Abstract
Free full text

Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue.
Abstract
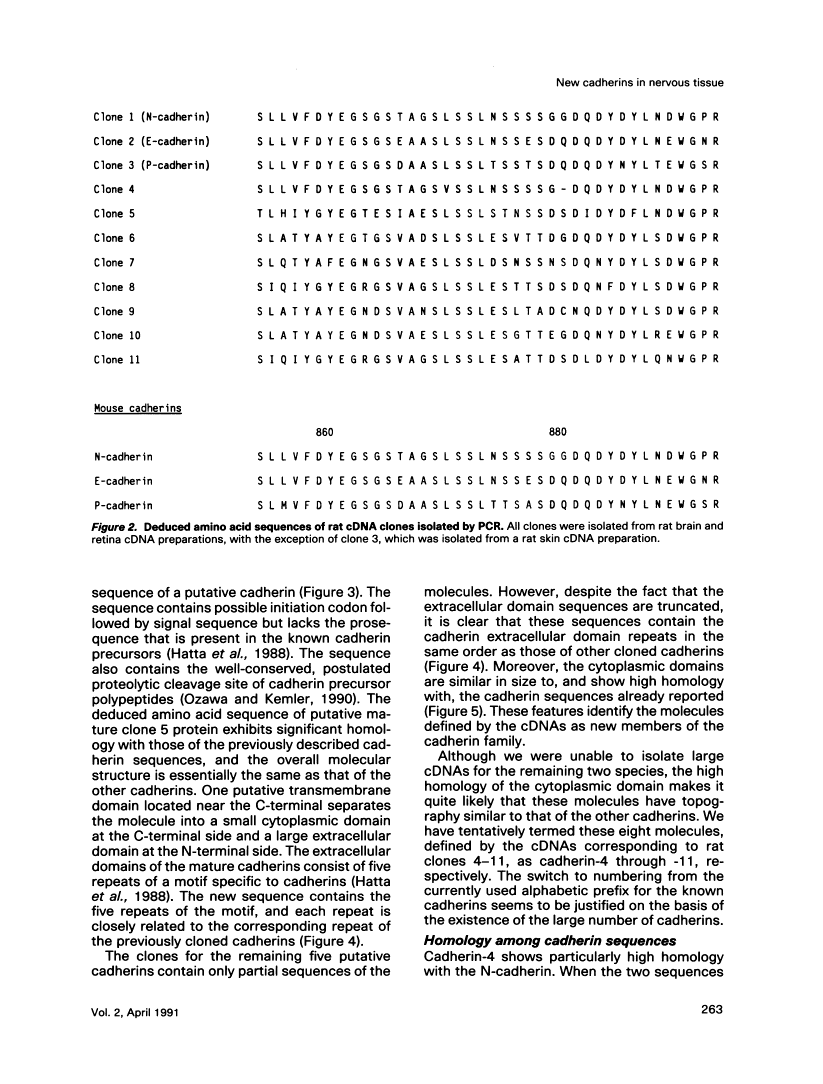
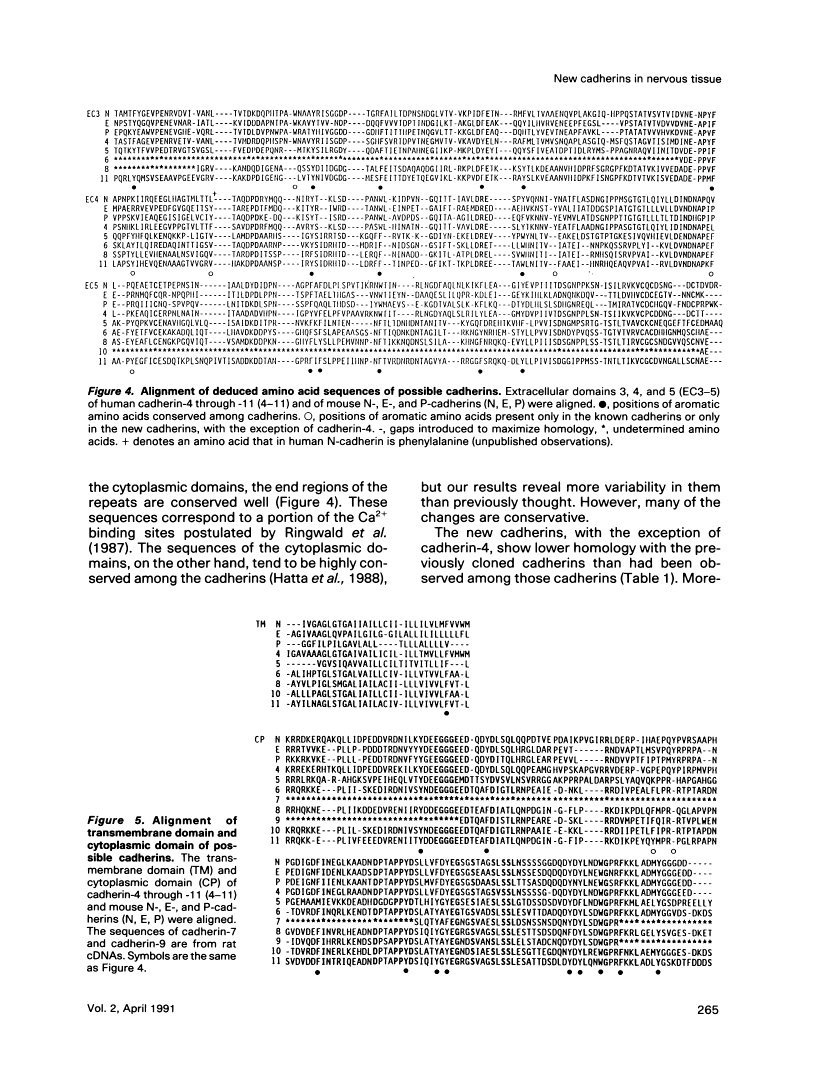
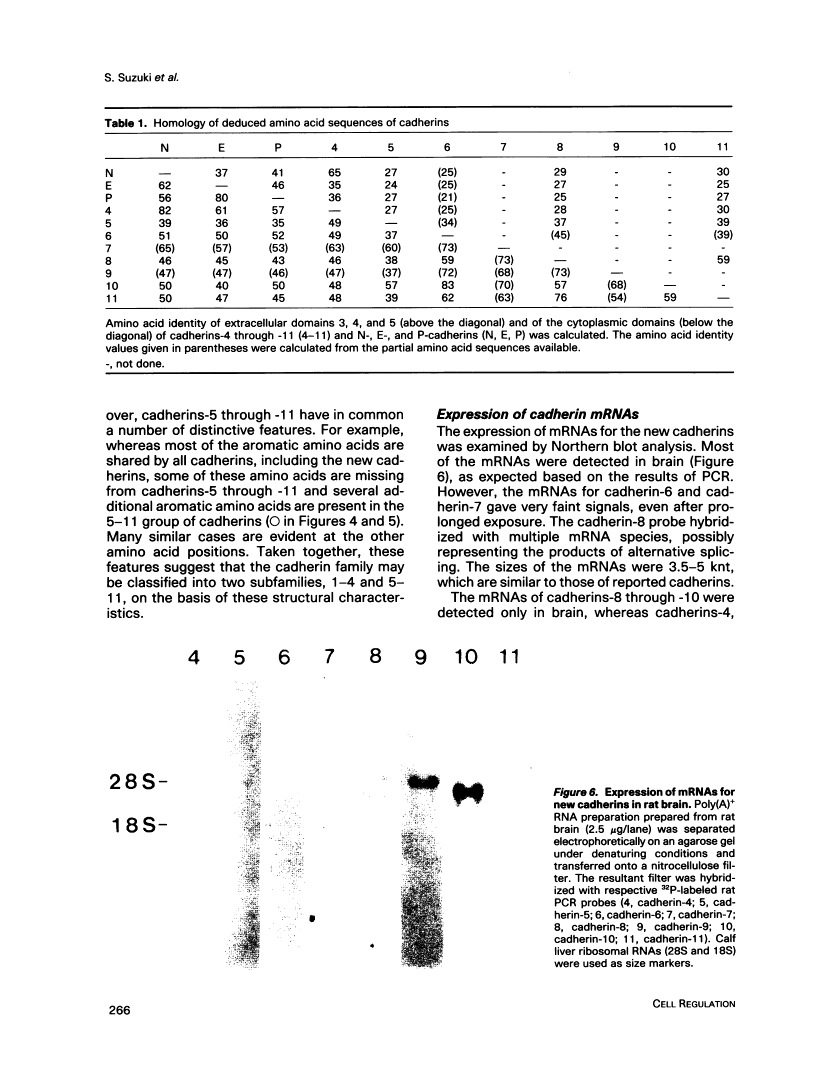
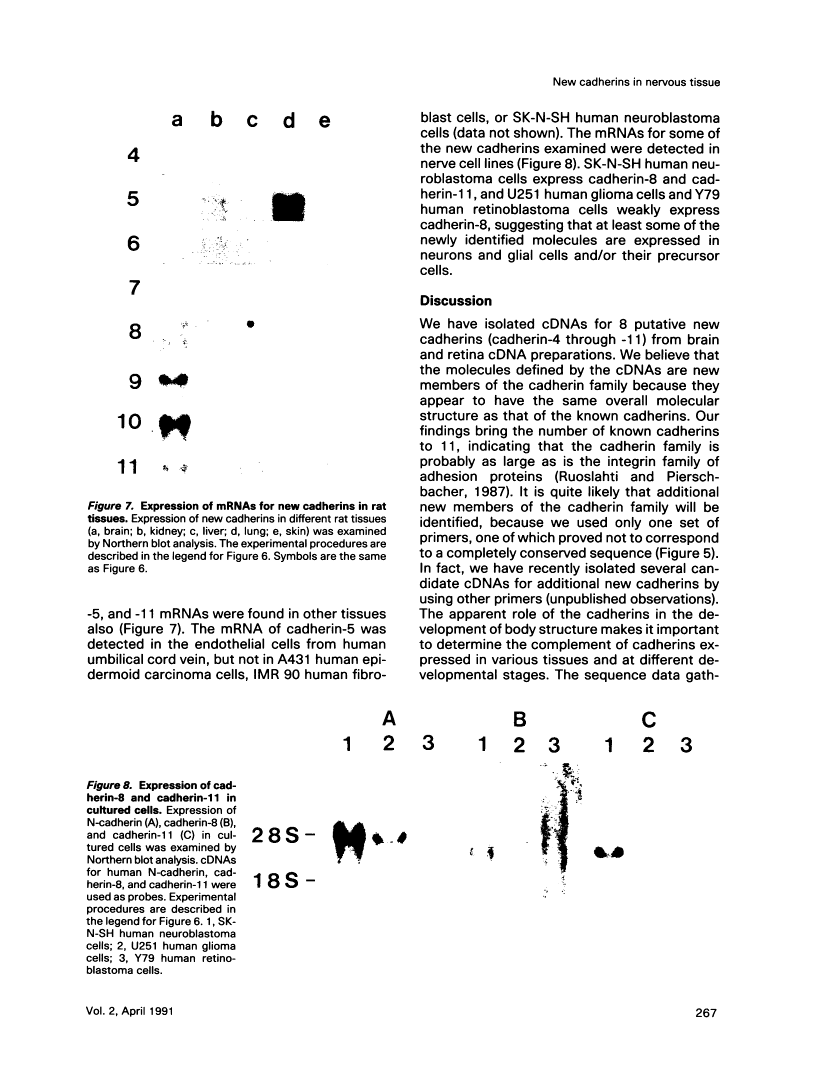
To examine the diversity of the cadherin family, we isolated cDNAs from brain and retina cDNA preparations with the aid of polymerase chain reaction. The products obtained included cDNAs for two of three known cadherins as well as eight distinct cDNAs, of which deduced amino acid sequences show significant similarity with the known cadherin sequences. Larger cDNA clones were isolated from human cDNA libraries for six of the eight new molecules. The deduced amino acid sequences show that the overall structure of these molecules is very similar to that of the known cadherins, indicating that these molecules are new members of the cadherin family. We have tentatively designated these cadherins as cadherin-4 through -11. The new molecules, with the exception of cadherin-4, exhibit features that distinguish them as a group from previously cloned cadherins; they may belong to a new subfamily of cadherins. Northern blot analysis showed that most of these cadherins are expressed mainly in brain, although some are expressed in other tissues as well. These findings show that the cadherin family of adhesion molecules is much larger than previously thought, and suggest that the new cadherins may play an important role in cell-cell interactions within the central nervous system.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
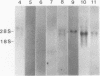
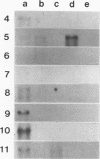
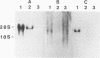
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. Adhesion molecules and animal development. Experientia. 1990 Jan 15;46(1):2–13. [Abstract] [Google Scholar]
- Behrens J, Birchmeier W, Goodman SL, Imhof BA. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985 Oct;101(4):1307–1315. [Europe PMC free article] [Abstract] [Google Scholar]
- Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990 Apr;110(4):1253–1260. [Europe PMC free article] [Abstract] [Google Scholar]
- Choi YS, Sehgal R, McCrea P, Gumbiner B. A cadherin-like protein in eggs and cleaving embryos of Xenopus laevis is expressed in oocytes in response to progesterone. J Cell Biol. 1990 May;110(5):1575–1582. [Europe PMC free article] [Abstract] [Google Scholar]
- Crittenden SL, Rutishauser U, Lilien J. Identification of two structural types of calcium-dependent adhesion molecules in the chicken embryo. Proc Natl Acad Sci U S A. 1988 May;85(10):3464–3468. [Europe PMC free article] [Abstract] [Google Scholar]
- Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. [Abstract] [Google Scholar]
- Damsky CH, Richa J, Solter D, Knudsen K, Buck CA. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell. 1983 Sep;34(2):455–466. [Abstract] [Google Scholar]
- Gallin WJ, Edelman GM, Cunningham BA. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. [Europe PMC free article] [Abstract] [Google Scholar]
- Gallin WJ, Sorkin BC, Edelman GM, Cunningham BA. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. [Europe PMC free article] [Abstract] [Google Scholar]
- Gould SJ, Subramani S, Scheffler IE. Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1934–1938. [Europe PMC free article] [Abstract] [Google Scholar]
- Grumet M, Edelman GM. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984 May;98(5):1746–1756. [Europe PMC free article] [Abstract] [Google Scholar]
- Harrelson AL, Goodman CS. Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science. 1988 Nov 4;242(4879):700–708. [Abstract] [Google Scholar]
- Hatta K, Nose A, Nagafuchi A, Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatta K, Okada TS, Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985 May;82(9):2789–2793. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987 Mar;120(1):215–227. [Abstract] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986 Apr 3;320(6061):447–449. [Abstract] [Google Scholar]
- Heimark RL, Degner M, Schwartz SM. Identification of a Ca2(+)-dependent cell-cell adhesion molecule in endothelial cells. J Cell Biol. 1990 May;110(5):1745–1756. [Europe PMC free article] [Abstract] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987 Dec;105(6 Pt 1):2501–2510. [Europe PMC free article] [Abstract] [Google Scholar]
- Jaffe SH, Friedlander DR, Matsuzaki F, Crossin KL, Cunningham BA, Edelman GM. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc Natl Acad Sci U S A. 1990 May;87(9):3589–3593. [Europe PMC free article] [Abstract] [Google Scholar]
- Keynes R, Cook G. Cell-cell repulsion: clues from the growth cone? Cell. 1990 Aug 24;62(4):609–610. [Abstract] [Google Scholar]
- Liaw CW, Cannon C, Power MD, Kiboneka PK, Rubin LL. Identification and cloning of two species of cadherins in bovine endothelial cells. EMBO J. 1990 Sep;9(9):2701–2708. [Europe PMC free article] [Abstract] [Google Scholar]
- McClay DR, Ettensohn CA. Cell adhesion in morphogenesis. Annu Rev Cell Biol. 1987;3:319–345. [Abstract] [Google Scholar]
- Millán JL. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [Abstract] [Google Scholar]
- Miyatani S, Shimamura K, Hatta M, Nagafuchi A, Nose A, Matsunaga M, Hatta K, Takeichi M. Neural cadherin: role in selective cell-cell adhesion. Science. 1989 Aug 11;245(4918):631–635. [Abstract] [Google Scholar]
- Moos M, Tacke R, Scherer H, Teplow D, Früh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. [Abstract] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. [Abstract] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. [Europe PMC free article] [Abstract] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989 Nov;1(1):37–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987 Dec 1;6(12):3655–3661. [Europe PMC free article] [Abstract] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986 Dec;103(6 Pt 2):2649–2658. [Europe PMC free article] [Abstract] [Google Scholar]
- Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. [Abstract] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. [Europe PMC free article] [Abstract] [Google Scholar]
- Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990 Oct;111(4):1645–1650. [Europe PMC free article] [Abstract] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988 Oct;107(4):1561–1573. [Europe PMC free article] [Abstract] [Google Scholar]
- Ringwald M, Schuh R, Vestweber D, Eistetter H, Lottspeich F, Engel J, Dölz R, Jähnig F, Epplen J, Mayer S, et al. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. [Abstract] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Shimoyama Y, Yoshida T, Terada M, Shimosato Y, Abe O, Hirohashi S. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989 Oct;109(4 Pt 1):1787–1794. [Europe PMC free article] [Abstract] [Google Scholar]
- Shirayoshi Y, Hatta K, Hosoda M, Tsunasawa S, Sakiyama F, Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986 Oct;5(10):2485–2488. [Europe PMC free article] [Abstract] [Google Scholar]
- Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990 Mar;9(3):757–763. [Europe PMC free article] [Abstract] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. [Abstract] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. [Abstract] [Google Scholar]
- Thomas PS. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. [Europe PMC free article] [Abstract] [Google Scholar]
- Vestweber D, Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984 May;152(1):169–178. [Abstract] [Google Scholar]
- Volk T, Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986 Oct;103(4):1441–1450. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoshida-Noro C, Suzuki N, Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984 Jan;101(1):19–27. [Abstract] [Google Scholar]
Associated Data
Articles from Cell Regulation are provided here courtesy of American Society for Cell Biology
Full text links
Read article at publisher's site: https://doi.org/10.1091/mbc.2.4.261
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc361775?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1091/mbc.2.4.261
Article citations
Cadherin-18 loss in prospermatogonia and spermatogonial stem cells enhances cell adhesion through a compensatory mechanism.
Zool Res, 45(5):1048-1060, 01 Sep 2024
Cited by: 0 articles | PMID: 39147719 | PMCID: PMC11491781
Coordinated linear and rotational movements of endothelial cells compartmentalized by VE-cadherin drive angiogenic sprouting.
iScience, 26(7):107051, 07 Jun 2023
Cited by: 3 articles | PMID: 37426350 | PMCID: PMC10329149
devCellPy is a machine learning-enabled pipeline for automated annotation of complex multilayered single-cell transcriptomic data.
Nat Commun, 13(1):5271, 07 Sep 2022
Cited by: 14 articles | PMID: 36071107 | PMCID: PMC9452519
Characterization of the roles of amphiregulin and transforming growth factor β1 in microvasculature-like formation in human granulosa-lutein cells.
Front Cell Dev Biol, 10:968166, 24 Aug 2022
Cited by: 2 articles | PMID: 36092732 | PMCID: PMC9448859
Simulated Microgravity Increases the Permeability of HUVEC Monolayer through Up-Regulation of Rap1GAP and Decreased Rap2 Activation.
Int J Mol Sci, 23(2):630, 06 Jan 2022
Cited by: 1 article | PMID: 35054818 | PMCID: PMC8776081
Go to all (206) article citations
Other citations
Wikipedia (Showing 9 of 9)
- https://en.wikipedia.org/wiki/CDH6
- https://en.wikipedia.org/wiki/CDH8
- https://en.wikipedia.org/wiki/CDH9
- https://en.wikipedia.org/wiki/T-cadherin
- https://en.wikipedia.org/wiki/CDH11
- https://en.wikipedia.org/wiki/CDH4
- https://en.wikipedia.org/wiki/CDH10
- https://en.wikipedia.org/wiki/CDH12
- https://en.wikipedia.org/wiki/VE-cadherin
Show less
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular cloning of the E-cadherin cDNAs from rabbit corneal epithelium.
Curr Eye Res, 14(12):1136-1145, 01 Dec 1995
Cited by: 5 articles | PMID: 8974843
Isolation and sequence analysis of human cadherin-6 complementary DNA for the full coding sequence and its expression in human carcinoma cells.
Cancer Res, 55(10):2206-2211, 01 May 1995
Cited by: 34 articles | PMID: 7743525
Identification of novel cadherins expressed in human melanoma cells.
J Invest Dermatol, 108(6):908-913, 01 Jun 1997
Cited by: 16 articles | PMID: 9182820
Phylogenetic analysis of the cadherin superfamily.
Bioessays, 14(11):743-748, 01 Nov 1992
Cited by: 27 articles | PMID: 1365887
Review