Abstract
Free full text

Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions
Abstract
The protocols described here efficiently direct human pluripotent stem cells (hPSCs) to functional cardiomyocytes in a completely defined, serum-free system by temporal modulation of regulators of canonical Wnt signaling. Appropriate temporal application of Gsk3 inhibitor followed by expression of β-catenin shRNA or a chemical Wnt inhibitor is sufficient to produce a high yield (0.8–1.3 million cardiomyocytes/cm2) of virtually pure (80%–98%) functional cardiomyocytes from multiple hPSC lines without cell sorting or selection. Characterization of differentiated cells is performed in qualitative (immunostaining) and quantitative (flow cytometry) manners to assess expression of cardiac transcription factors and myofilament proteins. Flow cytometry of BrdU incorporation or Ki67 expression in conjuction with cardiac sarcomere myosin protein expression can be used to determine the proliferative capacity of hPSC-derived cardiomyocytes. Functional human cardiomyocytes differentiated via these protocols may constitute a potential cell source for heart disease modeling, drug screening, and cell-based therapeutic applications.
INTRODUCTION
Directed differentiation of specific lineages from human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), is the first critical step toward constructing development or disease models, drug screening tools, or cellular therapies from hPSCs. Because postnatal cardiomyocytes have little or no regenerative capacity, very limited supplies of human cardiomyocytes are available at present. hPSCs could potentially provide an unlimited supply of cardiomyocytes from a single clonal source.
Initial efforts to differentiate hESCs into cardiomyocytes employed embryoid bodies (EBs) in medium containing fetal calf serum, but this method is inefficient, with the culture typically composed of less than 1% cardiomyocytes, and provides variable results in different hPSC lines1. Mouse END-2 (visceral endoderm-like) cell-conditioned medium has been shown to enhance cardiac differentiation in EBs2. The appropriate temporal addition of growth factors important in cardiovascular development, including fibroblast growth factor 2 (FGF2), transforming growth factor β (TGFβ) superfamily growth factors Activin A and BMP4, vascular endothelial growth factor (VEGF), and the Wnt inhibitor DKK-1, can enhance cardiomyocyte differentiation in EBs3. Monitoring the onset of KDR/c-kit3 or Flk1/PDGFRα4 expression during the differentiation protocol enables presentation of these differentiation factors at the appropriate developmental stage, resulting in relatively consistent cardiomyocyte yields in multiple hPSC lines4. In prior work, we reported that undifferentiated hPSC expansion conditions affects cardiomyocyte yield5–8. Pretreatment of hPSCs with a Gsk3 inhibitor before forming EBs greatly enhanced cardiac differentiation using serum-based EB differentiation7.
As an alternative to hPSC differentiation to cardiomyocytes via EBs, a monolayer-based directed differentiation platform was developed. This protocol sequentially exposes the hPSCs to Activin A and BMP4 in defined RPMI/B27 medium, and has been reported to be much more efficient than serum-based EB differentiation, generating greater than 30% cardiomyocytes in the H7 hESC line9, 10. However, the efficiency of the Activin A and BMP4 monolayer directed differentiation protocol is highly variable between cell lines and experimental repeats within the same line11. Here, we modified this protocol in two ways, optimizing Gsk3 inhibitor pretreatment concentration at the undifferentiated hPSC expansion stage and insulin concentration during the first 5 days of differentiation. We found that insulin, present in B27 supplement, greatly inhibits cardiomyocyte yield during the first 5 days of differentiation which is consistent with previous reports that insulin inhibits cardiac differentiation of hPSCs12, 13. We therefore use B27 supplement lacking insulin in the cardiomyocyte differentiation medium. We also found that Gsk3 inhibitor pretreatment of undifferentiated hPSCs is critical for robust cardiac differentiation. We obtained less than 1% cardiomyocytes using the original RPMI/B27 monolayer directed differentiation protocol in several hPSC lines (H9, H13, H14, 19-9-11, 6-9-9 and IMR90C4) that we tested in several experimental repeats (n>5). However, using B27 supplement without insulin and Gsk3 inhibitor pretreatment in the Activin A and BMP4 monolayer directed differentiation protocol generated 30% – 90% cardiomyocytes across these hPSC lines14. Neither B27 lacking insulin nor Gsk3 inhibitor pretreatment alone was sufficient for efficient cardiomyocyte differentiation in this protocol.
Consistent with our findings that hPSC pretreatment with a Gsk3 inhibitor greatly improved cardiac differentiation of hPSCs, Wnt signaling has also been shown to have a biphasic effect on cardiac development in zebrafish, mouse embryos, and mouse embryonic stem cells15, 16, with early Wnt signaling enhancing and later Wnt signaling repressing heart development. Because of the important temporal roles of Wnt/β-catenin on cardiac differentiation, we assessed whether modulation of Wnt/β-catenin signaling, in the absence of exogenous Activin A and BMP4, was sufficient to efficiently produce cardiomyocytes from hPSCs. We found that sequential activation of canonical Wnt signaling by Gsk3 inhibitor treatment and inhibition of Wnt signaling by inducible expression of β-catenin shRNA is sufficient to drive multiple hPSC lines to cardiomyocytes7. Small molecule inhibitors of Wnt ligand production (IWPs) also induced cardiac differentiation as effectively as β-catenin shRNA expression7. Other inhibitors of Wnt signaling, including IWR-1-endo17 and XAV93918, also have been show to promote cardiac differentiation of pluripotent stem cells.
Here we provide three protocols for efficient generation of functional human cardiomyocytes from hPSCs, the first utilizing TGFβ superfamily growth factors (protocol 1, GiAB) and the others employing small molecule activators of canonical Wnt signaling followed by shRNA of β-catenin expression (protocol 2, GiSB) or small molecule inhibitors of Wnt signaling (protocol 3, GiWi) in a growth factor-free system. Protocol 1 relies upon treatment of undifferentiated hPSCs with Gsk3 inhibitor in mTeSR1, followed by Activin A and BMP4 in RPMI/B27-insulin. The small molecule methods, protocols 2 and 3, use sequential treatment of Gsk3 inhibitors and Wnt signaling inhibitors (or inducible expression of β-catenin shRNA) to stimulate cardiogenesis. Compared with growth factor-induced cardiomyocyte differentiation, the small molecule approaches (protocol 2 and protocol 3) provide more robust cardiac differentiation, producing 82–98% cardiomyocytes from six hPSC lines. While inducible expression of β-catenin shRNA provides specific and facile temporal regulation of canonical Wnt signaling, this method (protocol 2) requires genetic modification of the hPSC line. Protocol 3, which uses Wnt signaling inhibitors instead of β-catenin shRNA, does not require genetic modification and is applicable to any existing hPSC line. All these three protocols can be performed under fully defined conditions with defined medium (RPMI/B27 without or with insulin) and defined substrates (Synthemax plates). These protocols will enable efficient production of human cardiomyocytes for development and disease research, drug screening and testing, and advancing cardiac cellular therapies.
Experimental design
Quality of hPSCs
The cardiac differentiation protocol critically depends on the quality of hPSCs, which in turn relies on the quality of matrix and medium, and the methods used to passage and maintain the hPSCs. We recommend mTeSR1 medium in conjunction with hPSC-qualified lots of Matrigel or the chemically defined surface Synthemax for hPSC expansion and maintenance in the undifferentiated state. An enzyme-free method of passaging the cells using Versene is recommended. The hPSCs cultivated in this manner should exhibit a uniform undifferentiated morphology (Fig. 1A). Undifferentiated hPSCs should express Oct4, Nanog, SSEA4, and TRA-1-80. The pluripotent marker Oct4 should be expressed in greater than 95% of the cells, as assessed by flow cytometry (Fig. 1B). Flow cytometry of Oct4 expression should be performed every three passages to validate the lack of differentiation of hPSCs. Partially differentiated hPSCs will diminish the cardiomyocyte differentiation efficiency in the protocols presented here.

Analysis of undifferentiated hPSCs. (a) H9 hESCs were cultured on Matrigel-coated 6-well plates in mTeSR1 for 2 days. Bright field images of the typical morphology of undifferentiated H9 colonies are shown. Scale bar = 100 μm. (b) Immunofluorescent staining for Oct4, Nanog, TRA-1-80 and SSEA4 was performed on undifferentiated H9 cells. Scale bar = 100 μm. (c) Flow cytometry analysis of Oct4 expression in H9 hESCs cultivated on Matrigel-coated plates in mTeSR1.
Induction of mesendoderm, cardiac mesoderm and cardiomyocytes
The hPSCs are initially cultured on Matrigel-coated plates or Synthemax plates in mTeSR1 medium until fully confluent. Differentiation is initiated by removing the mTeSR1 medium and adding RPMI/B27 medium lacking insulin and containing a Gsk3 inhibitor, such as CHIR99021. 24 hours of culture in this medium generates a high percentage of brachyury-expressing cells (>95% by flow cytometry) (Fig. 2A). In order to direct these brachyury-expressing mesendoderm progenitor cells to a cardiac fate, inhibition of canonical Wnt signaling either by β-catenin shRNA expression or Wnt signaling inhibitors, such as Porcupine inhibitors IWP2 or IWP4, is performed. Cardiac mesoderm cells spontaneously develop into functional contracting cardiomyocytes when cultured in RPMI/B27 medium.

Analysis of cardiomyocyte progenitor marker expression. (a) 19-9-11 iPSCs on Matrigel-coated 6-well plates were treated with 12 μM CHIR99021 for 24 hours. After 24 hours, flow cytometry analysis of brachyury expression was performed. The green histogram represents brachyury expression and the red histogram is an isotype control. (b) 19-9-11 iPSCs were cultured on Matrigel-coated 6-well plates in mTeSR1 for 4 days before exposure to 12 μM CH on day 0 and 5 μM IWP2 in RPMI/B27-insulin on day 3. At day 5, differentiated cells were singularized and replated on gelatin coated coverslips. At day 6, Isl1 expression was assessed by immunostaining and nucleus were stained with DAPI. Scale bar = 50 μm.
Characterization of human cardiomyocyte differentiation
Cardiac differentiation using either the growth factor or the small molecule-based differentiation protocols proceeds rapidly. A relatively pure (>95%) population of brachyury-expressing mesendoderm cells can be detected after one day of differentiation. Gene expression of the cardiac transcription factors NKX2.519 and ISL120, 21 begins at day 4, with the protein detectable at day 5 (Fig. 2B). Cardiac troponin T (cTnT) can be readily detected at day 8 of differentiation. The presence of cardiomyocytes can be easily established by visual observation of spontaneously contracting regions. The first beating cluster of cells can be observed between days 8 to day 10, depending on individual cell line used. Robust spontaneous contraction occurs by day 12. Cardiac marker protein expression after onset of contractions can also be assessed by immunostaining or quantified with flow cytometry. Here we provide procedures for performing these characterizations, including optimized immunostaining and flow cytometry methods, antibody sources, antibody dilutions, and combination of antibodies that facilitate dual staining.
MATERIALS
REAGENTS
0.25% Trypsin-EDTA (Life Technologies, cat. no. 25200-056)
16% formaldehyde (Polysciences, cat. no. 18814)
293TN cells (System Biosciences, LV900A-1)
CRITICAL: 293TN cells adhere weakly and minimal mechanical disturbance or contact with cold medium or buffers can cause their detachment, especially when they are confluent. Therefore, transfer of plates and medium changes should be performed very gently. Medium used should be warmed at 37 °C prior to addition to cells.
Accutase (Innovative Cell Technology, cat. no. AT104)
Activin A (R&D Systems, cat. no. 338-AC-050)
B27 supplement (Life Technologies, cat. no. 17504-044)
B27 supplement Minus Insulin (Life Technologies, cat. no. 0050129SA)
BrdU (Life Technologies, cat. no. B23151) ! CAUTION BrdU is a suspected mutagen and should be handled with care. Wear proper protective gear, laboratory coat and weigh under fume hood.
BMP4 (R&D Systems, cat. no. 314-BP-010)
bFGF (Life Technologies, cat. no. 13256-029)
β-mercaptoethanol (Sigma, cat. no. M7522) ! CAUTION β-mercaptoethanol is combustible, corrosive and toxic in case of ingestion and skin absorption; keep away from sources of ignition; and avoid direct contact with skin.
Bovine Serum Albumin (Sigma, cat. no. A9418)
CHIR99021 (Selleckchem, cat. no. S1263-25mg)
DMEM (Life Technologies, cat. no. 11965-092)
DMEM/F12 (Life Technologies, cat. no. 11330-057)
DMSO (Sigma, cat. no. D8418)
Doxycycline (Stemgent, cat. no. 04-0016)
FBS (Life Technologies, cat. no. 16000-044)
FuGENE HD Transfection Reagent (Promega, cat. no. E2311)
Gelatin (Type A, Porcine skin) (Sigma, G1890)
Gold Anti-fade reagent with DAPI (Life Technologies, cat. no. P-36931)
GlutaMAX (Life Technologies, cat. no. 35050-061)
Hydrochloric acid (Sigma, H1758)
IWP2 (Tocris, cat. no. 3533-10 mg)
IWP4 (Stemgent, cat. no. 04-0036)
KnockOut Serum Replacement (Life Technologies, cat. no. 10828-028)
L-glutamine (Life Technologies, cat. no. 25030)
RPMI (Life Technologies, cat. no. 11875-119)
mTeSR1 (STEMCELL Technologies, 05857)
Matrigel (BD Biosciences, cat. no. 354277)
MEM non-essential amino acid (Life Technologies, cat. no. 11140)
Non Fat Dry Milk (Bio-rad, cat. no. 170-6404XTU)
One Shot Stbl3 chemically competent E. coli (Life Technologies, cat. no. C7373-03)
Omniscript RT Kit (Qiagen, cat. no. 205111)
Opti-MEM (Life Technologies, cat. no. 51985-034)
PureLink HiPure Plasmid Midiprep Kit (Life Technologies, cat. no. K2100-04)
Puromycin (Invitrogen, A11138-02)
psPAX2 (Addgene, cat. no. 12260)
pMD2.G (Addgene, cat. no. 12259)
Phosphate Buffered Saline (Sigma, cat. no. D8537)
Puromycin (10 mg/ml) (Life Technologies, cat. no. A11138-03)
Qiagen EndoFree Plasmid Maxi Kit (Qiagen, cat. no. 12362)
QuantiTect SYBR Green PCR Kits (Qiagen, cat. no. 204141)
RNeasy Mini Kit (Qiagen, cat. no. 74104)
Sodium Pyruvate (Life Technologies, cat. no. 11360-070)
Triton X-100 (Sigma, cat. no. T8532)
Versene (Life Technologies, cat. no. 15040-066)
Y27632 (Tocris, cat. no. 1254)
EQUIPMENT
15- and 50-ml conical tubes (BD Biosciences, cat. nos. 352095 and 352073)
Synthemax plates (Corning, cat. no. 3978XX1)
6-, 12-, 24- and 96-well plates (Nunc, cat. nos. 140675, 150628, 142475 and 165306)
CL2 Centrifuge (Thermo Scientific, part no. 004260F)
Polypropylene conical tubes (15 ml; BD Biosciences, cat. no. 352097)
Polypropylene conical tubes (50 ml; BD Biosciences, cat. no. 352098)
Sterile biosafety cabinets
Liquid waste disposal system
Flow cytometry FACSCalibur (Becton Dickinson)
Sterilized Pasteur pipettes (Fisher, 13-678-20D)
Humidified tissue culture incubator (37 °C, 5% CO2)
Hemocytometer (Hausser Scientific, cat. no. 02-671-52)
Inverted phase contrast microscope (Nikon, ECLIPSE TS100)
Microcentrifuge tube (1.5 ml, Fisher Scientific, cat. no. 05-408-129)
VWR Scientific 1205 Dual Heated Water Bath Incubator (VWR, cat. no. 14405)
Serological pipettes 5-, 10- and 25-ml (Fisher Scientific, cat. nos. 13-678-11D, 13-678-11E and 13-678-11)
Stericup filtration system (Millipore, cat. no. SCGPU05RE, 500 ml)
Stericup filtration system (Millipore, cat. no. SCGPU02RE, 250 ml)
Steriflip filtration system (Millipore, cat. no. SCGP00525, 50 ml)
0.45 μm Steriflip-HV Filter Unit (Millipore, cat. no. SE1M003M00, 50 ml)
Coverslips (18mm) (Fisher Scientific, 12-545-100 and 12-545-101)
Flow round-bottom tube (5 ml) (BD Biosciences, cat. no. 352052)
Flow round-bottom tube caps (BD Biosciences, cat. no. 352032)
REAGENT SETUP
293TN medium In a sterile environment, mix 50 ml FBS, 5 ml sodium pyruvate, 2.5 ml GlutaMAX, and 450 ml DMEM. Filter the medium with a 500 ml Stericup filtration system. The medium can be stored at 4 °C for up to 2 months.
10% FBS DMEM medium In a sterile environment, mix 50 ml FBS and 450 ml DMEM. Filter the medium with a 500 ml Stericup filtration system. The medium can be stored at 4 °C for up to 2 months.
1% formaldehyde Add 62.5 μl 16% formaldehyde into 1 ml PBS. We do not recommend storing this solution.
4% formaldehyde Add 1 ml 16% formaldehyde into 3 ml PBS. We do not recommend storing this solution.
90% methanol Add 5 ml MilliQ water to 45 ml pure methanol and store at − 20 °C.
0.1% BSA Add 1 g BSA into 1000 ml PBS and filter it using Stericup filtration systems. Store at 4 °C for up to 6 months.
20 mM BrdU Add 16.28 ml DMSO to 100 mg BrdU to make 20 mM BrdU. Aliquot and store at −20 °C for up to 1 year.
CHIR99021 (36 mM) Add 1.49 ml DMSO to 25 mg CHIR99021. Aliquot and store at −20 °C for up to 1 year.
Doxycycline (1 mg/ml) Add 10 ml MilliQ water to 10 mg doxycycline. Aliquot into 100 μl samples and store at −20 °C for up to 1 year.
hESC medium (500 ml) In a sterile environment, mix 392.5 ml of DMEM/F12, 100 ml of KnockOut serum replacement, 5 ml of MEM non-essential amino acids, 2.5 ml of 200 mM L-glutamine, and 3.5 μl of 14.3 M β-mercaptoethanol (final concentration of 0.1 mM). The medium can be stored at 4 °C for up to 2 weeks.
Freezing medium (50 ml) In a sterile environment, mix 30 ml hESC medium, 15 ml FBS, 5 ml DMSO and 50 μl Y27632. The medium can be stored at 4 °C for up to 1 month.
RPMI/B27-insulin (510 ml) In a sterile environment, mix 500 ml of RPMI and 10 ml B27 supplement Minus Insulin. The medium can be stored at 4 °C for up to 1 month.
RPMI/B27-insulin + 12 μM CHIR99021 (24 ml) Add 8 μl of 36 mM CHIR99021 into 24 ml RPMI/B27-insulin. We do not recommend storing this medium.
RPMI/B27 (510 ml) In a sterile environment, mix 500 ml of RPMI and 10 ml of B27 supplement. The medium can be stored at 4 °C for up to 1 month.
RPMI20 (250 ml) In a sterile environment, mix 200 ml of RPMI and 50 ml FBS, then filter through a 250 ml Stericup filtration system. The medium can be stored at 4 °C for up to 1 month.
IWP2 (5 mM) Add 4.28 ml DMSO to 10 mg IWP2. Incubate the mixture at 37 °C for 10 minutes to dissolve the IWP2. Aliquot 100 μl samples into 1.5 ml tubes and store at −20 °C for up to 1 year.
IWP4 (5 mM) Add 0.805 ml DMSO to 2 mg IWP4. Incubate the mixture at 37 °C for 10 minutes to dissolve the IWP4. Aliquot 100 μl samples into 1.5 ml tubes and store at −20 °C for up to 1 year.
mTeSR1 + 1 μg/ml puromycin Add 5 μl of 10 mg/ml puromycin to 50 ml mTeSR1 medium. The medium can be stored at 4 °C for up to 2 weeks.
mTeSR1 + 1 μM CHIR99021 (24 ml) Add 0.67 μl 36 mM CHIR99021 into 24 ml RPMI/B27-insulin. The medium can be stored at 4 °C for up to 2 weeks.
5 mM Y27632 In a sterile environment, add 6.24 ml PBS to 10 mg Y27632. Aliquot 100 μl samples into 1.5 ml tubes and store at −20 °C for up to 1 year.
mTeSR1 + 5 μM Y27632 Add 50 μl of 5 mM Y27632 to 50 ml mTeSR1 (final concentration of Y27632 is 5 μM). Store at 4 °C for up to 2 weeks.
0.1% Gelatin (1000 ml) Add 1.0 g gelatin into 1000 ml MilliQ water and autoclave the solution at 121°C, 15 psi for 30 minutes. The solution can be stored at room temperature for up to 1 year.
FlowBuffer-1 (500 ml) Add 2.5 g BSA into 500 ml PBS and filter using a 500 ml Stericup filtration system. The medium can be stored at 4 °C for up to 6 months.
1% Triton X-100 solution (500 ml) Add 5 ml Triton X-100 into 495 ml PBS and shake the bottle to dissolve the Triton. The medium can be stored at 4 °C for up to 6 months.
FlowBuffer-2 (550 ml) Add 2.5 g BSA and 50 ml 1% Triton X-100 solution into 500 ml PBS and filter using a 500 ml Stericup filtration system. The medium can be stored at 4 °C for up to 6 months.
10% Triton X-100 solution (50 ml) Add 5 ml Triton X-100 into 45 ml PBS and shake the tube to dissolve the Triton. The medium can be stored at 4 °C for up to 6 months.
2N HCl/1% Triton solution (15 mL): Add 2.5 ml 12N HCl and 1.5 ml 10% Triton-X100 solution into 11 ml MilliQ water. We do not recommend storing this solution.
5% non fat dry milk, 0.4 % Triton X-100 Add 0.5 g non fat dry milk and 4 ml 1% Triton X-100 solution into 6 ml PBS. We do not recommend storing this solution.
Activin A (50 μg/ml) Dissolve 50 μg of Activin A in 1 ml of 0.1% (wt/vol) BSA solution. Store at −80 °C for up to 6 months.
BMP4 (10 μg/ml) Dissolve 10 μg of BMP4 in 1 ml of 0.1% (wt/vol) BSA solution. Store at −80 °C for up to 6 months.
bFGF (10 μg/ml) Dissolve 10 μg of bFGF in 1 ml of 0.1% (wt/vol) BSA solution. Store at −80 °C for up to 6 months.
GiAB d0 medium (12 ml) Add 24 μl of 50 μg/ml Activin A, 12 μl of 10 μg/ml bFGF, and 120 μl KnockOut Serum replacement into 12 ml RPMI/B27-insulin. We do not recommend storing this medium.
RPMI/B27-insulin + 5 ng/ml BMP4 (24 ml) Add 12 μl of 10 μg/ml BMP4 into 24 ml of RPMI/B27-insulin. We do not recommend storing this medium.
EQUIPMENT SETUP
Matrigel-coated plates
In a sterile hood, add 23 ml of cold (4 °C) DMEM/F12 to a 50 ml conical tube and keep it cold by placing it on ice. Remove one Matrigel aliquot (2 mg) from the freezer, and add 1 ml of cold DMEM/F12 to it. Gently pipette the Matrigel solution with a P1000 tip to thaw and dissolve the Matrigel. Immediately transfer the Matrigel solution to the 50 ml conical tube that contains 23 ml cold DMEM/F12. Immediately add 1 ml/well Matrigel in DMEM/F12 for 6-well plates, 0.5 ml/well for 12-well plates, 250 μl/well for 24-well plates, or 100 μl/well for 96-well plates. Allow the Matrigel to set for 30 minutes at room temperature before use. The Matrigel-coated plates can be stored at 4 °C for up to 3 weeks. CRITICAL: We recommend dissolving 0.5 mg Matrigel into 6 ml cold DMEM/F12. Use lots of Matrigel qualified by BD Biosciences for hESC/iPSC culture. Some lots of Matrigel do not support hPSC self-renewal. We also recommend the use of Synthemax plates as an alternative matrix for consistent hPSC maintenance.
0.1% gelatin coated coverslips
Autoclave the coverslips at 121°C, 15 psi for 30 minutes and place one sterile coverslip in each well of a 12-well plate. Add 1 ml of 0.1% gelatin per well and incubate at 37 °C overnight. Store the gelatin coated coverslips at 4 °C for up to 2 months.
PROCEDURE
Feeder-independent culture of hPSCs
- 1
Take a Matrigel-coated 6-well plate from 4 °C and place it at room temperature for 15 minutes to warm up the plate.
- 2
Remove a frozen cell vial from liquid N2 and immerse the vial in a 37 °C water bath without submerging the cap. Swirl the vial gently for 2–3 minutes until the contents are completely thawed.
- 3
Spray the vial with 70% ethanol and place it in tissue culture hood. Use a sterile 1 ml pipette to gently transfer the cells into a sterile 15 ml conical tube containing 5 ml of room temperature mTeSR1 medium.
- 4
Centrifuge the cells at 200 × g for 5 minutes. Aspirate and discard the supernatant with a sterilized pasteur pipette. Aspirate the liquid from the wells of the Matrigel-coated plate.
- 5
Resuspend the cell pellet in 6 ml mTeSR1 medium + 5 μM Y27632. Slowly add 2 ml the cell suspension into each well of the Matrigel-coated 6-well plate (1 vial to 3 wells). Put the plate back into the 37°C, 5% CO2 incubator. Move the plate in three quick, short, back-and-forth and side-to-side motions to disperse cells across the surface of the wells.
CRITICAL STEP: Including a ROCK inhibitor is very important for high hPSC recovery after freezing and thawing. We recommend preparing freezing medium as follows: 10% DMSO, 30% FBS, 60% hESC medium by volume, supplemented with 5 μM Y27632. We also recommend use of 5 μM Y27632 in the medium when culturing hPSCs for the first 24 hours after thawing.
- 6
The next day, aspirate the medium in each well and replace with 2 ml fresh room temperature mTeSR1 medium daily.
Passaging hPSCs using Versene
- 7
When the cells are 80–90% confluent (usually about 3 to 5 days after passaging, daily monitoring is necessary), aspirate the old medium then add 1 ml room temperature Versene to each well. At this point, 1 to 1.5 million cells should be present in each well.
- 8
Incubate the plate at 37 °C, 5% CO2 and wait for 4 minutes.
- 9
Aspirate the Versene then hold a 5 ml pipette filled with 3 ml mTeSR1 + 5 μM Y27632 perpendicular to the plate and dispense the medium over the surface of the plate well until all the cells are detached. After the cells are removed from the surface of the well, pool the contents of the well into a sterile conical tube containing 9 ml mTeSR1 + 5 μM Y27632 and gently mix 5–10 times using 5 ml pipette. Seed 2 ml of the cell suspension into each well of a Matrigel-coated or Synthemax 6-well plate (split ratio of 1:6 is performed here).
CRITICAL STEP: The split ratio is variable, though generally between 1:6 and 1:18 is appropriate when using Versene for passaging. A general rule is to observe the last split ratio and adjust the ratio according to the appearance of the hPSCs colonies. If the cells possess an undifferentiated morphology (colony diameter < 500 μm) and colonies have enough space (more than 10 μm) between them to expand, split using the same ratio; if they are overly dense and crowded (colony diameter > 500 μm, spacing between colonies <10 μm), increase the ratio; and if the cells are sparse (colony diameter < 50 μm, spacing between colonies > 500 μm), decrease the ratio.
- 10
Return the plate to the incubator after plating the cells. Move the plate in three quick, short, back-and-forth and side-to-side motions to disperse cells across the surface of the wells.
- 11
Each day aspirate the medium and add 2 ml of fresh room temperature mTeSR1 per well.
Cardiac differentiation with inducible β-catenin shRNA hPSC lines
Inducible β-catenin shRNA lentivirus production
- 12
Expand both of the plasmids (pXL002 and pXL004; pXL002 is for production of inducible β-catenin shRNA and pXL004 is for production of inducible scramble shRNA as negative control) used for lentiviral production in Stbl3 E. coli and extract the plasmids with the Qiagen EndoFree Plasmid Maxi Kit or PureLink HiPure Plasmid Midiprep Kit according to the manufacturer’s recommendations. Resuspend the plasmid in Elution Buffer at a concentration of approximately 1 μg/μl.
- 13
Day 1: Thaw a vial of 293TN cells quickly by incubating in a 37 °C waterbath. Transfer the contents into a 15 ml conical tube containing 9 ml of 293TN medium. Centrifuge the cells at 200 × g for 5 minutes. Discard the supernatant. Resuspend 1.5 million cells/ml in 6 ml 293TN medium and transfer into 3 wells of a 6-well plate (0.5 million cells per well). Incubate the plates at 37 °C, 5% CO2 for 24–48 h or until the cells reach 95–100% confluence.
CRITICAL STEP: It is recommended to initially prepare several frozen aliquots of 293TN cells for subsequent lentivirus production.
- 14
Day 2: Add 4.5 μg pXL002 (or pXL004), 3.0 μg psPAX2, and 1.5 μg pMD2.G to 450 μl Opti-MEM medium and incubate at room temperature for 5 minutes. Add 27 μl of FuGENE HD reagent. Mix carefully by pipetting 15 times with a P200 tip. Incubate for 10 – 15 minutes at room temperature. Add 450 μl of plasmid mixture into 3 ml 293TN medium prewarmed to 37 °C. Aspirate the old medium from the 6-well plate with 293TN cells and add 1 ml of 293TN medium containing Fugene/DNA complex to each well of the 6-well plate. Incubate at 37 °C, 5% CO2.
CRITICAL STEP: Add medium gently along the wall of the 6 well plate to 293TN cell since 293TN cells adhere weakly and directly mechanical disturbance or contact with medium can cause their detachment Addtion of medium along the wall will help the 293TN cells remained attached.
- 15
Day 3: 16 – 18 hours after addition of the plasmid mixture to the 293TN cells, aspirate the medium from each well and replace with 3 ml 293TN medium prewarmed to 37 °C. Incubate the plate at 37°C, 5% CO2 for 24 hours.
- 16
Day 4: Use a 5 ml pipette to collect the virus-containing supernatant, transfer to a 50 ml conical tube, and store at 4 °C. Add 3 ml of 293TN medium prewarmed to 37 °C to each well of the 6 well plate.
- 17
Repeat step 16 on day 5 and day 6. In total, you will obtain about 27 ml virus-containing supernatant. Immediately after collection, chill the virus supernatant on ice and store at 4°C.
- 18
Centrifuge the virus-containing medium at 2000 g at 4°C for 5 minutes to pellet cell debris, then filter the supernatant through a 0.45 μm filter.
- 19
Aliquot the lentiviral supernatant into microcentrifuge tubes at 1 ml/tube and store at −80°C.
Establish inducible β-catenin shRNA hPSC clones
- 20
hPSCs are cultured according to steps 1–11. When the cells are 80–90% confluent (usually about 3 to 5 days after passaging, daily monitoring is necessary), aspirate old mTeSR1 medium and add 1 ml Accutase per well to the 6-well plate. Incubate the plate at 37 °C, 5% CO2 for 10 minutes.
- 21
Use a 5 ml pipette to add 4 ml mTeSR1 to a 15 ml conical tube, then collect 1 ml Accutase with cells from one well of the 6-well plate and transfer to the 15 ml conical tube. Mix the cells with a 5 ml pipette and then collect 10 μl of the cell mixture with a P10 tip, transfer to a hemocytometer, and count the cells.
- 22
Centrifuge the cells at 200 × g for 5 minutes. Aspirate the supernatant and resuspend the cells at density of 0.4 million cells/ml in room temperature mTeSR1 + 5 μM Y27632.
- 23
Plate 1 ml of cells (0.4 million cells) per well in a Matrigel-coated 6-well plate and incubate the plate for 24 hours at 37 °C, 5% CO2. Remove a 1 ml virus aliquot from the −80 °C freezer and place it in a 4 °C refrigerator overnight.
- 24
The next day (Day 1), aspirate the medium from each well. Add 1 ml room temperature mTeSR1 + 5 μM Y27632 + 1 ml virus supernatant (pXL002 or pXL004) per well of 6-well plate.
- 25
Day 2, aspirate the medium and add 2 ml room temperature mTeSR1 per well.
- 26
Day 3 and day 4, add 2 ml room temperature mTeSR1 + 1 μg/ml puromycin to kill the non-transduced cells. Each day, aspirate the medium and replace with mTeSR1 + 1 μg/ml puromycin.
- 27
Day 5, aspirate the medium and then add 1 ml Accutase into the transduced well, and incubate at 37°C, 5% CO2 for 10 minutes. Add 1 ml of mTeSR1 into the transduced well and pool the cells in 15 ml tube. You will have 2 ml cell mixture in total per virus-transduced sample. Count 10 μl of each cell mixture using a hemocytometer.
- 28
Centrifuge the cells at 200 × g for 5 minutes and aspirate the supernatant. Several serial dilutions will be performed to obtain approximately 1 cell per 100 μl. Assuming x million cells in total, first resuspend the cells in 10x ml mTeSR1 to obtain a cell density of 105 cells/ml. Add 100 μl of the mixture into another new 10 ml mTeSR1 to obtain a cell density of 103 cells/ml. Then add 100 μl of the 103 cells/ml mixture into another 10 ml mTeSR1 + 5 μM Y27632 to obtain a cell density of 101 cells/ml (1 cell per 100 μl).
- 29
Add 100 μl of the 101 cells/ml suspension from step 28 into each well of a 96-well plate pre-coated with Matrigel. Under the microscope, check and mark the wells that contain only a single cell. Only the wells that contain exactly 1 cell will be subjected to further analysis. Approximately 50 wells will contain exactly 1 cell by this approach.
- 30
Place the 96-well plate in a 37°C, 5% CO2 incubator for 4 days without changing the medium.
- 31
On day 4, aspirate the medium and add 100 μl mTeSR1 + 5 μM Y27632 + 1 μg/ml puromycin. Repeat this medium change every other day until day 12.
- 32
On day 12, aspirate the medium and add 50 μl Accutase into each well that contains only one colony. Usually 5 to 10 wells per 96-well plate contain only a single colony derived from a single cell.
- 33
Incubate the plate in a 37°C, 5% CO2 incubator for 10 minutes. Transfer the 50 μl Accutase-cell mixture into 1 ml mTeSR1 + 5 μM Y27632 into one well of Matrigel coated 24-well plate with a P200 tip pipette. Incubate the plate at 37°C, 5% CO2 for 2 days without changing the medium.
- 34
On day 14, aspirate the medium and add 500 μl mTeSR1. Repeat this medium change every day until the cells reach 80% confluence.
- 35
Instructions on how to passage and expand these cells can be found in steps 7 – 11.
- 36
In order to identify the best inducible knockdown clones, aspirate the old medium and add 2 ml mTeSR1 + 4 μl of 1 mg/ml doxcycline into the well of 6-well plate daily for 3 days.
- 37
Aspirate the medium, add 1 ml Accutase, and incubate at 37°C, 5% CO2 for 10 minutes. Add 1 ml of mTeSR1 into the well and pool all of the cells in 15 ml tube. Centrifuge the cells at 200 × g for 5 minutes
- 38
Aspirate the supernatant and extract the RNA using the Qiagen RNeasy Mini Kit according to the manufacturer’s instructions. Then generate cDNA from the mRNA with the Omniscript RT Kit according to the manufacturer’s instructions. Perform quantitative PCR with the QuantiTect SYBR Green PCR Kit according to the manufacturer’s recommendations.
Primers for quantitative RT-PCR GAPDH F: 5′ GTGGACCTGACCTGCCGTCT 3′
R: 5′ GGAGGAGTGGGTGTCGCTGT 3′Size 152 bp CTNNB1 F: 5′ CCCACTAATGTCCAGCGTTT 3′
R: 5′ AACGCATGATAGCGTGTCTG 3′Size 217 bp The knockdown efficiency of β-catenin can be measured by quantitative PCR comparing expression in the inducible knockdown cells to non-transduced cells and inducible scramble shRNA transduced cells. The best knockdown efficiency clone will be expanded for further cardiac differentiation. Clones that exhibit at least 80% knockdown efficiency of β-catenin are sufficient for robust cardiac differentiation using the protocol described below.
Cardiac differentiation with Gsk3 inhibitor and inducible shRNA of β-catenin (GiSB protocol)
- 39
Culture the β-catenin shRNA clone cells produced in steps 12–38 on Matrigel-coated or Synthemax 6-well plates in mTeSR1 medium to 80–90% confluence using instructions provided in steps 7–11. Aspirate the medium and add 1 ml of room temperature Accutase to each well. Put the plate in a 37°C, 5% CO2 incubatorand wait for 8 minutes.
- 40
Add 0.5 ml of mTeSR1 into each well and pool all of the cells in a 15 ml conical. Count the total cell number with a hemocytometer. Centrifuge the cells at 200 × g for 5 minutes.
- 41
Aspirate the supernatant, resuspend the cells in mTeSR1 + 5 μM Y27632 at a cell density of 2 million cells/ml, and plate 0.5, 0.75, 1.0, 1.25, or 1.5 million cells/well in each well of a 12-well plate. Add mTeSR1 + 5 μM Y27632 medium to each well to make a final volume of 1 ml in each well of the 12-well plate. This time point corresponds to day −4.
CRITICAL STEP: The starting seeding cell density is very critical for efficient cardiac differentiation. The initial plating density and/or the time of expansion prior to initiation of differentiation may require optimization for different cell lines or expansion conditions. We recommend plating at a cell density of 0.5 million cells per well of 12-well plate and increasing this stepwise to 1.5 million for your specific hPSC lines in your first experiment and expanding the cells for 4 days prior to initiation of differentiation. Once you have identified the optimal seeding density for your specific hPSC lines, you can use this seeding density for subsequent differentiation experiments.
- 42
Day −3, day −2, and day −1, aspirate the medium and replace with 2 ml room temperature mTeSR1 per well of the 12-well plate.
- 43
Day 0, prepare 12 μM CHIR99021 RPMI/B27-insulin. You will need 2 ml RPMI/B27-insulin for each well of the 12 well plate, so 24 ml to differentiate cells in all 12 wells. Add 8 μl of 36 mM CHIR99021 into 24 ml RPMI/B27-insulin medium to make 12 μM CHIR99021 RPMI/B27-insulin medium. Aspirate the old medium and then add 2 ml RPMI/B27-insulin with CHIR99021 to each well of the 12-well plate and record the time.
CRITICAL STEP: Recording the time when you added RPMI/B27-insulin with CHIR99021 is important since exactly 24 hours later you need to change the medium. Though we identified 12 μM CHIR99021 as the optimal concentration for the six lines that we tested, other lines may respond to CHIR99021 treatment differently. Thus, optimization of CHIR99021 concentration may be required. We recommend testing 6–14 μM CHIR99021.
- 45
Day 1, aspirate the medium from each well of the 12-well plate and replace with 2 ml room temperature RPMI/B27-insulin. Put the plate back into the 37°C, 5% CO2 incubator.
- 46
Day 1.5 (exactly 36 hours post addition of CHIR99021), add 4 μl of 1 mg/ml doxcycline per well of 12-well plate with a P10 tip and pipette to mix. Put the plate back into the 37°C, 5% CO2 incubator.
CRITICAL STEP: Cardiomyocyte differentiation is very sensitive to the timing of Wnt pathway modulation. During small molecule directed differentiation, optimization of timing of Wnt pathway regulators can generate up to 98% cTnT+ cardiomyocytes. In order to achieve a high purity of cardiomyocytes by β-catenin knockdown, dox addition must be initiated within 1–2 hours of the 36 hour post-differentiation optimum.
CRITICAL STEP: Do not change the medium between day 1 and day 5.
- 47
Day 5, Aspirate the medium from each well of the 12-well plate and add 2 ml/well room temperature RPMI/B27-insulin. Put the plate back into the 37°C, 5% CO2 incubator.
- 48
Day 7 and every 3 days following, aspirate the medium from each well and add 2 ml/well room temperature RPMI/B27 medium. Put the plate back into the 37°C, 5% CO2 incubator.
Robust spontaneous contraction should occur by day 12. Two typical spontaneously contracting results from 19-9-11 inducible β-catenin shRNA line are shown in Movie S1 (day 15 cardiomyocytes) and Movie S2 (day 180 cardiomyocytes).
Cardiac differentiation with Gsk3 inhibitor and Wnt inhibitor (GiWi protocol)
Because we use small molecule inhibitors of Wnt signaling instead of β-catenin shRNA in the GiWi protocol, transgenic modification of hPSC to inducibly express β-catenin shRNA is not required for this protocol. GiWi protocol is applicable to any existing hPSC line. A summary of this protocol is shown in Fig. 3.
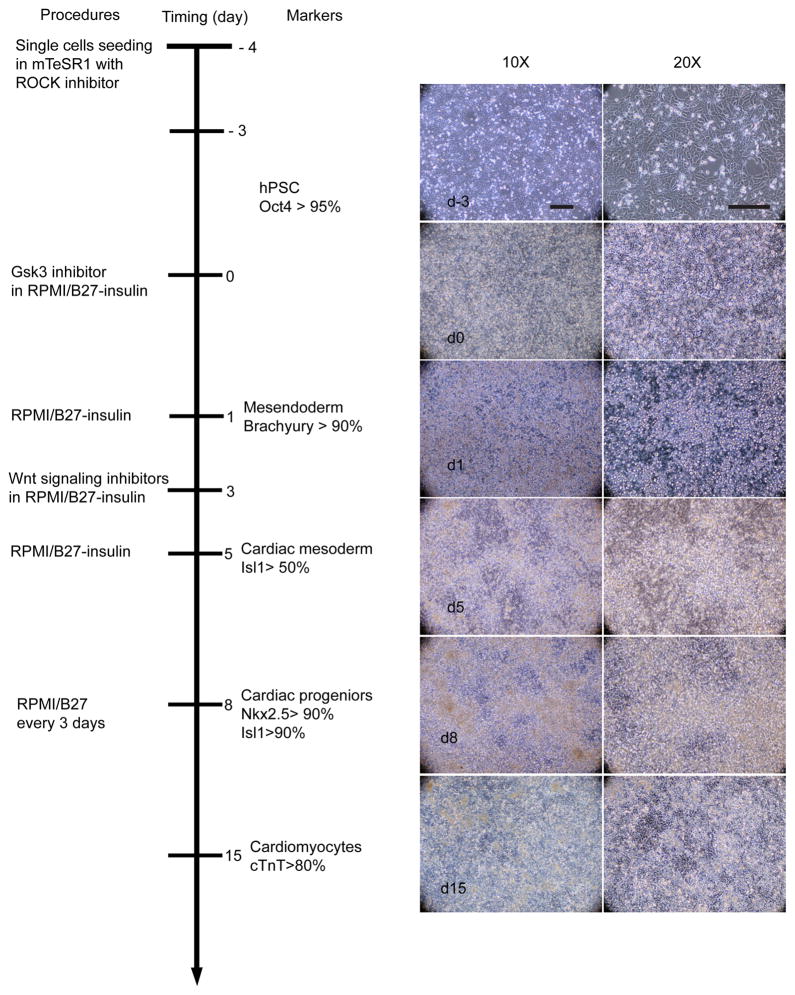
Schematic of protocol to differentiate of cardiomyocytes from hPSCs with small molecule modulators of canonical Wnt signaling. Bright field images of the typical morphology of day −3, day 0, day 1, day 5, day 8, day 15 cells from 19-9-11 are shown at 10X and 20X magnifications. Scale bar = 100 μm.
- 49
Culture the hPSCs on Matrigel-coated or Synthemax 6-well plates in mTeSR1 medium to 80–90% confluence using instructions provided in steps 7–11. Aspirate the medium and add 1 ml of room temperature Accutase to each well. Put the plate in a 37°C, 5% CO2 incubatorand wait for 8 minutes.
- 50
Add 0.5 ml of mTeSR1 into each well of the 12-well plate and pool all of the cells into a 15 ml conical. Count the total cell number with a hemocytometer. Centrifuge the cells at 200 × g for 5 minutes.
- 51
Aspirate the supernatant, resuspend the cells in mTeSR1 + 5 μM Y27632 at a cell density of 2 million cells/ml, and plate 0.5, 0.75, 1.0, 1.25, or 1.5 million cells/well in each well of a 12-well plate. Add mTeSR1 + 5 μM Y27632 medium to each well to make a final volume of 1 ml in each well of the 12-well plate. This time point corresponds to day −4.
CRITICAL STEP: The starting seeding cell density is very critical for efficient cardiac differentiation. The initial plating density and/or the time of expansion prior to initiation of differentiation may require optimization for different cell lines or expansion conditions. We recommend plating at a cell density of 0.5 million cells per well of 12-well plate and increasing this stepwise to 1.5 million for your specific hPSC lines in your first experiment and expanding the cells for 4 days prior to initiation of differentiation. Once you have identified the optimal seeding density for your specific hPSC lines, you can use this seeding density for subsequent differentiation experiments.
- 52
Day −3, day −2, and day −1, aspirate the medium and replace with 2 ml room temperature mTeSR1 per well of the 12-well plate.
- 53
Day 0, prepare 12 μM CHIR99021 RPMI/B27-insulin. You will need 2 ml RPMI/B27-insulin for each well of the 12 well plate, so 24 ml to differentiate cells in all 12 wells. Add 8 μl of 36 mM CHIR99021 into 24 ml RPMI/B27-insulin medium to make 12 μM CHIR99021 RPMI/B27-insulin medium. Aspirate the old medium and then add 2 ml RPMI/B27-insulin with CHIR99021 to each well of the 12-well plate and record the time.
CRITICAL STEP: Recording the time when you finished adding RPMI/B27-insulin with CHIR99021 is important since exactly 24 hours later you need to change the medium. Though we identified 12 μM CHIR99021 as the optimal concentration for the six lines that we tested, other lines may respond to CHIR99021 treatment differently. Thus, optimization of CHIR99021 concentration may be required. We recommend testing 6–14 μM CHIR99021.
- 54
Day 1, aspirate the medium from each well of the 12-well plate and replace with 2 ml room temperature RPMI/B27-insulin. Put the plate back into the 37°C, 5% CO2 incubator.
- 55
Day 3 (72 hours post addition of CHIR99021), prepare combined medium: use a 5 ml pipette to collect 1 ml medium from the 12-well plate and mix it with 1 ml of fresh RPMI/B27-insulin medium in a 15 ml conical tube. This 2 ml medium is called combined medium. Add 2 μl of 5 mM IWP2 (final concentration is 5 μM) into the 2 ml combined medium. Prior to aspirating, gently rock the plate back and forth to get cell debris into suspension, ensuring that the cell debris will be removed after aspiration. Aspirate the remaining 1 ml medium from each well of the 12-well plate, then add 2 ml/well of the combined medium containing IWP2 to each well.
- 56
Day 5, Aspirate the medium from each well of the 12-well plate and add 2 ml/well room temperature RPMI/B27-insulin. Put the plate back into the 37°C, 5% CO2 incubator.
- 57
Day 7 and every 3 days following, Aspirate the medium from each well of the 12-well plate and add 2 ml/well room temperature RPMI/B27 medium. Put the plate back into the 37°C, 5% CO2 incubator. Robust spontaneous contraction should occur by day 12. The cells can be maintained with this spontaneously beating phenotype for more than six months.
Greater than 80% cardiomyocytes were obtained in the six hESC and iPSC lines that we tested using GiSB or GiWi protocols (Table 1).
Table 1
Percent of cTnT+ cardiomyocytes present at day 15 post differentiation via small molecule directed differentiation methods
| Cell line | Gsk3 inhibitor + shRNA | Gsk3 inhibitor + IWPs |
|---|---|---|
| H9 | 85.0 ± 2.9% | 82.7 ± 2.3% |
| H13 | NA | 85.6 ± 1.8% |
| H14 | NA | 85.57 ± 4.17% |
| 19-9-11 | 97.5 ± 0.5% | 95.2 ± 1.1% |
| 6-9-9 | 86.3 ± 2.6% | 89.5 ± 2.1% |
| IMR90C4 | 83.8 ± 3.1% | 91.0 ± 3.5% |
NA = not available
Data present as mean ± SD (standard derivation) of three independent experiments
Scientists may be interested in comparing the small molecule-derived cardiomyocytes with cardiomyocytes differentiated from hPSCs using growth factors. A brief outline for generating cardiomyocytes via our activin/BMP method is provided in Box 1.
Characterization of hPSC derived cardiomyocytes
Sarcomere immunostaining analysis of hPSC derived cardiomyocytes
- 58
Day 20 post-addition of RPMI/B27-insulin with CHIR99021, wash the differentiated cells with 1 ml PBS per well in a 12-well plate. Aspirate the PBS, add 1 ml 0.25% Trypsin-EDTA per well, and incubate in a 37°C, 5% CO2 incubator for 5 minutes.
- 59
Pipette 5–10 times with a P1000 tip to singularize the cells and then transfer the 1 ml cell mixture into a 15 ml conical containing 2 ml RPMI20 medium.
- 60
Count the cells with a hemocytometer, centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatant.
- 61
Resuspend the cell pellet in RPMI20 + 5 μM Y27632 at a concentration of 100,000 cells/ml. Plate 1 ml of the resuspended cell solution in each well of a 12-well plate containing a 0.1% gelatin-coated coverslip. Incubate the plate at 37°C, 5% CO2 for 2 days without medium change to allow cell attachment.
- 62
After two days, aspirate the medium and add 1 ml of PBS per well to wash the cells. Aspirate the 1 ml PBS.
- 63
Add 0.5 ml of 4% formaldehyde per well and incubate for 15 minutes at room temperature to fix the cells. Aspirate the formaldehyde solution and then add 1 ml of PBS per well and aspirate to rinse the cells.
- 64
Add 400 μl 5% non-fat dry milk, 0.4 % Triton X-100 in PBS per well and then add primary antibodies into individual wells according to Table 2. Incubate at room temperature for 1 hour. Antibodies include but are not restricted to cardiac troponin T, troponin I, MLC2a, MLC2v, sarcomere myosin (MF20), cardiac transcription factors Nkx2.5, Isl1, Tbx5.
Table 2
Antibodies used for characterization of hPSCs derived cardiomyocytes
Antibody Isotype/Source/cat. no./clone Concentration Smooth Muscle Actin mouse IgG2a/Lab Vision/ms-113-p/1A4 1:100 Cardiac Troponin T mouse IgG1/Lab Vision/ms-295-p1/13-11 1:200 Cardiac Troponin I rabbit IgG/Santa Cruz/sc-15368/H-170 1:100 MF20 mouse IgG2b/DSHB/MF20 1:20 MLC2v rabbit polyclonal/ProteinTech Group/PTG10906-1-AP 1:200 MLC2a mouse IgG2b/Synaptic systems, 311011, Clone: 56F5 1:200 α-actinin mouse IgG1/Sigma/A7811/Clone: EA-53 1:500 Brachyury goat polyclonal IgG/R&D, AF2085 1:100 ISL1 mouse IgG2b/DSHB/39.4D5-s 1:20 NKX2-5 rabbit IgG/Santa Cruz/sc-14033/H-114 1:100 TBX5 rabbit IgG/Sigma/HPA008786/R02421 1:200 Ki67 mouse IgG1/BD Bioscience/550609 1:100 BrdU mouse IgG1/Invitrogen/A21300/PRB-1 1:100 All secondary antibodies are from Life technologies Alexa 488 Goat anti Ms IgG1/A-21121 1:1000 Alexa 488 Goat anti Rb IgG/A-11008 1:1000 Alexa 594 Goat anti Ms IgG2a/A-21135 1:1000 Alexa 594 Goat anti Ms IgG2b/A-21145 1:1000 Alexa 594 Goat anti Rb IgG/A-11012 1:1000 Alexa 647 Goat anti Ms IgG2a/A-21241 1:1000 Alexa 647 Goat anti Ms IgG2b/A-21242 1:1000 Alexa 647 Goat anti Rb IgG/A-21244 1:1000 - 65
Add 1 ml of PBS to each well and then aspirate the PBS. Repeat this wash three times.
- 66
Dilute the secondary antibodies specific to the primary IgG subtype at 1:1000 in 5% milk, 0.4% Triton X-100. Add 400 μl of secondary antibody solution to each well and then incubate for 20 minutes at room temperature in the dark.
- 67
Add 1 ml of PBS to each well and then aspirate the PBS. Repeat this wash three times.
- 68
Seal the coverslips with Gold Anti-fade reagent with DAPI to glass slides. Examine the slides with an epifluorescence microscope. Typical α-actinin and MLC2a patterns of iPSC-derived cardiomyocytes are shown in Fig. 4.
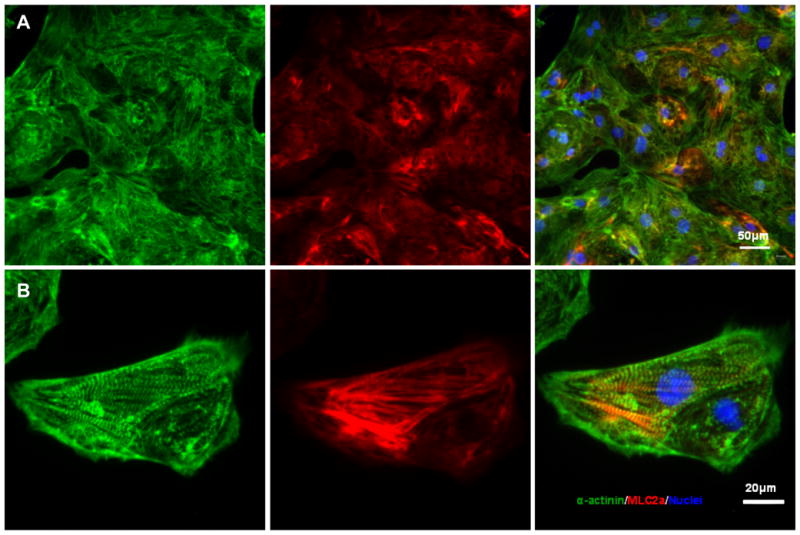
Structural characterization of cardiomyocytes generated from hPSCs via small molecule modulation of Wnt signaling (a–b) Cardiomyocytes were generated from 19-9-11 iPSCs using the protocol described in Fig. 3, with 12 μM CH treatment at day 0 and 5 μM IWP2 treatment at day 3. At day 20, cells were individualized and replated on 0.1% gelatin coated coverslips. Immunostaining for α-actinin (green) and MLC2a (red) shows sarcomere organization. Nuclei were stained with DAPI (a) Scale bar = 50 μm. (b) Scale bar = 20 μm.
Flow cytometry analysis of hPSC derived cardiomyocytes
We recommend flow cytometry for quantitative analysis of the purity of hPSC-derived cardiomyocytes. Antibody combinations of cTnT/SMA, cTnT/MLC2a, and MF20/Ki67 are recommended for double staining.
- 69
Day 20 post-addition of RPMI/B27-insulin with CHIR99021, wash the differentiated cells with 1 ml PBS per well in a 12-well plate. Aspirate the PBS, add 1 ml 0.25% Trypsin-EDTA per well, and incubate in a 37°C, 5% CO2 incubator for 5 minutes.
- 70
Pipette 5–10 times with a P1000 tip to singularize the cells and then transfer the 1 ml cell mixture into a 15 ml conical containing 2 ml RPMI20 medium.
- 71
Count the cells with a hemocytometer, centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatant.
- 72
Add 1 ml of 1% formaldehyde to resuspend the cell pellet and then incubate at room temperature for 20 minutes. Next, centrifuge the cells at 200 × g for 5 minutes, aspirate the supernatant, and then resuspend the fixed cells in 1 ml of 90% cold methanol per tube. Incubate at 4 °C for 15 minutes. Calculate the cell density based on the cell count obtained in step 71.
- 73
Add 0.5 million cells into a 15 ml tube containing 2 ml FlowBuffer-1, centrifuge the cells at 200 × g for 5 minutes, and then aspirate the supernatant. Repeat this wash two times to remove the methanol.
- 74
Resuspend the cell pellet in 100 μl of FlowBuffer-2 with the appropriate dilution of primary antibody. Antibody combinations of cTnT/SMA, cTnT/MLC2a, and MF20/Ki67 are recommended for double staining. Incubate for 1 hour at room temperature or at 4 °C overnight.
- 75
Wash the cells with 2 ml of FlowBuffer-2 and resuspend the cell pellet in 100 μl of FlowBuffer-2 containing 1:1000 dilution of secondary antibody. Incubate for 30 minutes at room temperature in the dark.
- 76
Wash the cells with 2 ml FlowBuffer-2 twice, resuspend the cell pellet in 300 μl FlowBuffer-1, and transfer into flow round-bottom tubes. Place the flow tubes on ice and perform the flow cytometric analysis with a FACSCaliber. Fig. 5 provides representative results of cTnT/SMA, cTnT/MLC2a, and MF20/Ki67 double staining of day 20 human iPSC 19-9-11 derived-cardiomyocytes differentiated via GiWi protocol.
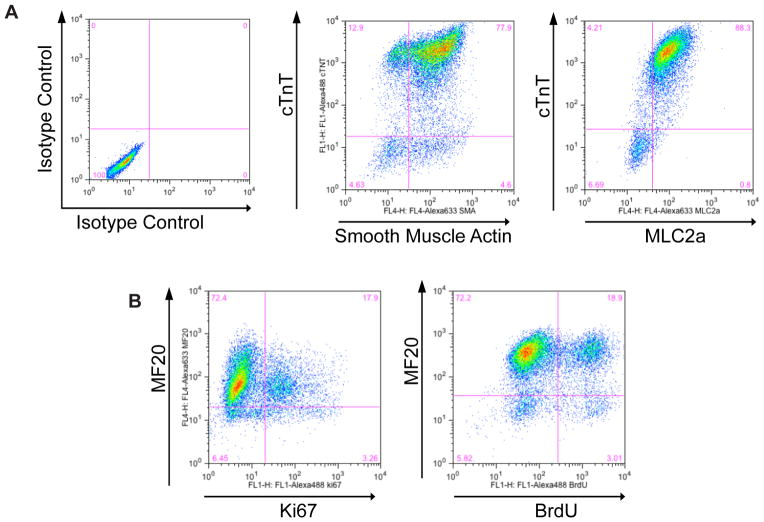
Quantitative analysis of cardiomyocytes differentiated from hPSCs via small molecule modulation of Wnt signaling (a–b) Cardiomyocytes were generated from 19-9-11 iPSCs using the protocol described in Fig. 3, with 12 μM CH treatment at day 0 and 5 μM IWP2 treatment at day 3. At day 20, cells were analyzed for (a) cTnT/SMA and cTnT/MLC2a expression, and (b) MF20/Ki67 and MF20/BrdU incorporation by flow cytometry.
BrdU and MF20 analysis
The protocol for analysis of BrdU and MF20 is different from other combinations of antibodies.
- 77
Day 20 post-addition of RPMI/B27-insulin with CHIR99021 using the GiWi protocol, add 2 μl of 20 mM BrdU to one well of the 12-well plate and incubate at 37 °C 5% CO2 for 17 hours22.
- 78
Singularize the cells with 0.25% Trypsin-EDTA and inactivate the trypsin with 10% FBS in DMEM. (Steps 69 – 71)
- 79
Resuspend the cells in 1 ml of −20 °C 90% methanol and incubate for 15 minutes at 4 °C.
CRITICAL STEP: Do NOT use 1% formaldehyde to fix the cells. Directly resuspend the cell pellet in 1 ml of −20 °C 90% methanol.
- 80
Add 2 ml FlowBuffer-1 to each cell pellet, centrifuge the cells at 200 × g for 5 minutes, and then aspirate the supernatant. Repeat this step two times to remove the methanol.
- 81
Resuspend the cells in 1 ml of freshly prepared 2N HCl/1% Triton solution. Incubate at room temperature for 20 minutes.
- 82
Centrifuge at 200 × g for 5 minutes, aspirate the supernatant, and then resuspend the cell pellet in 1 ml of 0.1 M sodium tetraborate. Centrifuge at 200 × g for 5 minutes and then aspirate the supernatant.
- 83
Add 2 ml of FlowBuffer-1 per tube and centrifuge at 200 × g for 5 minutes. Aspirate the supernatant. Repeat this step one time to remove the sodium tetraborate.
- 84
Follow steps 74 – 76 using MF20 and BrdU antibodies. A typical result from BrdU and MF20 flow cytometry analysis is shown in Fig. 5.
TIMING
Steps 1–5, thawing hPSCs: 30 minutes
Step 6, daily maintenance of hPSCs: 4 days
Steps 7–10, passaging hPSCs: 10 minutes
Step 11, daily maintenance of hPSCs: 4 days
Steps 12–19, production of inducible β-catenin shRNA virus: 8 days
Steps 20–39, generation of inducible β-catenin shRNA hPSC lines: 21 days
Steps 40–48, cardiac differentiation with Gsk3 inhibitor and inducible β-catenin shRNA: 14 days
Steps 49–57, cardiac differentiation with Gsk3 inhibitor and Wnt signaling inhibitor: 14 days
Steps 58–68, immunostaining analysis of hPSC derived cardiomyocytes: 3 hours
Steps 69–84, flow cytometric analysis of hPSC derived cardiomyocytes: 2 days
Box 1, directed differentiation with Gsk3 inhibitor, Activin A and BMP4 (GiAB protocol): 14 days
TROUBLESHOOTING
Troubleshooting advice can be found in Table 3.
Table 3
Troubleshooting
| Step | Problem | Possible reasons | Solution |
|---|---|---|---|
| 6 | Poor attachment of hPSCs on Matrigel-coated plates | No ROCK inhibitor included in step 5 | Include a ROCK inhibitor, (e.g. Y27632) in thawing medium |
| 14 | Detachment of 293TN cells | Cold 293TN medium is used in step 13 or cells are mechanically detached during handling or medium changes | Prewarm the 293TN medium to 37°C before use; perform transfer of 293TN cell plates and medium changes very gently |
| 45, 54 | Cell death or detachment of hPSCs after exposure to CHIR99021 for 24 hours | Initial cell seeding density is not optimal or too high of a CHIR99021 concentration is used, or CHIR99021 application time is too long | Optimize initial cell seeding density in Steps 41, 51; optimize CHIR99021 (6–14 μM) for your specific lines; reduce CHIR99021 application time |
| 48 | No spontanously contracting cells at day 12; low % cTnT+ cells at day 15 | Initial cell seeding density is not optimal or dox addition dose and/or time is not optimal; efficient brachyury or Nkx2.5 expression is not induced | Optimize initial cell seeding density in Steps 41; add 2 μg/ml dox exactly at 36 hours post differentiation |
| 57 | No spontanously contracting cells at day 12; low % cTnT+ cells at day 15 | Initial cell seeding density is not optimal or IWP2 addition dose and/or time is not optimal; efficient brachyury or Nkx2.5 expression is not induced | Optimize initial cell seeding density in Steps 41; add 5 μM IWP2 on day 3 post differentiation |
| Box 1-8 | No spontanously contracting cells at day 12; low % cTnT+ cells at day 15 | Initial cell seeding density is not optimal or day 1 BMP4 concentration is not optimal; efficient brachyury or Nkx2.5 expression is not induced | Optimize initial cell seeding density; optimize BMP4 (0–10 ng/ml) for your specific lines |
ANTICIPATED RESULTS
This protocol presents a rapid and efficient (80%–98% cTnT+ cells after two weeks) method for the generation of functional cardiomyocytes from multiple hPSC lines. Before starting the differentiation protocol, well-maintained hPSCs should have a high ratio of nucleus to cytoplasm, prominent nucleoli morphology (Fig. 1A) and be uniformly positive for pluripotency markers, including Oct4, Nanog, TRA-1-80 and SSEA4 (Fig. 1B–C). 24 hours after initiation of differentiation with CHIR99021, at least 90% of the total differentiated cells should express brachyury, the mesendoderm marker (Fig. 2A). Mesendoderm differentiation below 90% could be caused by poor quality of the starting hPSCs. After 5–6 days of differentiation, cells will express the cardiac progenitor marker protein Isl1 (Fig. 2B). The first beating cluster of cells can be observed between day 8 to day 10, depending on the individual cell line used. Robust spontaneous contraction occurs by day 12 in all hPSCs that we tested. Cardiac sarcomere proteins, such as α-actinin, MLC2a, cTnT, will be expressed in more than 80% of the differentiated cells (Fig. 3–5) by day 15. About 20% of the day 20 cardiomyocytes should show proliferative capacity via Ki67 or BrdU incorporation analysis (Fig. 5B). We typically generate approximately 3–5 million cardiomyocytes (80%–98% cTnT+) per well of a 12-well plate (surface area = 3.8 cm2) resulting a total density of day 15 cardiomyocytes equal to 0.8–1.3 million cardiomyocytes/cm2.
Acknowledgments
This study was supported by NIH grants R01 EB007534 and U01 HL099773 and NSF grant EFRI 0735903.
Footnotes
AUTHOR CONTRIBUTIONS
X.L. designed and performed experiments, analyzed data and wrote the paper; J.Z., S.M.A., K.Z., L.H., X.B. and C.H. contributed to the development of this protocol. T.J.K. and S.P.P. supervised the project, wrote and approved the final paper.
COMPETING FINANCIAL INTERESTS
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing. All the other authors declare no competing financial interests.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nprot.2012.150
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3612968?pdf=render
Citations & impact
Impact metrics
Article citations
TMEM182 inhibits myocardial differentiation of human iPS cells by maintaining the activated state of Wnt/β-catenin signaling through an increase in ILK expression.
FASEB Bioadv, 6(11):565-579, 14 Oct 2024
Cited by: 0 articles | PMID: 39512841 | PMCID: PMC11539028
Hypertrophic cardiomyopathy-associated mutations drive stromal activation via EGFR-mediated paracrine signaling.
Sci Adv, 10(42):eadi6927, 16 Oct 2024
Cited by: 0 articles | PMID: 39413182 | PMCID: PMC11482324
A novel 3D cardiac microtissue model for investigation of cardiovascular complications in rheumatoid arthritis.
Stem Cell Res Ther, 15(1):382, 29 Oct 2024
Cited by: 0 articles | PMID: 39468575 | PMCID: PMC11520781
Spaceflight-induced contractile and mitochondrial dysfunction in an automated heart-on-a-chip platform.
Proc Natl Acad Sci U S A, 121(40):e2404644121, 23 Sep 2024
Cited by: 1 article | PMID: 39312653
Maturation of pluripotent stem cell-derived cardiomyocytes: limitations and challenges from metabolic aspects.
Stem Cell Res Ther, 15(1):354, 08 Oct 2024
Cited by: 0 articles | PMID: 39380099 | PMCID: PMC11462682
Review Free full text in Europe PMC
Go to all (935) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
HPA - The Human Protein Atlas
- (1 citation) HPA - HPA008786
Nucleotide Sequences (3)
- (1 citation) ENA - A21300
- (1 citation) ENA - R02421
- (1 citation) ENA - PTG10906
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling.
Proc Natl Acad Sci U S A, 109(27):E1848-57, 29 May 2012
Cited by: 1005 articles | PMID: 22645348 | PMCID: PMC3390875
Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions.
Nat Protoc, 12(9):1890-1900, 17 Aug 2017
Cited by: 32 articles | PMID: 28817124 | PMCID: PMC5630264
A Universal and Robust Integrated Platform for the Scalable Production of Human Cardiomyocytes From Pluripotent Stem Cells.
Stem Cells Transl Med, 4(12):1482-1494, 28 Oct 2015
Cited by: 67 articles | PMID: 26511653 | PMCID: PMC4675501
Human cardiomyocyte generation from pluripotent stem cells: A state-of-art.
Life Sci, 145:98-113, 10 Dec 2015
Cited by: 45 articles | PMID: 26682938
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA014520
NHLBI NIH HHS (1)
Grant ID: U01 HL099773
NIBIB NIH HHS (1)
Grant ID: R01 EB007534





