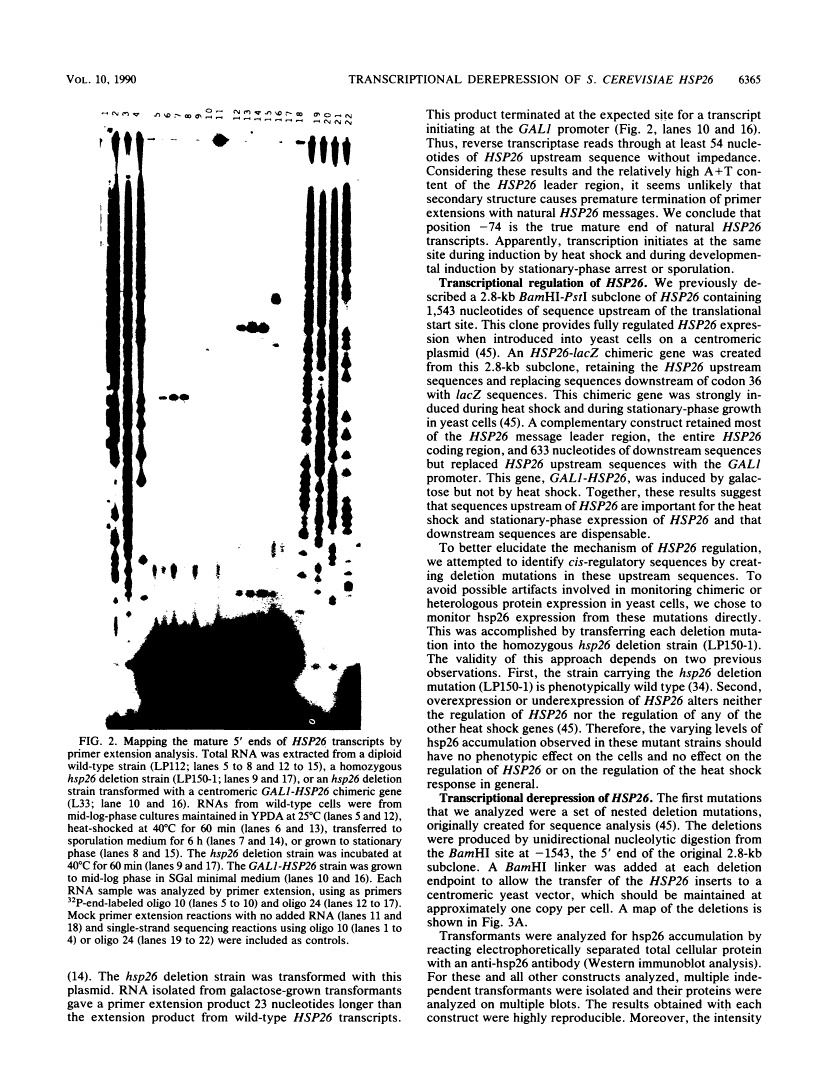
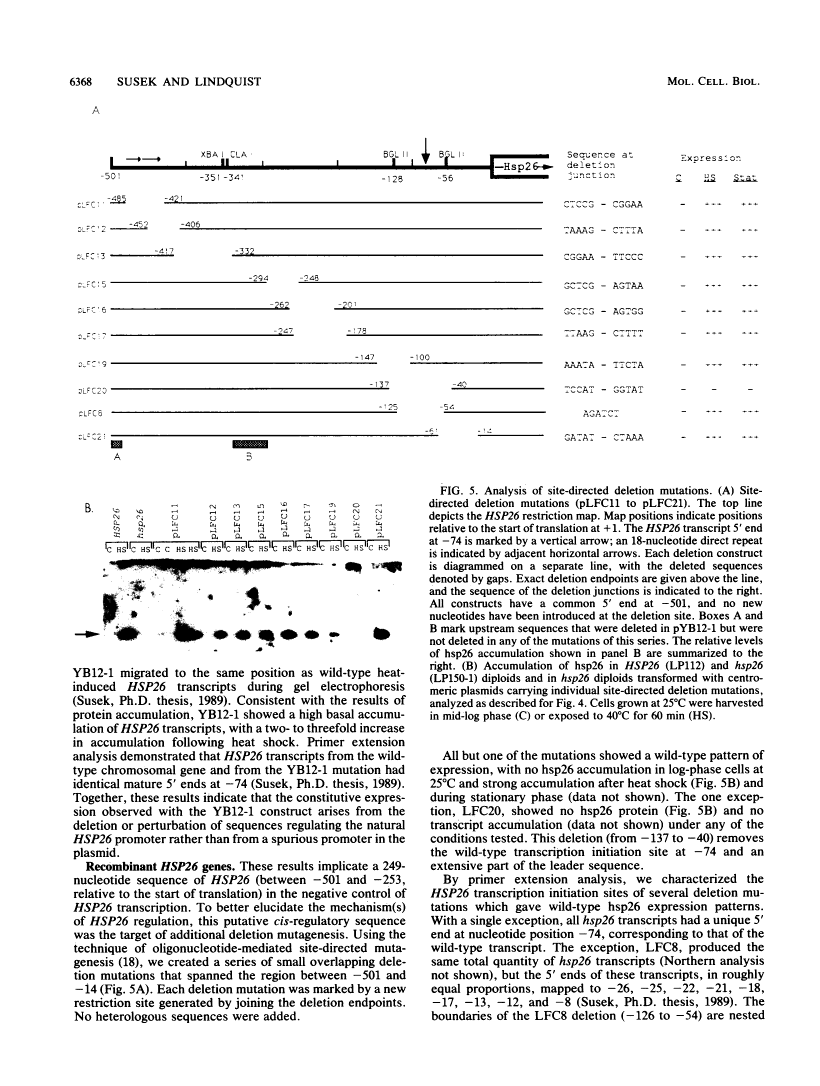
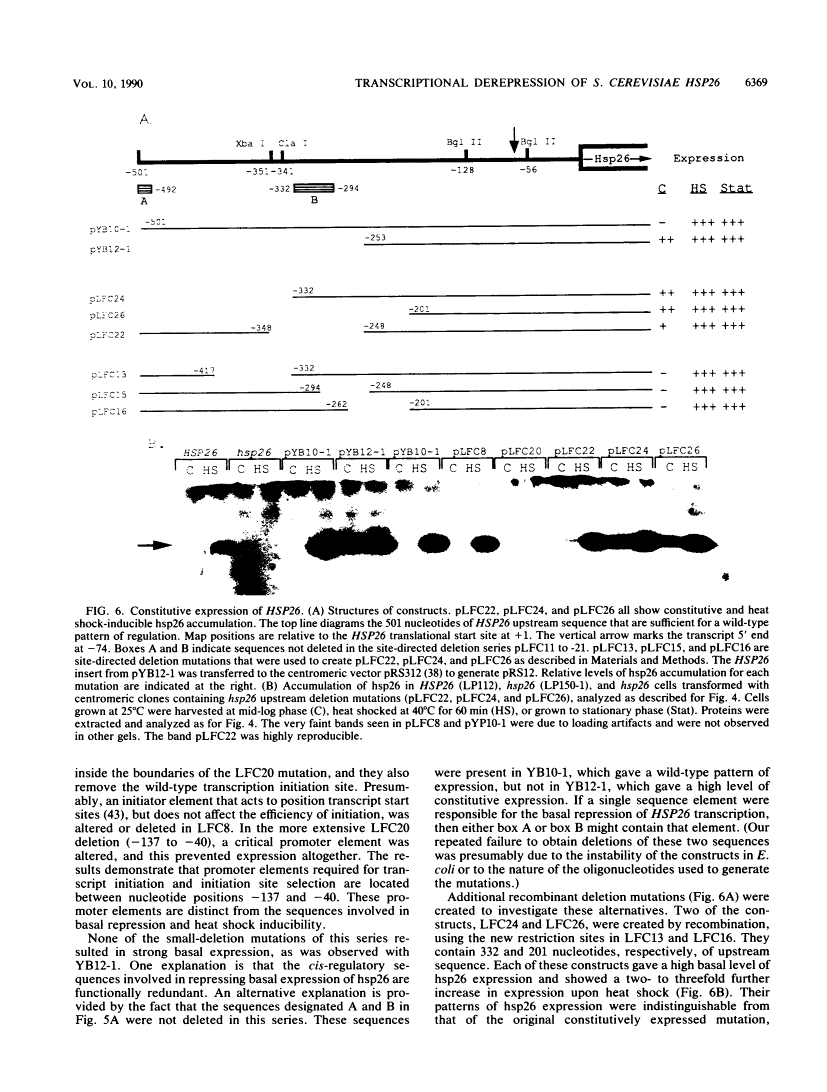
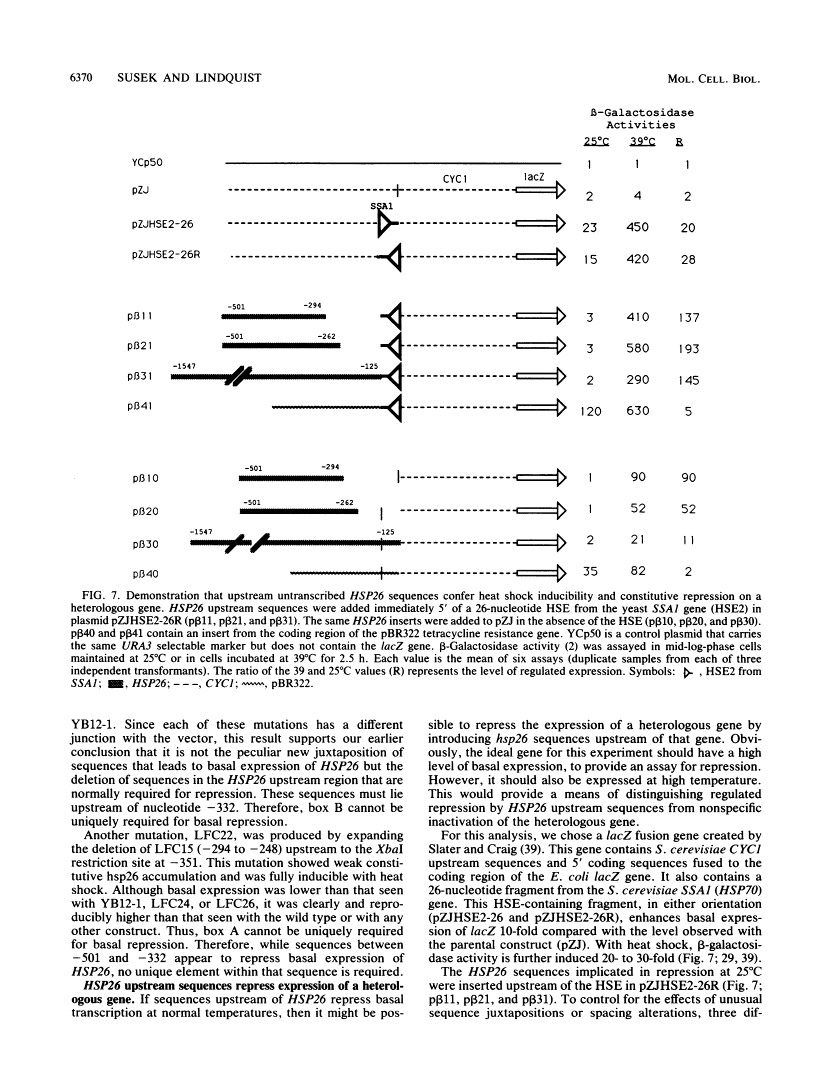
Abstract
Free full text

Transcriptional derepression of the Saccharomyces cerevisiae HSP26 gene during heat shock.
Abstract
hsp26, the small heat shock protein of Saccharomyces cerevisiae, accumulates in response to heat and other types of stress. It also accumulates during the normal course of development, as cells enter stationary phase growth or begin to sporulate (S. Kurtz, J. Rossi, L. Petko, and S. Lindquist, Science 231:1154-1157, 1986). Analysis of deletion and insertion mutations demonstrated that transcriptional control plays a critical role in regulating HSP26 expression. The HSP26 promoter was found to be complex and appears to contain repressing elements as well as activating elements. Several upstream deletion mutations resulted in strong constitutive expression of HSP26. Furthermore, upstream sequences from the HSP26 gene repressed the constitutive expression of a heterologous heat shock gene. We propose that basal repression and heat-induced depression of transcription play major roles in regulating the expression of HSP26. None of the recombinant constructs that we analyzed separated cis-regulatory sequences responsible for heat shock regulation from those responsible for developmental regulation of HSP26. Depression of HSP26 transcription may be the general mechanism of HSP26 induction in yeast cells. This regulatory scheme is very different from that described for the regulation of most other heat shock genes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
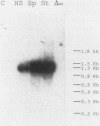
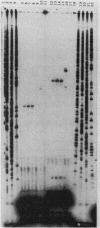
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J, Ananthan J, Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol. 1988 Sep;8(9):3761–3769. [Europe PMC free article] [Abstract] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen RS, Meselson M. Separate regulatory elements for the heat-inducible and ovarian expression of the Drosophila hsp26 gene. Cell. 1985 Dec;43(3 Pt 2):737–746. [Abstract] [Google Scholar]
- Corces V, Pellicer A. Identification of sequences involved in the transcriptional control of a Drosophila heat-shock gene. J Biol Chem. 1984 Dec 10;259(23):14812–14817. [Abstract] [Google Scholar]
- Glaser RL, Lis JT. Multiple, compensatory regulatory elements specify spermatocyte-specific expression of the Drosophila melanogaster hsp26 gene. Mol Cell Biol. 1990 Jan;10(1):131–137. [Europe PMC free article] [Abstract] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. [Abstract] [Google Scholar]
- Henikoff S, Eghtedarzadeh MK. Conserved arrangement of nested genes at the Drosophila Gart locus. Genetics. 1987 Dec;117(4):711–725. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoffman EP, Gerring SL, Corces VG. The ovarian, ecdysterone, and heat-shock-responsive promoters of the Drosophila melanogaster hsp27 gene react very differently to perturbations of DNA sequence. Mol Cell Biol. 1987 Mar;7(3):973–981. [Europe PMC free article] [Abstract] [Google Scholar]
- Hultmark D, Klemenz R, Gehring WJ. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. [Abstract] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. [Europe PMC free article] [Abstract] [Google Scholar]
- Jakobsen BK, Pelham HR. Constitutive binding of yeast heat shock factor to DNA in vivo. Mol Cell Biol. 1988 Nov;8(11):5040–5042. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnston M, Davis RW. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelly SE, Cartwright IL. Perturbation of chromatin architecture on ecdysterone induction of Drosophila melanogaster small heat shock protein genes. Mol Cell Biol. 1989 Jan;9(1):332–335. [Europe PMC free article] [Abstract] [Google Scholar]
- Kingston RE, Schuetz TJ, Larin Z. Heat-inducible human factor that binds to a human hsp70 promoter. Mol Cell Biol. 1987 Apr;7(4):1530–1534. [Europe PMC free article] [Abstract] [Google Scholar]
- Klemenz R, Gehring WJ. Sequence requirement for expression of the Drosophila melanogaster heat shock protein hsp22 gene during heat shock and normal development. Mol Cell Biol. 1986 Jun;6(6):2011–2019. [Europe PMC free article] [Abstract] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurtz S, Rossi J, Petko L, Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. [Abstract] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. [Abstract] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. [Abstract] [Google Scholar]
- McDaniel D, Caplan AJ, Lee MS, Adams CC, Fishel BR, Gross DS, Garrard WT. Basal-level expression of the yeast HSP82 gene requires a heat shock regulatory element. Mol Cell Biol. 1989 Nov;9(11):4789–4798. [Europe PMC free article] [Abstract] [Google Scholar]
- McMaster GK, Carmichael GG. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. [Europe PMC free article] [Abstract] [Google Scholar]
- Munro S, Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. [Abstract] [Google Scholar]
- Park HO, Craig EA. Positive and negative regulation of basal expression of a yeast HSP70 gene. Mol Cell Biol. 1989 May;9(5):2025–2033. [Europe PMC free article] [Abstract] [Google Scholar]
- Parker CS, Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. [Abstract] [Google Scholar]
- Pelham HR. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. [Abstract] [Google Scholar]
- Perisic O, Xiao H, Lis JT. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. [Abstract] [Google Scholar]
- Petersen R, Lindquist S. The Drosophila hsp70 message is rapidly degraded at normal temperatures and stabilized by heat shock. Gene. 1988 Dec 10;72(1-2):161–168. [Abstract] [Google Scholar]
- Petko L, Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. [Abstract] [Google Scholar]
- Riddihough G, Pelham HR. Activation of the Drosophila hsp27 promoter by heat shock and by ecdysone involves independent and remote regulatory sequences. EMBO J. 1986 Jul;5(7):1653–1658. [Europe PMC free article] [Abstract] [Google Scholar]
- Rossi JM, Lindquist S. The intracellular location of yeast heat-shock protein 26 varies with metabolism. J Cell Biol. 1989 Feb;108(2):425–439. [Europe PMC free article] [Abstract] [Google Scholar]
- Rougvie AE, Lis JT. The RNA polymerase II molecule at the 5' end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988 Sep 9;54(6):795–804. [Abstract] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Slater MR, Craig EA. Transcriptional regulation of an hsp70 heat shock gene in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987 May;7(5):1906–1916. [Europe PMC free article] [Abstract] [Google Scholar]
- Sorger PK, Lewis MJ, Pelham HR. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. [Abstract] [Google Scholar]
- Sorger PK, Pelham HR. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987 Oct;6(10):3035–3041. [Europe PMC free article] [Abstract] [Google Scholar]
- Sorger PK, Pelham HR. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. [Abstract] [Google Scholar]
- Struhl K. Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell. 1987 May 8;49(3):295–297. [Abstract] [Google Scholar]
- Sumrada RA, Cooper TG. Ubiquitous upstream repression sequences control activation of the inducible arginase gene in yeast. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3997–4001. [Europe PMC free article] [Abstract] [Google Scholar]
- Susek RE, Lindquist SL. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. [Europe PMC free article] [Abstract] [Google Scholar]
- Werner-Washburne M, Becker J, Kosic-Smithers J, Craig EA. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. [Europe PMC free article] [Abstract] [Google Scholar]
- Wiederrecht G, Seto D, Parker CS. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988 Sep 9;54(6):841–853. [Abstract] [Google Scholar]
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984 Sep 6;311(5981):81–84. [Abstract] [Google Scholar]
- Xiao H, Lis JT. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. [Abstract] [Google Scholar]
- Zagursky RJ, Berman ML. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. [Abstract] [Google Scholar]
- Zimarino V, Tsai C, Wu C. Complex modes of heat shock factor activation. Mol Cell Biol. 1990 Feb;10(2):752–759. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimarino V, Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. Nature. 327(6124):727–730. [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.10.12.6362-6373.1990
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/10/12/6362
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/10/12/6362
Citations & impact
Impact metrics
Citations of article over time
Article citations
Phosphorylation activates the yeast small heat shock protein Hsp26 by weakening domain contacts in the oligomer ensemble.
Nat Commun, 12(1):6697, 18 Nov 2021
Cited by: 9 articles | PMID: 34795272 | PMCID: PMC8602628
Linking the dynamics of chromatin occupancy and transcription with predictive models.
Genome Res, 31(6):1035-1046, 23 Apr 2021
Cited by: 7 articles | PMID: 33893157 | PMCID: PMC8168580
Assessing regulatory features of the current transcriptional network of Saccharomyces cerevisiae.
Sci Rep, 10(1):17744, 20 Oct 2020
Cited by: 5 articles | PMID: 33082399 | PMCID: PMC7575604
A chemical compound inhibiting the Aha1-Hsp90 chaperone complex.
J Biol Chem, 292(41):17073-17083, 28 Aug 2017
Cited by: 23 articles | PMID: 28851842 | PMCID: PMC5641884
Biodegradation of zearalenone by Saccharomyces cerevisiae: Possible involvement of ZEN responsive proteins of the yeast.
J Proteomics, 143:416-423, 22 Apr 2016
Cited by: 21 articles | PMID: 27109348
Go to all (28) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
(CT)n (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene.
Mol Cell Biol, 13(5):2802-2814, 01 May 1993
Cited by: 154 articles | PMID: 8474442 | PMCID: PMC359663
Evidence for a heat shock transcription factor-independent mechanism for heat shock induction of transcription in Saccharomyces cerevisiae.
Proc Natl Acad Sci U S A, 87(17):6550-6554, 01 Sep 1990
Cited by: 35 articles | PMID: 2118651 | PMCID: PMC54574
Structure and regulation of the SSA4 HSP70 gene of Saccharomyces cerevisiae.
J Biol Chem, 265(31):18912-18921, 01 Nov 1990
Cited by: 57 articles | PMID: 2121731
A distal heat shock element promotes the rapid response to heat shock of the HSP26 gene in the yeast Saccharomyces cerevisiae.
J Biol Chem, 268(10):7442-7448, 01 Apr 1993
Cited by: 22 articles | PMID: 8463277
Funding
Funders who supported this work.
NIGMS NIH HHS (2)
Grant ID: GM07183
Grant ID: GM35482