (CID) to activate iCasp9 [6,24]. H9-iCasp9 cells with silencing of caspase-3 or stably expressing wild type Bcl-2 or Bcl-xL, or mutant Bcl-2 or Bcl-xL resistant to proteolytic cleavage by caspases (Bcl-2D/A or Bcl-xLD/A), have been reported [24]. JMR cells and JMR cells reconstituted with caspase-9 (JMR-C9) have been described [31,32].
Western blotting
Cells were lysed in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1× protease inhibitor cocktail from Roche, 2 μM z-VAD-fmk) at different time points for Western blotting as described [6,24]. The following primary antibodies were used for Western blotting: mouse monoclonal antibodies to Smac, cytochrome c and Bcl-2 (BD Biosciences, San Jose, CA), ICAD and caspase-9 (Medical and Biological Laboratories, Woburn, MA), HA (Covance, Princeton, NJ) and α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal antibody to caspase-3 (BD Biosciences), Bcl-xL and GAPDH (Cell Signaling Technology, Danvers, MA) and VDAC1 (Abcam, Cambridge, MA); and goat polyclonal antibody to actin (Santa Cruz Biotechnology). The blots were then incubated with HRP-conjugated secondary antibodies (Southern Biotech, Birmingham, AL and GE Healthcare, Piscataway, NJ), followed by development with the SuperSignal West Dura substrate (Thermo Scientific, Waltham, MA).
Subcellular fractionation
Cells were suspended in permeabilization buffer (75 mM KCl, 1 mM NaH2PO4, 8 mM Na2HPO4, 250 mM sucrose, 1× protease inhibitor cocktail from Roche, μM z-VAD-fmk) containing 150 μg/ml digitonin and fractionated into the cytosolic and particulate fractions as described [24,44]. The particulate pellet was then re-suspended in lysis buffer on ice for 30 min and centrifuged at 16,000 × g at 4° for 10 min. The supernatant was then collected for Western blot analyses of the particulate fraction.
Immunocytochemistry
Cells were cultured for the indicated times with or without 10 nM CID to induce apoptosis. For the last 30 minutes of culture, cells were stained with 100 nM of MitoTracker Deep Red (Invitrogen, Carlsbad, CA). Cells were washed and centrifuged onto poly-L-lysine-coated slides using a Shandon Cytospin IV (Thermo Scientific). Cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), permeabilized with 0.15% Triton X-100 and stained with DAPI. Cells were visualized with a Zeiss Axioplan fluorescent microscope (Zeiss, Munich, Germany).
Flow cytometric analyses of sub-diploid nuclei
Cells were cultured for the indicated times with or without apoptotic stimuli. Cells were then collected, washed once in PBS and re-suspended in hypotonic staining buffer (0.1% sodium citrate, 0.1% Triton X-100 and 50 μg/mL propidium iodide) at 4° in the dark overnight as described [32]. Cells were analyzed using an LSRII flow cytometer (BD Biosciences).
Cell death assay
Cells (20,000/well) were plated in 96-well plates and cultured with a Smac mimetic [45] or solvent control for 2 h. Cells were then treated with CID for 24 h and stained with propidium iodide (PI). The percentage of cell loss was quantitated by flow cytometry as described [6,24].
Caspase activity assay
Caspase activation was determined using the Caspase-Glo 3/7 Kit (Promega, Madison, WI) according to the manufacturer's instructions. Cells (10,000/well) were plated in 96-well flat bottom plates and treated with a Smac mimetic [45] or solvent control for 2 h, followed by treatment with CID for 6 h. The Caspase-Glo 3/7 reagent was added to cells and the plate was incubated for 1 h at room temperature. Luminescence was measured using a FLUOstar OPTIMA plate reader (BMG Labtech, Offenburg, Germany). Results are shown in Relative Light Units (RLU).
Measurement of mitochondrial membrane potential
Cells were cultured for the indicated times. During the last 20 minutes of culture, cells were incubated with 25 nM of tetramethylrhodamine ethyl ester (TMRE; Invitrogen). Cells were collected, washed and mitochondrial membrane potential was quantitated on a LSRII flow cytometer (BD Bioscience).
Results
Bcl-2D/A or Bcl-xLD/A inhibits caspase-9-induced nuclear damage
Previously, we have established a H9 T cell line that stably expresses an inducible caspase-9 (iCasp9) by replacing the prodomain of caspase-9 with a mutated FK506-binding protein [6,24]. The iCasp9 is expressed at a level comparable to endogenous caspase-9 [24] and can be activated by chemically-induced dimerization with a dimeric FK506 analog [43]. We have demonstrated that the direct activation of caspase-9 results in feedback disruption of the mitochondria through cleavage of anti-apoptotic Bcl-2 family proteins, including Bcl-2 and BclxL [24].
Caspase-mediated nuclear fragmentation is characteristic of apoptotic cell death [34]. To determine if caspase-mediated feedback disruption of the mitochondria contributes to nuclear damages during apoptosis, we stably expressed wild type or cleavage-resistant Bcl-2 or Bcl-xL with aspartate to alanine substitutions at the caspase-cleavage sites (Bcl-2D/A or Bcl-xLD/A, respectively) in iCasp9 cells [24]. We have shown that the aspartate to alanine substitutions renders Bcl-2D/A and Bcl-xLD/A resistant to caspase-mediated cleavage [24]. We found that Bcl-2D/A or Bcl-xLD/A potently inhibited nuclear fragmentation after the dimerization of caspase-9 (Figure 1A). In addition, Bcl-2D/A or Bcl-xLD/A diminished the appearance of sub-diploid cells after the activation of caspase-9 (Figure 1B). These results suggest that caspase-9-mediated cleavage of anti-apoptotic Bcl-2 family proteins promotes DNA fragmentation and nuclear damage.
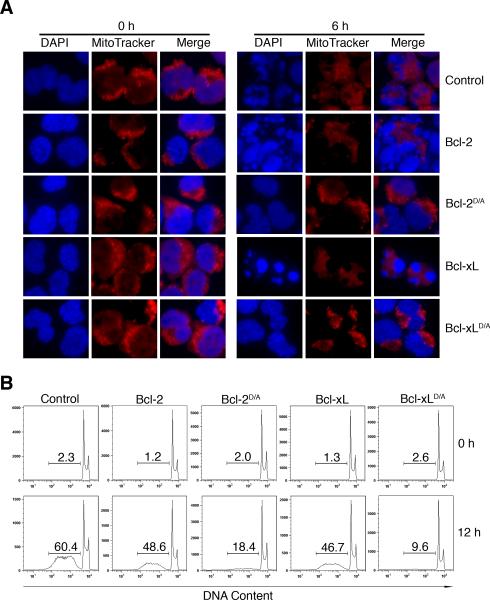
(A) Control H9-iCasp9 cells, or H9-iCasp9 cells expressing wild type Bcl-2 or Bcl-xL, or mutant Bcl-2 or Bcl-xL (Bcl-2D/A or Bcl-xLD/A), were cultured 6 h in the presence or absence of 10 nM of the divalent FK506 analog, AP20187, as a chemical-inducer of dimerization (CID) to activate iCasp9 [6,24]. Nuclear morphology was visualized by staining with DAPI and mitochondria were visualized using MitoTracker Deep Red. (B) Cells as in (A) were cultured for 12 h in the presence or absence of 10 nM of CID. Sub-diploid DNA content was measured by staining with propidium iodide. Results are representative of at least two experiments.
Bcl-2D/A or Bcl-xLD/A inhibits the cleavage of ICAD after activation of caspase-9
The activation of caspase-activated DNase (CAD) is sufficient to induce DNA fragmentation [38,40]. CAD is kept inactive in cells through interaction with inhibitor of CAD (ICAD) until ICAD is proteolytically cleaved by caspases [37,38,39]. We therefore examined whether the cleavage of ICAD is inhibited by the expression of Bcl-2D/A or Bcl-xLD/A.
ICAD is expressed in two isoforms, ICAD long (ICAD-L) and ICAD short (ICAD-S), both of which can be cleaved by caspases [39]. After activation of caspase-9 by dimerization, the processing of ICAD in the presence of Bcl-2D/A or Bcl-xLD/A was dramatically reduced (Figure 2). Because ICAD is present in stoichiometric excess relative to CAD [46,47], low levels of caspase-mediated cleavage of ICAD in cells expressing Bcl-2D/A or Bcl-xLD/A are unlikely to cause nuclear fragmentation. Our results suggest that caspase-9-induced amplification of mitochondrial disruption is important for promoting the cleavage of ICAD to release CAD for DNA fragmentation.
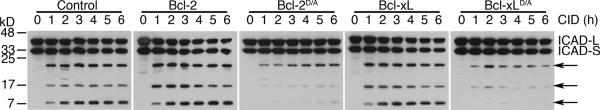
The indicated cell lines were treated with 10 nM of CID for 0 to 6 h, followed by Western blotting for ICAD. The antibody used here detects the long and short isoforms of ICAD (ICAD-L and ICAD-S, respectively). Arrows indicate the caspase-processed forms of ICAD. Results are representative of two experiments.
Bcl-2D/A or Bcl-xLD/A inhibits the activation of endogenous caspase-9 and caspase-3 after the dimerization of caspase-9
Decreased caspase-9-induced processing of ICAD in cells expressing cleavage-resistant Bcl-2D/A or Bcl-xLD/A suggests decreased activation of caspases in these cells. To analyze processing on endogenous caspases-9, we utilized an antibody that recognizes the N-terminus of caspase-9 that is lacking in iCasp9. We found that expression of Bcl-2D/A or Bcl-xLD/A inhibited the cleavage of endogenous caspase-9 after the dimerization of caspase-9 (Figure 3, upper panels). In addition, Bcl-2D/A or Bcl-xLD/A inhibited caspase-3 activation after dimerization of caspase-9 (Figure 3, lower panels). Together, these results suggest that caspase-9 induced feedback disruption of mitochondria is important for optimal activation of caspases.
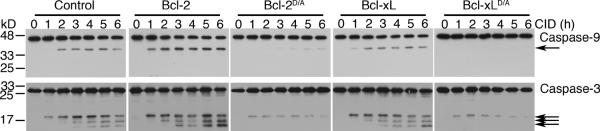
The indicated H9-iCasp9 cells lines were treated with 10 nM of CID, followed by Western blotting with anti-caspase-9 or anti-caspase-3 antibodies. Arrows indicates processed caspases. Results are representative of two experiments.
Bcl-2D/A or Bcl-xLD/A inhibits caspase-9-induced release of Smac and cytochrome c
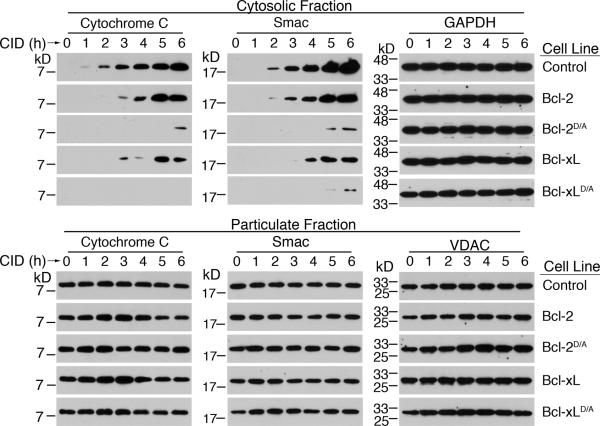
Decreased release of mitochondrial apoptotic effectors involved in caspase-mediated cell death in cells expressing Bcl-2D/A or Bcl-xLD/A could explain the decreased activation of caspases observed in these cells. We performed subcellular fractionation to determine the release of mitochondrial apoptotic proteins into the cytosol after direct activation of caspase-9. We have previously detected the translocation of cytochrome c into the cytosol after the activation of caspase-9 [24], but did not examine the release of Smac. IAP-family proteins can bind to caspase-9 and caspase-3 and prevent caspase activation, whereas Smac can bind to and inhibit IAP-family proteins to promote caspase activity [17,18,20,21,48,49]. Significantly, IAP-family proteins can inhibit caspase-3 directly [50]. Thus, the release of Smac may be critical for allowing complete caspase activation after the activation of caspase-9. We found that the direct activation of caspase-9 promoted the release of Smac into the cytosol (Figure 4). These results suggest that activation of caspase-9 promotes the release of mitochondrial proteins that trigger caspase activation.
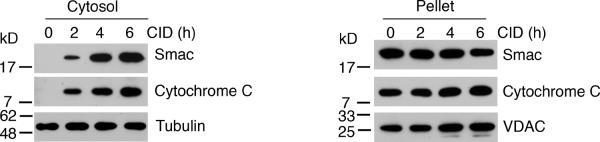
Control H9-iCasp9 cells, or H9-iCasp9 cells expressing either wild type or cleavage-resistant Bcl-2 or Bcl-xL, were treated with 10 nM of CID for the indicated times, followed by isolation of the cytosolic and particulate fractions. Western blotting was performed for the indicated proteins. Membranes were probed with anti-GAPDH or anti-VDAC antibodies to confirm equal loading. Results are representative of three experiments.
We then determined if the expression of cleavage-resistant Bcl-2D/A or Bcl-xLD/A inhibited the release of Smac and cytochrome c. We found that Bcl-2D/A or Bcl-xLD/A inhibited the release of Smac from the mitochondria after caspase-9 activation (Figure 4). In addition, consistent with previous findings [24], we observed that the expression of Bcl-2D/A or Bcl-xLD/A significantly reduced the translocation of cytochrome c into the cytosol after the activation of caspase-9 by dimerization (Figure 4). Our results suggest that the caspase-9 signaling cascade is important for the cleavage of anti-apoptotic Bcl-2 family proteins to promote the release of cytochrome c and Smac.
Knockdown of caspase-3 inhibits caspase-9-mediated mitochondrial disruption
Caspase-3 is the primary mediator of apoptosis signaling by caspase-9 [28,51]. Indeed, we have previously observed that knockdown of caspase-3 inhibits caspase-9-induced cleavage of Bcl-2 and Bcl-xL, release of cytochrome c into the cytosol and loss of mitochondrial membrane potential [24]. We confirmed that silencing of caspase-3 (Figure 5A) inhibited the loss of mitochondrial membrane potential downstream of the dimerization of caspase-9 (Figure 5B). In addition, we found that silencing of caspase-3 inhibited caspase-9-induced release of cytochrome c and Smac, as well as the activation of endogenous caspase-9 (Figure 5, C and D). Together, these results suggest that caspase-3 is critical for mediating caspase-9-induced amplification of mitochondrial disruption.
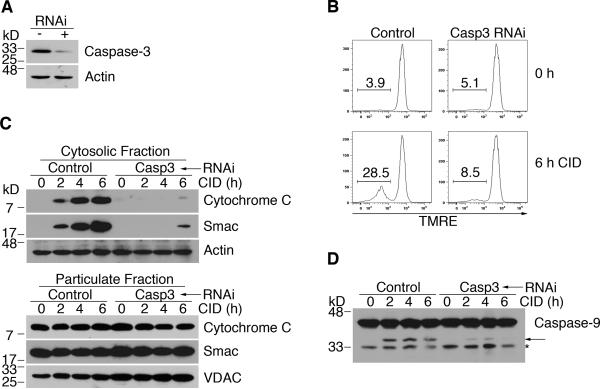
(A) Control H9-iCasp9 cells and cells expressing siRNA to silence caspase-3 were used for Western blot for casapse-3 or actin. (B) Cells as in (A) were treated with 10 nM CID and TMRE staining was performed at the indicated times to measure mitochondrial membrane potential. (C) Cells were fractionated at the indicated times. Western blotting for cytochrome c and Smac was performed. Membranes were re-probed with anti-actin or anti-VDAC for equal loading controls. (D) Cells were treated with CID and lysed for Western blotting at the times indicated. Arrow denotes processed caspase-9. Asterisk indicates degraded or non-specific protein. Results are representative of at least two experiments.
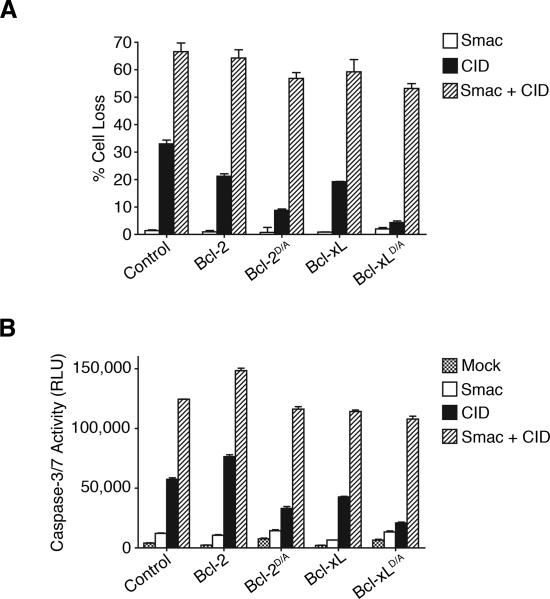
A Smac mimetic overcomes the inhibition by Bcl-2D/A or Bcl-xLD/A to restore caspase-9-mediated cell death
Expression of Bcl-2D/A or Bcl-xLD/A inhibited caspase-9-induced release of Smac and cytochrome c (Figure 4), and the activation of endogenous caspase-9 and caspase-3 (Figure 3). Because Smac functions to increase the activation of caspase-3 and caspase-9, we hypothesized that promoting Smac functions may bypass the requirement for the feedback amplification of mitochondrial disruption. Although the addition of a synthetic Smac mimetic [45] alone did not have significant effects, the Smac mimetic synergized with caspase-9 dimerization to promote cell death in iCasp9 cells (Figure 6A). Significantly, the Smac mimetic overcame the inhibitory effect of Bcl-2D/A or Bcl-xLD/A and promoted cell death induced by dimerization of caspase-9 (Figure 6A). Similar effects were observed with caspase activation (Figure 6B). These results suggest that caspase-mediated feedback disruption of mitochondria could promote caspase activation and apoptosis by facilitating the release of proteins with Smac-like functions (Figures 4 and and66).
(A) Cells were treated with 3 μM of the Smac mimetic and 10 nM of CID as indicated. Percentages of cell death (Mean ± standard deviations from triplicate wells) are displayed from a representative experiment. (B) Cells were treated as in (A) and caspase-3-like activity (Mean ± standard deviations from triplicate wells) was measured in a luminescence-based assay (Caspase-Glo 3/7, Promega). RLU: Relative Light Units.
Caspase-9 deficiency inhibits mitochondrial disruption
To support our data generated using iCasp9 cells that caspase-9 can amplify mitochondrial disruption, we utilized a caspase-9-deficient Jurkat T cell line, JMR, as well as JMR cells reconstituted with caspase-9 (JMR-C9) (Figure 7A) [31,32]. Caspase-9-deficient JMR cells released significantly less cytochrome c and Smac after treatment with UV-irradiation compared to caspase-9-sufficient JMR-C9 cells (Figure 7B), suggesting that caspase-9 signaling is important for promoting the release of apoptosis signaling molecules from mitochondria. Consistent with previous observations [31,32], we observed that deficiency of caspase-9 inhibited loss of mitochondrial membrane potential after UV-irradiation (Figure 7C). As expected, caspase-9-deficient JMR cells did not show cleavage of Bcl-2, Bcl-xL or ICAD after treatment with UV-irradiation (Figure 7D), nor did these cells exhibit DNA fragmentation (Figure 7E). These results support the conclusions that caspase-9 signaling is important for inducing a feedback loop to amplify mitochondrial disruption, thereby promoting the optimal activation of caspases for the execution of apoptosis.
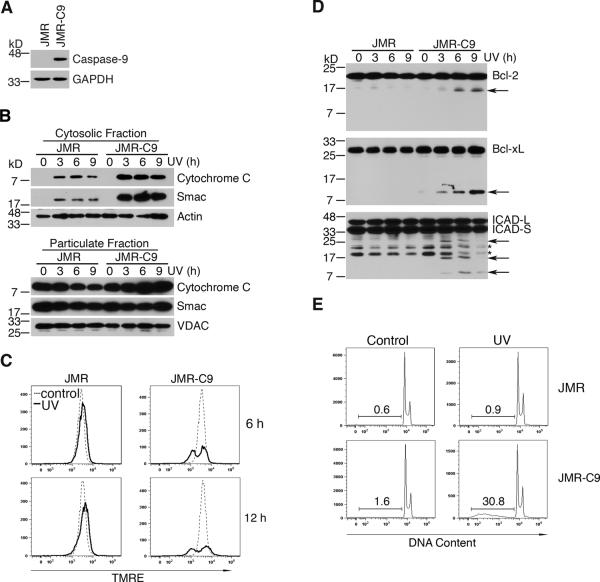
(A) JMR cells and JMR cells reconstituted with caspase-9 (JMR-C9) were lysed for caspase-9 Western blot. Membrane was re-probed with anti-GAPDH as a loading control. (B) JMR or JMR-C9 cells were treated with 500 mJ/cm2 UV-irradiation, followed by culture for 3, 6 or 9 h. Cells were fractionated into cytosolic and particulate preparations at the indicated times. Western blotting was performed for the indicated proteins. Membranes were re-probed with anti-actin or anti-VDAC antibodies as loading controls. (C) Mitochondrial membrane potential of JMR and JMR-C9 cells was determined at the indicated times using TMRE. (D) JMR and JMR-C9 cells treated as in (B) were used for Western blotting. Membranes were probed with the indicated antibodies. Arrows denote caspase-cleaved protein fragments. Asterisks denote degraded or non-specific protein bands. (E) Cells treated as indicated were cultured for 16 h. DNA fragmentation was measured by analyzing sub-diploid DNA content with propidium iodide. Results are representative of at least two experiments.
Discussion
In this study, we determined the signaling events after specific activation of caspase-9 in H9 T cells by chemically-induced dimerization. We found that cleavage-resistant Bcl-2D/A or Bcl-xLD/A suppressed caspase-9-induced cleavage of ICAD and nuclear damages. Caspase-9-mediated feedback amplification of mitochondrial disruption promoted the activation of endogenous caspase-9 and caspase-3. Decreased activation of caspases in cells expressing Bcl-2D/A or Bcl-xLD/A correlated with decreased release of Smac and cytochrome c. Addition of a Smac mimetic bypassed the inhibitory effects of Bcl-2D/A or Bcl-xLD/A and promoted caspase-9-induced caspase activation and apoptosis. In agreement with the data obtained by chemically-induced dimerization of caspase-9, JMR cells that lack caspase-9 displayed significant defects in the loss of mitochondrial membrane potential and the release of cytochrome c and Smac after UV-irradiation. Together, these results suggest that the caspase-9-induced signaling cascade cleaves anti-apoptotic Bcl-2 and Bcl-xL, leading to amplification of mitochondrial disruption (Figure 8). Such feedback amplification of mitochondrial disruption facilitates the release of Smac, which may promote the activation of caspases and the execution of apoptosis.
Various stress signals promote the release of Smac and cytochrome c from the mitochondria to promote the activation of caspase-9. (A) Without caspase-mediated feedback disruption of the mitochondria through cleavage of anti-apoptotic Bcl-2 family proteins, members of the IAP family inhibit the full activation of caspases and thus the execution of apoptosis. (B) Caspase-mediated cleavage of anti-apoptotic Bcl-2 family proteins promotes the complete disruption of the outer mitochondrial membrane, furthering the release Smac and cytochrome c. Complete release of Smac and cytochrome c results in the full activation of caspases and apoptosis.
Previous studies have determined that cleavage-resistant Bcl-2 or Bcl-xL protects cells from apoptosis induced by cytokine withdrawal and chemotherapeutics to a greater extent than their wild type counterparts [24,25,26,27]. Indeed, we found Bcl-2D/A or Bcl-xLD/A were much more potent inhibitors of caspase-9-mediated apoptosis than their wild type counterparts. The increased anti-apoptotic ability of cleavage-resistant Bcl-2 or Bcl-xL may be due to an inability to be converted into a pro-apoptotic cleavage product [25,26]. How cleaved Bcl-2 and Bcl-xL act as a pro-apoptotic protein is not fully understood, although it should be noted, cleavage removes the Bcl-2 homology 4 (BH4) domain that is specific to anti-apoptotic Bcl-2 family proteins from the BH1-3 domains [25,26]. The resulting C-terminal cleavage product thus resembles the multi-domain pro-apoptotic proteins Bax and Bak [52]. While endogenous Bcl-2 and Bcl-xL may be cleaved following caspase-9 activation in cells expressing Bcl-2D/A or Bcl-xLD/A, it is likely the presence of cleavage-resistant Bcl-2D/A or Bcl-xLD/A counters the action of these pro-apoptotic fragments.
The addition of a Smac mimetic restored caspase-9-induced cell death in cells expressing Bcl-2D/A or Bcl-xLD/A (Figure 6). These findings suggest that inhibition of IAPs by Smac following feedback disruption of mitochondria is important for optimal caspase activation downstream of caspase-9. This underscores the potency of IAP proteins in preventing low levels of caspase activation from inducing cell death in the absence of extensive mitochondrial damages. This is similar to the situation in sympathetic neurons, which release cytochrome c without resulting in caspase activation due to X-linked IAP (XIAP)-mediated inhibition of caspases [53]. To overcome IAP-mediated inhibition, caspases may promote the release of Smac through cleavage of anti-apoptotic Bcl-2 family proteins (Figure 4), or directly cleave IAP proteins [54,55,56]. Alternatively, caspases may cleave other cellular proteins to expose hidden IAP-binding motifs and lead to sequestration of IAP proteins [57].
Although the release of mitochondrial apoptotic mediators initially occurs upstream of caspase activation, recent studies suggest that caspase activation can further mitochondrial disruption. Deficiency of caspase-9 or inhibition of caspases hinders loss of mitochondrial membrane potential after apoptotic stimuli [28,29,31,32,33]. Additionally, mouse embryonic fibroblasts lacking effector caspases-3 and -7 display decreased release of AIF and cytochrome c during apoptosis [30]. Moreover, cells with decreased activation of caspases due to deficiency of Smac or cytochrome c show defective release of other mitochondrial apoptotic effector proteins [58,59,60]. Our work suggests that caspases can facilitate the release of mitochondrial apoptotic effectors, such as Smac, through the cleavage of anti-apoptotic Bcl-2 family proteins, increasing the activation of caspases to ensure the induction of cell death in leukemia and lymphoma.
The ability for a Smac mimetic to promote apoptosis by overcoming the inhibitory effects of Bcl-2D/A or Bcl-xLD/A has important implications for cancer therapy. Chemotherapeutic drugs typically induce mitochondria-dependent apoptosis [61,62,63]. In cancer cells with increased anti-apoptotic Bcl-2 family members [64], decreased expression of caspases [65], lower Smac expression [66], or increases in IAP proteins [67,68], the feedback loop for mitochondrial disruption is potentially defective, resulting in insufficient caspase activation and defects in apoptosis. A Smac mimetic may overcome the requirement for caspase-dependent feedback disruption of the mitochondria and promote the activation of caspases.
Acknowledgements
We thank Dr. Xiaodong Wang for the Smac mimetic, and ARIAD pharmaceuticals for AP20187. This work was supported by grants from the NIH (J.W. and M.C.).
Abbreviations
| iCasp9 | inducible caspase-9 |
| CID | chemical-inducer of dimerization |
| AIF | apoptosis-inducing factor |
| Endo G | endonuclease G |
| HtrA2 | high temperature requirement protein A2 |
| Smac/DIABLO | second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI |
| IAP | inhibitor of apoptosis |
| XIAP | X-linked IAP |
| CAD/DFF40 | caspase-activated DNase/DNA fragmentation factor 40 kDa |
| ICAD/DFF45 | inhibitor of CAD/DNA fragmentation factor 45 kDa |
| VDAC | voltage-dependent anion channel |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HA | hemagglutinin |
| TMRE | tetramethylrhodamine ethyl ester |
| zVAD-fmk | carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone |
| UV | ultraviolet. |
References
Full text links
Read article at publisher's site: https://doi.org/10.4172/2155-9899.1000126
Read article for free, from open access legal sources, via Unpaywall:
https://www.omicsonline.org/promotion-of-caspase-activation-by-caspase-9-mediated-feedback-amplification-of-mitochondrial-damage-2155-9899.1000126.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Synthesized Gold Nanoparticles with Moringa Oleifera leaf Extract Induce Mitotic Arrest (G2/M phase) and Apoptosis in Dalton's Lymphoma Cells.
Cell Biochem Biophys, 82(2):1043-1059, 02 May 2024
Cited by: 0 articles | PMID: 38696103
Pathogen-driven nucleotide overload triggers mitochondria-centered cell death in phagocytes.
PLoS Pathog, 19(12):e1011892, 29 Dec 2023
Cited by: 0 articles | PMID: 38157331 | PMCID: PMC10756532
Iodine-Biofortified Lettuce Can Promote Mitochondrial Dependent Pathway of Apoptosis in Human Gastrointestinal Cancer Cells.
Int J Mol Sci, 24(12):9869, 07 Jun 2023
Cited by: 5 articles | PMID: 37373017 | PMCID: PMC10298746
Live imaging of apoptotic signaling flow using tunable combinatorial FRET-based bioprobes for cell population analysis of caspase cascades.
Sci Rep, 12(1):21160, 07 Dec 2022
Cited by: 2 articles | PMID: 36476686 | PMCID: PMC9729311
Role of apoptotic inhibitors, viability, and differentiation in low oxygen tension of mesenchymal stem cells cultured in a rat model of ovarian failure.
F1000Res, 12:24, 09 Jan 2023
Cited by: 1 article | PMID: 38644927 | PMCID: PMC11031646
Go to all (18) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells.
Oncogene, 23(26):4523-4535, 01 Jun 2004
Cited by: 56 articles | PMID: 15064710
Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members.
J Biol Chem, 282(46):33888-33895, 24 Sep 2007
Cited by: 73 articles | PMID: 17893147
Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor beta in rat fetal hepatocytes.
Hepatology, 34(3):548-556, 01 Sep 2001
Cited by: 73 articles | PMID: 11526541
Differentiation-induced HL-60 cell apoptosis: a mechanism independent of mitochondrial disruption?
Apoptosis, 9(3):345-352, 01 May 2004
Cited by: 9 articles | PMID: 15258466
Funding
Funders who supported this work.
NIAID NIH HHS (1)
Grant ID: R01 AI074949
NIDDK NIH HHS (1)
Grant ID: R01 DK083164
NIGMS NIH HHS (1)
Grant ID: R01 GM087710




