Abstract
Free full text

Induction of ICOS+CXCR3+CXCR5+ TH Cells Correlates with Antibody Responses to Influenza Vaccination
Associated Data
Abstract
Seasonal influenza vaccine protects 60 to 90% of healthy young adults from influenza infection. The immunological events that lead to the induction of protective antibody responses remain poorly understood in humans. We identified the type of CD4+ T cells associated with protective antibody responses after seasonal influenza vaccinations. The administration of trivalent split-virus influenza vaccines induced a temporary increase of CD4+ T cells expressing ICOS, which peaked at day 7, as did plasmablasts. The induction of ICOS was largely restricted to CD4+ T cells co-expressing the chemokine receptors CXCR3 and CXCR5, a subpopulation of circulating memory T follicular helper cells. Up to 60% of these ICOS+CXCR3+CXCR5+CD4+ T cells were specific for influenza antigens and expressed interleukin-2 (IL-2), IL-10, IL-21, and interferon-γ upon antigen stimulation. The increase of ICOS+CXCR3+CXCR5+CD4+ T cells in blood correlated with the increase of preexisting antibody titers, but not with the induction of primary antibody responses. Consistently, purified ICOS+CXCR3+CXCR5+CD4+ T cells efficiently induced memory B cells, but not naïve B cells, to differentiate into plasma cells that produce influenza-specific antibodies ex vivo. Thus, the emergence of blood ICOS+CXCR3+CXCR5+CD4+ T cells correlates with the development of protective antibody responses generated by memory B cells upon seasonal influenza vaccination.
INTRODUCTION
Influenza vaccines provide protection mainly by generating high-affinity antibodies against hemagglutinin, thereby preventing virus entry (1, 2). Immunological events that lead to the development of protective immunity after vaccinations remain largely unknown. Antibody response requires CD4+ helper T (TH) cells, most particularly a TH subset, T follicular helper (TFH) cells (3, 4). TFH cells are essential for the generation of high-affinity memory B cells through the germinal center (GC) reaction (3–5). TFH cells express the chemokine (C-X-C) receptor 5 (CXCR5) (6–9), which guides their migration into B cell follicles. Inducible costimulator (ICOS), expressed at high density by TFH cells in human tonsils (9), plays a critical role for their development (10–12) and functions (13, 14). TFH cells support the differentiation and survival of GC B cells (15, 16) through the secretion of interleukin-21 (IL-21) (17, 18). Tonsillar TFH cells express the transcription repressor B cell lymphoma 6 (Bcl-6) (9, 18–20), which is essential for TFH cell generation in vivo (21–23). In addition to GC response, CD4+ T cells also provide help to B cells at extrafollicular sites and induce their differentiation into plasma cells that contribute to the early generation of specific antibodies after antigen challenge (24). Extrafollicular helper cells appear to share developmental mechanisms, phenotypes, and functional properties with TFH cells (18, 25–27).
CXCR5+CD4+ T cells are also found in human blood and share functional properties with TFH cells (28, 29). This is also supported by the observations that subjects who show severely impaired GC formation through deficiency of CD40 ligand or ICOS display substantially fewer circulating CXCR5+CD4+ T cells (11). We have previously shown that human blood CXCR5+CD4+ T cells are composed of subsets that differentially express the chemokine receptors CXCR3 and CCR6, and display different functions (28). For example, CXCR3+CCR6− cells produce interferon-γ (IFN-γ), whereas the CXCR3−CCR6+ cells produce IL-17A (28). At variance with TFH cells in secondary lymphoid organs, blood CXCR5+CD4+ T cells are in a resting state and do not express ICOS (28, 29). In patients with clinically active autoimmune diseases, such as systemic lupus erythematosus, blood CXCR5+CD4+ T cells express ICOS (30), suggesting that they are activated.
Here, we hypothesized that the detailed phenotypical analysis on blood CXCR5+CD4+ T cells and their subsets might provide insights regarding the mechanistics by which influenza vaccinations induce protective antibody responses. Here, we show evidence that ICOS+CXCR3+CXCR5+CD4+ T cells emerging in blood 7 days after influenza vaccination contribute to the development of antibody responses by providing help to memory B cells.
RESULTS
Influenza vaccination induces ICOS on CXCR3+CXCR5+CD4+ T cells
Initially, two cohorts of healthy subjects were accrued in this study. A nonadjuvanted trivalent split seasonal influenza vaccine (Fluzone) was administered to a cohort of healthy adults (n = 12, called adult cohort) during winter 2009/2010 and to a cohort of healthy children (n = 19, called children cohort) during winter 2010/2011. The two vaccines shared the influenza B strain (B/Brisbane/60/2008-like). The influenza H3N2 strains were different [2009/2010: A/Brisbane/10/2007 (H3N2)–like; 2010/2011: A/Perth/16/2009 (H3N2)–like] but largely similar (for example, the identity of hemagglutinin sequences was 98%). However, only the 2010/2011 vaccine contained a component derived from swine-origin H1N1 influenza strain [A/California/7/2009 (H1N1)–like], a pandemic strain in the year 2009 to 2010 (31); the 2009/2010 vaccine contained A/Brisbane/59/2007 (H1N1)–like strain.
The percentage of total CD4+ T cells as well as CXCR5+CD4+ T cells in blood did not change at any time points after vaccination (days 1, 3, 7, 10, 14, 21, and 28) (fig. S1; the gating strategy is shown in Fig. 1A). However, the frequency of CD4+ T cells expressing ICOS increased after vaccination and peaked on day 7 (Fig. 1B). The up-regulation of ICOS was largely confined to CXCR5+CD4+ T cells because the frequency of ICOS+ cells within the memory CXCR5−CD4+ T cells did not change (Fig. 1, C and D). Furthermore, up-regulation of ICOS expression was found to be restricted to CXCR5+CD4+ T cells that coexpressed CXCR3 but not CCR6 (Fig. 2, A and B). Notably, CXCR3+CCR6+ cells, a minor cell population within CXCR3+CXCR5+CD4+ T cells (28), did not up-regulate ICOS expression (fig. S2). Therefore, in this article, we refer to CXCR3+CCR6−CXCR5+ cells as CXCR3+CXCR5+ cells for simplicity. The extent of ICOS expression within CXCR3+CXCR5+CD4+ T cells varied among subjects (in adults: mean range, 2.9 to 34.4%; in children: range, 4.9 to 45.5%). The induced ICOS+CXCR3+CXCR5+CD4+ T cells coexpressed high levels of PD-1, but did not express detectable levels of Bcl-6 (Fig. 2C), and thus were distinct from bona fide TFH cells present in secondary lymphoid organs (3, 4, 18).
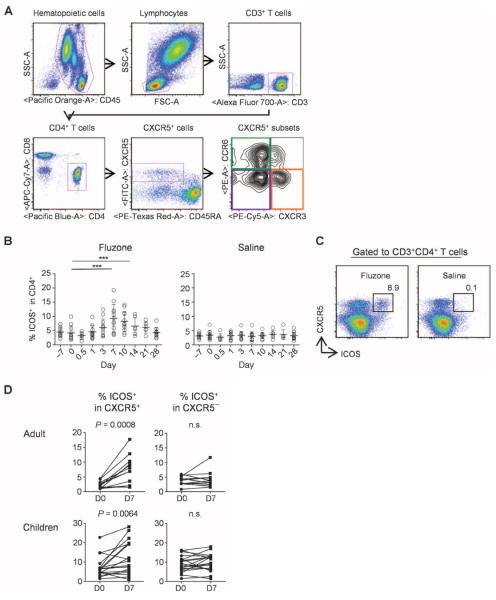
Seasonal influenza vaccines induce ICOS+CXCR5+ cells in blood. (A) Gating strategy for the analysis of blood CXCR5+CD4+ T cell subsets. (B) Percentage of ICOS+ cells within CD4+ T cells. Fresh blood samples were obtained from a healthy adult cohort (n = 12) before and after vaccination with trivalent seasonal influenza vaccine (Fluzone 2009/2010). The right panel shows data from six adults who received injection of normal saline. One-way analysis of variance (ANOVA). ***P < 0.001, paired t test. (C) A representative result of expression of ICOS on CXCR5+CD4+ T cells on day 7 after influenza vaccination or injection of normal saline. (D) Percentage of ICOS+ cells within CXCR5+ and CXCR5−CD4+ T cell compartments before and after influenza vaccination. Paired t test. n.s., not significant.
ICOS is expressed by CXCR3+CXCR5+CD4+ T cells. (A) ICOS expression on CXCR5+ TH subsets before and 7 days after influenza vaccination. A representative result is shown. (B) Percentage of ICOS+ cells in CXCR5+ TH subsets before and 7 days after influenza vaccination in the two cohorts. Paired t test. (C) Expression of PD-1 and Bcl-6 by ICOS+CXCR3+CXCR5+CD4+ T cells (red), ICOS−CXCR3+CXCR5+CD4+ T cells (blue), and CXCR3−CD4+ T cells (gray). A representative of three experiments is shown.
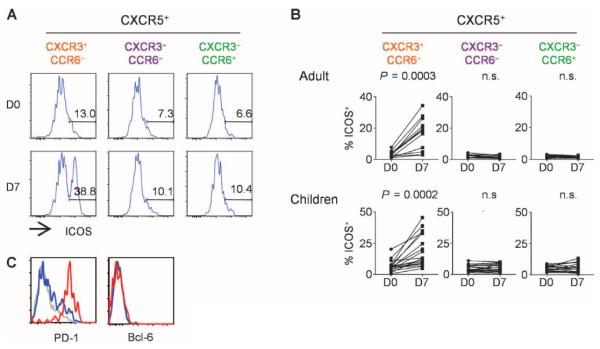
Influenza vaccination also resulted in the altered composition of blood CXCR5+CD4+ T cells. In both cohorts, the frequency of CD4+ T cells co-expressing CXCR3 and CXCR5 increased at day 7 after vaccination, whereas the frequency of cells coexpressing CCR6 and CXCR5 decreased (Fig. 3A). The increased expression of CXCR3 within the CXCR5+ cell population positively correlated with the increased expression of ICOS on CXCR5+CXCR3+ cells (Fig. 3B), suggesting that the two events were associated.
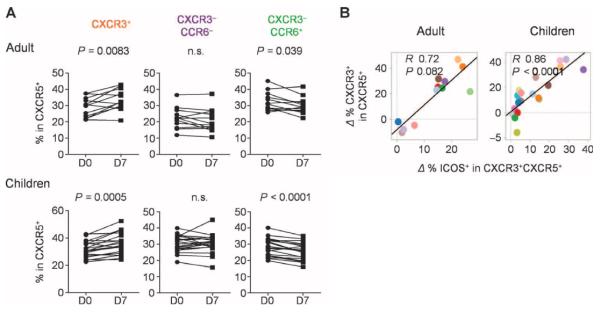
Increase of CXCR3+CXCR5+CD4+ T cells after influenza vaccination. (A) Increase of CXCR3+ cells in CXCR5+CD4+ T cell populations. The composition of the indicated subsets within the CXCR5+CD4+ T cells before and 7 days after influenza vaccination in the two cohorts. Paired t test. (B) Correlation between the increase of CXCR3+ cells in CXCR5+CD4+ T cells (y axis) and the increase of ICOS on CXCR3+CXCR5+CD4+ cells.
Thus, trivalent split seasonal influenza vaccination results in ICOS expression on CXCR3+CXCR5+CD4+ T cells in blood across ages and vaccine components.
Emergence of ICOS+CXCR3+CXCR5+CD4+ T cells correlates with the development of antibody responses
We wondered whether the emergence of ICOS+CXCR3+CXCR5+CD4+ T cells might correlate with the development of protective antibody responses. Hemagglutinin inhibition (HI) and virus neutralization (VN) antibody titers against each influenza virus strains incorporated in the vaccines were measured at baseline and at day 28 after vaccination (figs. S3 and S4). We determine the global antibody responses as the maximum fold increase of the titers from the baseline among the three influenza strains in the vaccine. The increase of blood ICOS+CXCR3+CXCR5+CD4+ T cells positively correlated with the fold increase of global antibody titers for both adults and children (Fig. 4A). Furthermore, the absolute number of ICOS+CXCR3+CXCR5+CD4+ T cells in blood measured at day 7 alone also correlated with the global antibody responses in both adult and children cohorts (Fig. 4B). No other parameters on blood TH subsets, including the frequency of CXCR5+CD4+ T cells or that of ICOS+ cells among CXCR5−CD4+ T cell subsets, showed a significant correlation in both cohorts (table S1).
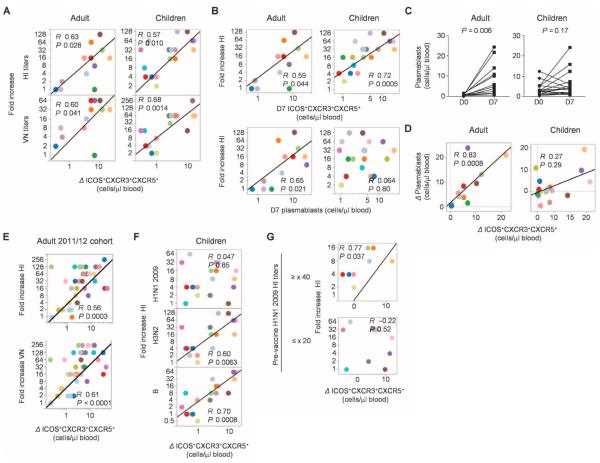
ICOS+CXCR3+CXCR5+CD4+ T cells correlate with antibody responses. (A) Correlation between the increase of global antibody (HI and VN) titers and the increase of ICOS+CXCR3+CXCR5+CD4+ T cells. (B) Correlation between the increase of global HI antibody titers and absolute number of ICOS+CXCR3+CXCR5+CD4+ T cells at day 7 (top) and CD38+CD27+ plasmablasts at day 7 (bottom). (C) Absolute number of plasmablasts in blood before and 7 days after influenza vaccination in the two cohorts. Paired t test. (D) Correlation between the increase in the absolute number of ICOS+CXCR3+CXCR5+CD4+ T cells in blood and the increase in the absolute number of CD38+CD27+ plasmablasts. (E) Correlation between the increase of global antibody (HI and VN) titers and the increase of ICOS+CXCR3+CXCR5+CD4+ T cells in the 2011/2012 adult cohort. n = 37. (F) Correlation between the increase of HI titers against three different hemagglutinin included in the vaccines and the increase of ICOS+ CXCR3+CXCR5+CD4+ T cells in the children cohort. (G) Correlation between the increase of HI titers against H1N1 2009 and the increase of ICOS+CXCR3+CXCR5+CD4+ T cells in children who did not carry preexisting antibodies (bottom) and in children who showed preexisting specific antibodies (HI titer ≥ ×40, top).
The number of plasmablasts (CD38+CD27+CD19+) in blood increased with variable magnitude at day 7 after vaccination in the adult cohort (Fig. 4C, left), an observation consistent with previous studies (32, 33). In this cohort, the absolute number of plasmablasts at day 7 correlated with the global antibody responses (Fig. 4B). Furthermore, the increase of blood plasmablasts showed a strong correlation with the increase of ICOS+CXCR3+CXCR5+CD4+ T cells (Fig. 4D, left; R = 0.83, P = 0.0008). In the children cohort, however, four subjects showed relatively high numbers of plasmablasts (>5 plasmablasts/μl of blood) at baseline, and six subjects showed decrease in the number of plasmablasts after influenza vaccination (Fig. 4C, right). The absolute number of plasmablasts at day 7 did not show a correlation with the increase of global antibody titers in this cohort (Fig. 4B). Although there was a trend for the increase of ICOS+CXCR3+CXCR5+CD4+ T cells to positively correlate with the increase of plasmablasts, it did not reach statistical significance (Fig. 4D, right). However, when four subjects who had 5 plasmablasts/ml of blood at baseline were removed from the analysis, the two parameters showed a significant correlation (fig. S5; R = 0.77, P = 0.0014), whereas the absolute number of plasmablasts at day 7 did not show a correlation.
To validate our observation, we obtained blood samples from another adult cohort during the 2011/2012 winter. Fluzone 2011/2012 was administered to 37 healthy adults, and the correlation between the increase of blood ICOS+CXCR3+CXCR5+CD4+ T cells at day 7 and the fold increase of global antibody titers at day 28 was analyzed. In Fig. 4E, the two parameters showed a strong positive correlation (HI: R = 0.56, P = 0.0003; VN: R = 0.61, P < 0.0001). Furthermore, the absolute number of ICOS+CXCR3+CXCR5+CD4+ T cells at day 7 also showed the positive correlation (HI: R = 0.54, P = 0.0005; VN: R = 0.57, P = 0.0002).
Collectively, these data from three independent cohorts of vaccinees show that the emergence of ICOS+CXCR3+CXCR5+CD4+ T cells at day 7 correlates with the magnitude of global protective antibody responses in seasonal influenza vaccination.
ICOS+CXCR3+CXCR5+CD4+ T cell numbers correlate with recall antibody responses
The increase of ICOS+CXCR3+CXCR5+ CD4+ T cells positively correlated with the increased antibody titers against each viral strain in the vaccines (fig. S6 and table S2), with the exception in the children cohort of titers against swine-origin H1N1 2009 (Fig. 4F). Considering that seasonal influenza vaccines induce antibody responses mainly through a boost of recall response (34), this lack of correlation might indeed reflect the lack of H1N1 2009–specific memory in some children. This is consistent with the fact that these children had not been vaccinated or naturally exposed to the H1N1 virus before being vaccinated in that 2010/2011 winter. Indeed, there was no correlation between H1N1 2009 HI titer increase and the induction of ICOS+CXCR3+CXCR5+CD4+ T cells in those children who did not carry preexisting H1N1 2009 HI antibodies (R = −0.22, P = 0.52, n = 11). In contrast, a positive correlation was observed in children who had preexisting specific antibodies (R = 0.77, P = 0.037, n = 8; HI titer ≥ ×40) (Fig. 4G). Furthermore, a similar observation was made in the adult validation cohort. Thus, in the adult 2011/2012 cohort, there was a correlation between H1N1 2009 HI titer increase and the induction of ICOS+CXCR3+CXCR5+CD4+ T cells in subjects who had preexisting or cross-reactive antibodies for H1N1 2009 HI (fig. S7; either HI or VN titer ≥ ×40, n = 20; HI: R = 0.70, P = 0.0009; VN: R = 0.75, P = 0.0001) but not in subjects who did not have these preexisting antibodies (both HI and VN titer below detection limit, n = 10; HI: R = −0.33, P = 0.35; VN: R = −0.27, P = 0.45). These data support that the increase of ICOS+CXCR3+CXCR5+CD4+ T cells contributes to a boost of preexisting antibody titers but not to the induction of primary antibody responses.
Induced ICOS+CXCR3+CXCR5+CD4+ T cells recognize influenza antigens
The increase of ICOS+CXCR3+CXCR5+CD4+ T cells after vaccination suggests that they might have undergone proliferation. Indeed, ~40 to 60% of these cells expressed Ki67 (Fig. 5A), indicating that they were in cell cycle. To test their specificity for vaccine antigens, we stimulated peripheral blood mononuclear cells (PBMCs) at day 7 after vaccination for 6 hours with Fluzone or killed flu virus (PR8) in the presence of brefeldin A and monensin. The expression of intracytoplasmic cytokines was analyzed together with that of CD154, because this permits distinction of antigen-specific CD4+ T cells from antigen nonspecific cells (35). Both stimulations induced a fraction of CXCR5+CD4+ T cells to express CD154, demonstrating the presence of influenza antigen–specific T cells (Fig. 5B). CD154 expression was independent of soluble factors secreted during the stimulation because inhibition of their secretion from any cells by adding Golgi inhibitors before the stimulation did not alter the frequency of CD154+ cells (fig. S8). Among CXCR5+CD4+ T cells, the frequency of CD154+ cells was much higher within ICOS+ cells than within ICOS− cells (Fig. 5C). Upon stimulation with Fluzone and PR8 virus, about 40 and 12% of ICOS+CXCR5+ cells expressed CD154, respectively. The frequency of CD154+ cells within ICOS+ cells was much higher among CXCR5+CD4+ T cells than among CXCR5−CD4+ T cells (Fig. 5C). ICOS+CXCR5+ cells did not express CD154 in response to control cytomegalovirus (CMV) peptides (fig. S9), thus confirming antigen specificity. The CD154+ICOS+CXCR5+CD4+ T cells originated from ICOS+CXCR5+ cells because ICOS−CXCR5−CD4+ T cells did not up-regulate ICOS or CXCR5 during 6 hours of in vitro activation [fig. S10 and (28)]. These observations show that the induced ICOS+CXCR5+CD4+ T cells contained influenza-specific cells.
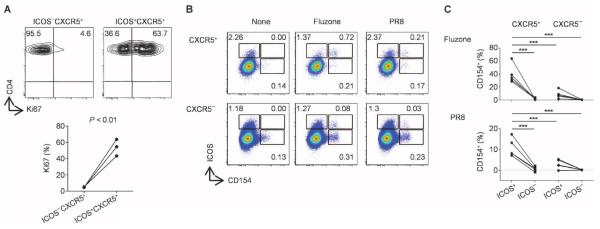
ICOS+CXCR3+CXCR5+CD4+ T cells are specific for influenza antigens. (A) Ki67 expression by ICOS+CXCR3+CXCR5+CD4+ T cells at day 7 after vaccination (n = 3). A representative result is shown on the top panels. (B) CD154 assay. PBMCs obtained at day 7 after vaccination were stimulated with either Fluzone or heat-killed PR8 influenza virus for 6 hours. CD154+ cells represent antigen-specific cells. Gated to CXCR5+CD4+ and CXCR5−CD4+ T cells. A representative from experiments with four donors. (C) The percentage of CD154+ cells within ICOS+ and ICOS− cells among the CXCR5+ and CXCR5−CD4+ T cells upon stimulation with Fluzone (top) and PR8 virus (bottom). n = 4. ***P < 0.001, one-way ANOVA.
Induced ICOS+CXCR3+CXCR5+CD4+ T cells express multiple cytokines
CXCR5+CD4+ T cells that expressed CD154 upon influenza antigen stimulation were found to coexpress several cytokines, including IFN-γ, IL-2, IL-10, and IL-21 (Fig. 6A). Among the CD154+CXCR5+CD4+ T cells, these cytokines were expressed dominantly by ICOS+ cells (Fig. 6B). About 70% of CD154+ICOS+CXCR5+CD4+ T cells expressed IFN-γ, 50% of cells expressed IL-2, and 40% of cells expressed IL-21 (Fig. 6C). Furthermore, about 20% of CD154+ICOS+CXCR5+CD4+ T cells were found to coexpress IL-2, IFN-γ, and IL-21 (Fig. 6D). In contrast, very few CD154+ICOS−CXCR5+CD4+ T cells expressed IL-21 (Fig. 6, B and C), and most lacked expression of any of the tested cytokines (Fig. 6D). Comparable results were obtained upon stimulation with PR8 virus (fig. S11). The cytokine expression pattern of CD154+ICOS− cells within the CXCR5−CD4+ T cells was very similar to that of CD154+ICOS−CXCR5+CD4+ T cells (fig. S12).
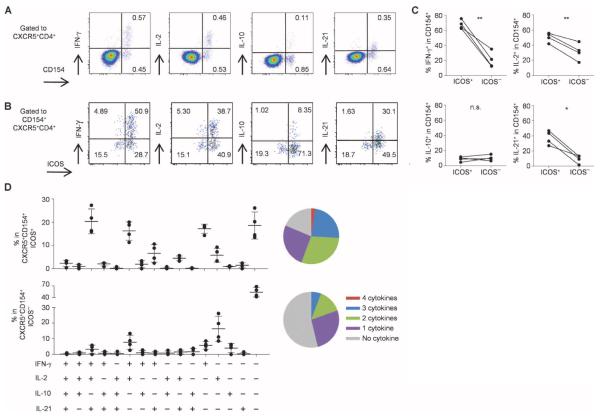
ICOS+CXCR3+CXCR5+CD4+ T cells express multiple cytokines including IL-21. (A) Intracytoplasmic cytokine expression by CXCR5+CD4+ T cells stimulated with Fluzone. A representative from experiments with four donors. (B) Cytokine expression by CD154+CXCR5+CD4+ T cells induced by Fluzone stimulation. Expression of the indicated cytokines and ICOS. A representative from experiments with four donors. (C) Frequency of cytokine-expressing cells among CD154+ICOS+CXCR5+ and ICOS−CXCR5+CD4+ T cells induced by Fluzone stimulation. n = 4. Paired t test. **P < 0.01, *P < 0.05. (D) Cytokine expression pattern of CD154+ICOS+CXCR5+ and ICOS−CXCR5+CD4+ T cells induced by Fluzone stimulation. n = 4. The numbers of expressed cytokines are shown on the right in pie charts.
Thus, the cytokine expression profiles were largely distinct between influenza-specific ICOS+ and ICOS−CXCR5+CD4+ T cells, and ICOS+ cells expressed a broader range of cytokines, including IL-21.
Induced ICOS+CXCR3+CXCR5+CD4+ T cells efficiently help memory B cells
To analyze the capacity of ICOS+CXCR3+CXCR5+CD4+ T cells to help B cells, we isolated these cells from blood samples at day 7 after vaccination and cultured them with both naïve and memory B cells from the same blood samples. Staphylococcal enterotoxin B, a super-antigen, was added to the cultures to enhance T and B cell interactions (28). Naïve B cells cultured with ICOS+CXCR3+CXCR5+CD4+ T cells did not proliferate or differentiate into antibody-secreting cells (CD38hiCD138− plasmablasts and CD38hiCD138+ plasma cells) (Fig. 7, A and B). In contrast, consistent with our previous study (28), naïve B cells cultured with CXCR3−CCR6− and CXCR3−CCR6+CXCR5+ cells proliferated and differentiated into antibody-secreting cells.
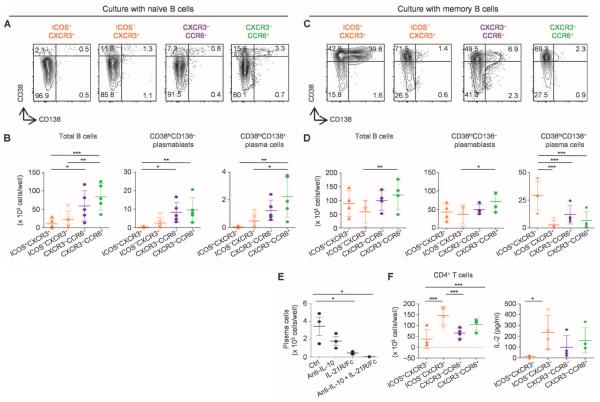
ICOS+CXCR3+CXCR5+CD4+ T cells help memory B cells. (A and B) Differentiation of naïve B cells cocultured with CXCR5+ TH subsets. Naïve B cells cultured for 12 days with the indicated CXCR5+ TH subsets (from day 7 after vaccination) were analyzed for the expression of CD38 and CD138. (A) Representative result of four experiments. (B) The number of total B cells, plasmablasts, and plasma cells in the cultures was determined on day 12. n = 4. ***P < 0.001, **P < 0.01, *P < 0.05, one-way ANOVA. (C and D) Memory B cell differentiation. Memory B cells cultured for 6 days with the indicated CXCR5+ TH subsets were analyzed for the expression of CD38 and CD138. (C) Representative result of four experiments. (D) The number of total B cells, plasmablasts, and plasma cells in the cultures was determined on day 6. n = 4. One-way ANOVA. Blocking of IL-10 and IL-21. (E) Blocking reagents were added to the cultures of memory B cells and ICOS+CXCR3+CXCR5+CD4+ T cells. The number of plasma cells induced in the cultured wells was determined. n = 3. One-way ANOVA. (F) T cell recovery and IL-2 production. The indicated CXCR5+ TH subsets were cultured with memory B cells. The number of recovered viable CD4+ T cells at day 6 (left) and IL-2 levels in the supernatants at day 2 (right) are shown. n = 4. One-way ANOVA.
However, ICOS+CXCR3+CXCR5+CD4+T cells were the most efficient among the tested TH subsets at inducing memory B cells to differentiate into plasma cells (Fig. 7, C and D). The induction of plasma cell differentiation was dependent on IL-10 and IL-21 because the blocking of both cytokines completely inhibited the generation of plasma cells (Fig. 7E) as well as immunoglobulin (Ig) production (fig. S13). The recovery of ICOS+CXCR3+CXCR5+CD4+ T cells after coculture with memory B cells was lower than that of ICOS−CXCR3+CXCR5+CD4+T cells (Fig. 7F). Furthermore, the IL-2 levels in ICOS+CXCR3+CXCR5+CD4+ T cell culture supernatants were lower than those of ICOS−CXCR3+CXCR5+CD4+ T cell cultures (Fig. 7F). Therefore, the superior function of ICOS+CXCR3+CXCR5+CD4+ T cells in the induction of memory B cell differentiation toward plasma cells was not due to their superior survival and/or proliferation in culture.
Last, we determined whether ICOS+CXCR3+CXCR5+CD4+ T cells were capable of inducing specific antibody responses. Sorted ICOS+CXCR3+CXCR5+CD4+ T cells were cocultured with memory B cells loaded with Fluzone. Consistent with Ki67 expression (Fig. 5A), ICOS+CXCR3+CXCR5+CD4+ T cells underwent considerable proliferation and cytokine secretion upon coculture with Fluzone-loaded memory B cells (Fig. 8, A and B). Furthermore, ICOS+CXCR3+CXCR5+CD4+ T cells efficiently induced memory B cells to proliferate and differentiate into plasma cells (Fig. 8, C and D), producing influenza-specific IgG (Fig. 8E). Together, these observations show that ICOS+CXCR3+CXCR5+CD4+ T cells induced by influenza vaccination recognize influenza antigens and are capable of inducing memory B cells to produce influenza-specific Igs.

ICOS+CXCR3+CXCR5+CD4+ T cells induce antigen-specific antibody response. (A) Proliferation of CD4+ T cells. CXCR5+ TH subsets were cultured with memory B cells loaded with Fluzone. The number of CD4+ T cells in the cultures was determined on day 6. Two independent experiments. (B) Cytokine levels on day 2 of culture supernatants. A representative of two experiments. (C and D) The expression of CD38 and CD138 on B cells was determined on day 6. (C) Representative result of three experiments. (D) The number of total B cells, plasmablasts, and plasma cells in the cultures was determined on day 6. n = 3. One-way ANOVA. (E) Influenza-specific IgG levels on day 6. n = 3.
DISCUSSION
Administration of seasonal trivalent split influenza vaccines provides protection from influenza in about 60 to 90% of vaccinees (2, 36). Impaired generation of neutralizing antibodies causes inadequate protection. Our study shows that the induction of ICOS+CXCR3+CXCR5+CD4+ T cells represents one of the key immunological events associated with protective antibody responses after seasonal influenza vaccination. The emergence of ICOS+CXCR3+CXCR5+CD4+ T cells in blood at day 7 after vaccination correlated with the increase of preexisting antibodies but not with the induction of primary antibody responses. Furthermore, our in vitro studies show that the induced ICOS+CXCR3+CXCR5+CD4+ T cells efficiently induced memory B cells to differentiate into plasma cells. These ICOS+CXCR3+CXCR5+CD4+ T cells were, however, unable to help naïve B cells. These observations suggest that the ICOS+CXCR3+CXCR5+CD4+ T cells induced by split influenza vaccines contribute to the antibody response primarily through boosting memory responses. This is consistent with recent data showing that split influenza vaccines, including ones for H1N1 2009, induce antibody responses mainly via activation of memory B cells (34, 37).
Another important finding in our study is that the detailed phenotypic analysis of blood CXCR5+CD4+ T cells after vaccinations provides a parameter that correlates with the magnitude of antibody responses. A recent study showed that the increase of blood CXCR5+CD4+ T cells in HIV-infected donors at day 28 after vaccination with the 2009 H1N1 vaccine was associated with the generation of antibody responses (38). However, we did not observe an increase of blood CXCR5+CD4+ T cells at day 28 in our cohort (fig. S1). Thus, their conclusion might mainly apply to subjects infected with HIV, a virus that induces immunodeficiency. Our study suggests that the analysis of the expression of ICOS on blood CXCR5+CD4+ T cells [and their subsets (28)] and their kinetics might provide a better parameter that correlates with generation of antibody responses.
ICOS+CXCR3+CXCR5+CD4+ T cells induced by influenza vaccination expressed Ki67 and thus were in cell cycle. Furthermore, this cell population was enriched with cells specific for influenza antigens. These observations suggest that ICOS+CXCR3+CXCR5+CD4+ T cells were induced through the interaction with antigen-presenting cells carrying influenza antigens, possibly including memory B cells. Although such interaction most likely occurs in secondary lymphoid organs, the sites where the ICOS+CXCR3+CXCR5+CD4+ T cells contribute to antibody responses might not be limited to the draining lymph nodes. Mouse studies with pulmonary influenza models showed that memory B cells and effector T cells migrate to the lung via CXCR3, and CXCR3+ memory B cells persist at sites for a long time (39, 40). Furthermore, numerous bona fide TFH cells were found in the lung infected by influenza virus (41). Therefore, it is possible that the induced ICOS+ CXCR3+CXCR5+CD4+ T cells might migrate to the lung via CXCR3, particularly when active inflammatory responses are ongoing, and that they help the generation of influenza-specific antibody responses through interaction with resident memory B cells.
Whether the blood ICOS+CXCR3+CXCR5+CD4+ T cells are derived from TFH cells that provided help to B cells in GCs, or from TFH-committed cells that provided help outside GCs, remains unclear. Although they share phenotypic and functional properties with TFH cells (such as the expression of ICOS and PD-1, and the capacity to help B cells through IL-21 secretion), they lack the expression of Bcl-6 and thus do not appear to represent bona fide TFH cells. Whether blood ICOS+CXCR3+CXCR5+CD4+ T cells are derived from naïve T cells or influenza-specific memory T cells also remains to be established. Addressing these questions will be very challenging in humans and will require animal models, provided they show the same transient expression of ICOS on blood CXCR3+CXCR5+CD4+ T cells.
Nonetheless, it is intriguing that the emergence of plasmablasts and ICOS+CXCR3+CXCR5+CD4+ T cells in blood peaks at the same time after influenza vaccination (at day 7) (33). This suggests that these cells develop in a similar kinetics. It is also notable that the emergence of ICOS+CXCR3+CXCR5+CD4+ T cells can be detected even at 3 days after vaccination in some subjects. Therefore, induction of such T cells might be a limiting factor for the generation of plasmablasts and plasma cells. Accordingly, it will be important to identify pathways and/or adjuvants that promote their generation.
Although the current split influenza vaccines provide protection in many cases, our study suggests that this vaccine design might be suboptimal in some conditions, in particular where primary antibody responses are required, such as immunization to young children or against H5N1 avian influenza viruses. This hypothesis is also supported by the observations that a split influenza vaccine does not immunize naïve mice, unless adjuvants stimulating Toll-like receptors are coadministered (42). In the same line, recent studies in humans show that conjugation of adjuvants (for instance, with MF59) with influenza vaccines significantly enhances the diversity and the affinity of antibody responses (43). Therefore, such adjuvant might be required to induce different types of TH cells, which efficiently induce primary antibody responses.
MATERIALS AND METHODS
Clinical samples
Blood samples were obtained from healthy control subjects before and after the administration of a nonadjuvanted trivalent split seasonal influenza vaccine (Fluzone, Sanofi Pasteur). A cohort of 12 healthy adults received vaccination in 2009/2010 winter, another cohort of 37 healthy adults received vaccination in 2011/2012 winter, and a cohort of 20 healthy children received vaccination in 2010/2011 winter. The study was approved by the Institutional Review Boards of Baylor Health Care System and Nationwide Children's Hospital. Informed consent was obtained from subjects, parents, or legal guardians.
Flow cytometry
Whole-blood samples (200 μl) were incubated with the indicated antibodies and Live/Dead Fixable Aqua (Invitrogen) for 15 min at room temperature. The following monoclonal antibodies were used: CXCR5 (1G10), CD3 (UCHT1), CD8 (SK1), CD4 (RPA-T4), CCR6 (11A9), CXCR3 (1C6/CXCR3), ICOS (C398.4A), CD45RA (2H4), and CD45 (HI30). Stained cells were acquired on a BD LSRII.
Antibody assays
HI and VN antibody titers for each strain of influenza virus incorporated in the vaccines were determined at baseline and at day 28 after vaccination.
CD154 assay
PBMCs at day 7 after vaccination were stimulated for 6 hours with Fluzone or killed flu virus (PR8) in the presence of brefeldin A and monensin. Cells were subsequently incubated with anti- CD3 (UCHT1), anti-CD4 (S3.5), anti-CXCR5 (RF8B2), anti-ICOS (C398.4A), and Aqua Live/Dead (Invitrogen). After permeabilization, cells were stained intracellularly with anti–IL-2 (MQ1-17H12), anti–IL-10 (JES3-9D7), anti–IL-21 (3A3-N21), anti–IFN-γ (4S.B3), and CD154 (24–31).
In vitro cell culture
Sorted CD4+CXCR5+ TH populations (2 × 104 cells per well) were cocultured with naïve B cells (2 × 104 cells per well) for 12 days and with memory B cells (2 × 104 cells per well) for 6 days in complete RPMI 1640 medium in the presence of staphylococcal enterotoxin B (1 μg/ml; Sigma-Aldrich) in 96-well U-bottomed plates.
Assessment of influenza-specific CD4+ T cell proliferation and cytokine secretion
Sorted CD4+ TH subsets (5 × 104 cells per well) were cocultured with memory B cells (5 × 104 cells per well) loaded with Fluzone. Culture supernatants were harvested at day 2, and cytokines were measured by Luminex. At day 6, the cultured cells were analyzed by fluorescence-activated cell sorting.
Statistical analysis
Statistical significance was assessed with one-way ANOVA followed by a Bonferroni multiple comparisons or a Student's t test. Pearson R and P values were determined to analyze the correlations between the indicated two parameters.
Acknowledgments
We would like to thank healthy individuals involved in this study; S. Burkeholder, L. Walters, and J. Fay for blood apheresis and PBMC isolation; S. Coquery, N. Loof, C. Boudreaux, and K. Kayembe for cell sorting; and C. Samuelsen for administration.
Funding: This study is supported by NIH grants U19 AI089987 and U19 082715, and funding from Baylor Health Care System.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/5/176/176ra32/DC1 Materials and Methods
Author contributions: S.-E.B. performed most of the in vitro experiments. S.L., E.F., A.M., and V.P. collected and analyzed children samples. G.O. analyzed flow data. N.S. and C.M. developed panels for CD154 assay, performed the experiments, and analyzed the data. C.H. organized and managed the vaccine study and the collection of adult samples. R.A.A. and A.G.-S. analyzed antibody titers. G.O., D.B., and H.X. contributed to the statistical analysis. S.-E.B., J.B., and H.U. wrote the manuscript. J.B., A.K.P., O.R., and H.U. designed the study and reviewed the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
Full text links
Read article at publisher's site: https://doi.org/10.1126/scitranslmed.3005191
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3621097?pdf=render
Citations & impact
Impact metrics
Article citations
Role of circulating T follicular helper subsets following Ty21a immunization and oral challenge with wild type <i>S</i>. Typhi in humans.
Front Immunol, 15:1384642, 12 Sep 2024
Cited by: 0 articles | PMID: 39328410 | PMCID: PMC11424897
Generation of antigen-specific memory CD4 T cells by heterologous immunization enhances the magnitude of the germinal center response upon influenza infection.
PLoS Pathog, 20(9):e1011639, 16 Sep 2024
Cited by: 1 article | PMID: 39283916 | PMCID: PMC11404825
TFH cell responses endure in human lymph nodes after vaccination.
Nat Immunol, 25(9):1510-1512, 01 Sep 2024
Cited by: 0 articles | PMID: 39164478
Atypical and non-classical CD45RBlo memory B cells are the majority of circulating SARS-CoV-2 specific B cells following mRNA vaccination or COVID-19.
Nat Commun, 15(1):6811, 09 Aug 2024
Cited by: 1 article | PMID: 39122676 | PMCID: PMC11315995
Adaptive Cellular Responses following SARS-CoV-2 Vaccination in Primary Antibody Deficiency Patients.
Pathogens, 13(6):514, 18 Jun 2024
Cited by: 0 articles | PMID: 38921811 | PMCID: PMC11206773
Go to all (373) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Early Rise of Blood T Follicular Helper Cell Subsets and Baseline Immunity as Predictors of Persisting Late Functional Antibody Responses to Vaccination in Humans.
PLoS One, 11(6):e0157066, 23 Jun 2016
Cited by: 32 articles | PMID: 27336786 | PMCID: PMC4918887
Circulating CXCR5⁺CD4⁺ T Follicular-Like Helper Cell and Memory B Cell Responses to Human Papillomavirus Vaccines.
PLoS One, 10(9):e0137195, 02 Sep 2015
Cited by: 29 articles | PMID: 26333070 | PMCID: PMC4557948
ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination.
Sci Rep, 6:26494, 27 May 2016
Cited by: 109 articles | PMID: 27231124 | PMCID: PMC4882544
The origins, function, and regulation of T follicular helper cells.
J Exp Med, 209(7):1241-1253, 01 Jul 2012
Cited by: 337 articles | PMID: 22753927 | PMCID: PMC3405510
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000090
NIAID NIH HHS (2)
Grant ID: U19 AI082715
Grant ID: U19 AI089987
PHS HHS (1)
Grant ID: U19 082715





