Abstract
Free full text

The role of fibrosis in Duchenne muscular dystrophy
Abstract
Muscular dystrophies such as Duchenne muscular dystrophy (DMD) are usually approached as dysfunctions of the affected skeletal myofibres and their force transmission. Comparatively little attention has been given to the increase in connective tissue (fibrosis) which accompanies these muscular changes. Interestingly, an increase in endomysial tissue is apparent long before any muscular degeneration can be observed. Fibrosis is the result of a reactive or reparative process involving mechanical, humoral and cellular factors. Originating from vulnerable myofibres, muscle cell necrosis and inflammatory processes are present in DMD. Muscular recovery is limited due to the limited number and capacity of satellite cells. Hence, a proactive and multimodal approach is necessary in order to activate protective mechanisms and to hinder catabolic and tissue degrading pathways.
Several avenues are discussed in terms of potential antifibrotic therapy approaches. These include pharmaceutical, nutritional, exercise-based and other mechanostimulatory modalities (such as massage or yoga-like stretching) with the intention of exerting an anti-inflammatory and antifibrotic effect on the affected muscular tissues. A preventive intervention at an early age is crucial, based on the early and seemingly non-reversible nature of the fibrotic tissue changes. Since consistent assessment is essential, different measurement technologies are discussed.
Evidence for fibrotic tissue changes in DMD
Most of the emphasis in muscular dystrophies – in research as well as treatment – has been on the degeneration of skeletal muscle fibres. Comparatively little attention has been given to the pathogenesis of the well-developed fibrosis and fat replacement in the affected muscle tissues. Possibly this lack of attention would have been different if the suggestion of Guillaume-Benjamin Amand Duchenne to call the respective pathology 'paralytic myosclerosis' (1) would have been taken over. In fact this type of pathology had been called 'pseudohypertrophic muscular dystrophy' for several decades prior to the suggestion of John Walton and Frederick Nattrass in 1954 to use the term 'Duchenne dystrophy' which has now become the prevailing fashion (2).
It is generally assumed that the proliferation of connective tissue is a secondary phenomenon, and many physicians regard it simply as a compensatory replacement of lost muscle. However, even several decades ago several researchers reported on an increase in endomysial tissue volume before any apparent muscle degeneration. They proposed that the prolific increase of collagenous connective tissue could be a significant co-determining factor in this myofascial pathology, possibly including an adverse effect on the nutrition of the enclosed muscle cells (3, 4).
A longitudinal study of 25 DMD patients with an average follow-up time of over 10 years examined the correlation of the severity of the pathology and different pathological features, including myofibre atrophy, necrosis, and fatty degeneration. Severity was gauged by muscle strength and age at loss of ambulation. The study concluded that endomysial fibrosis was the only myopathologic parameter that significantly correlated with poor motor outcome (5).
Muscle tissue has only limited potential for recovery. In DMD, constant myofibre breakdown cannot be fully compensated for by satellite cell proliferation. Inflammatory processes following muscular necrosis lead to fibrotic remodelling and finally fatty cell replacement. In DMD this phenomenon is often first seen in the posterior calf musculature, which is prone to overtraining because of its function as anti-gravity stabilizer (Fig. 1).
Extracellular matrix (ECM) as an overlooked factor
The recent shift in attention towards the role of connective tissue in muscular dystrophies is not a singular phenomenon. Similar developments are now being observed with other myopathies. While the focus in neuromuscular research has long been the myofibrils, the cytoskeleton and the cell membrane, the attention has gradually shifted towards the ECM. This shift was based on the growing recognition that the ECM is an extremely dynamic complex of molecules that closely interacts with sarcolemmal, nuclear and cytoskeletal elements (6).
The architecture of the ECM can roughly be compared to composite plastics in material science, with a gelatinous ground substance (made up of glycoproteins and proteoglycans) being reinforced by stiffer fibrous proteins. This matrix builds a supramolecular network that can transmit contractile muscle forces while maintaining tissue integrity. It provides intramuscular continuations of neurovascular tracts in which blood vessels and nerve branches are embedded. In addition, this integral matrix mediates the development and physiological behaviour of muscle cells. While in the past the ECM had been regarded as amorphous scaffolding for providing mechanical support, recent findings emphasise the crucial importance of the ECM in transmembrane signalling as well as in developmental and regenerative processes (6, 7). The ECM is now increasingly being recognised as a very dynamic structure that constantly modifies its viscoelastic properties and adapts to changes in physiological as well as mechanical demands (8).
Detailed analysis of the importance of epimuscular force transmission has also lead to significant improvements in the understanding and treatment of spastic pareses. While in the past the focus was mainly on the 'primary cause', e.g. altered neuromuscular interaction, recent investigations have revealed that clinical behaviour in these children is often more crucially influenced by additional changes observed within the muscular connective tissues. Recognition of an often dramatically increased epimuscular force transmission to antagonistic muscles (via increased endomysial and perimysial cross links) has now lead to encouraging surgical advances in this field (9, 10).
It is therefore hardly surprising that an overlap between myopathies and connective tissue diseases is increasingly being recognised, involving their molecular dynamics as well as clinical expression (6). For example the involvement of collagen type VI has been demonstrated for many congenital muscular dystrophies, as reviewed by Schessl et al. (11). We therefore suggest that the following observations and recommendations regarding a stronger inclusion of connective tissue components in muscular dystrophies may be viewed as part of a similar shift of attention within the larger field of neuromuscular research.
Fibrosis in DMD
Generally, fibrosis is referred to as the replacement of normal tissue with scar tissue. This means that fibrous connective tissue is the result of a reactive or reparative process. The following paragraph explains the link between the primary muscle disease DMD and fibrosis.
The genetic cause of DMD is an x-chromosomal mutation of the dystrophin gene. Dystrophin mechanically stabilises myofibres by linking the cytoskeleton to the basal lamina through the dystroglycan complex. Dysfunction or lack of dystrophin leads to instability of muscle fibre membranes. As a result, the cells are less resistant to mechanical shear and prone to excess influx of electrolytes such as calcium and sodium. An increase of intracellular sodium leads to ATP depletion, because Na+/K+ pumps need to operate at full capacity. Water molecules accompanying sodium produce cellular oedema. At rest the extracellular calcium ion concentration exceeds the cytoplasmic calcium ion concentration by a factor of 10,000. This gradient promotes calcium overload, resulting in mitochondrial uncoupling and the production of reactive oxygen species (ROS) such as oxygen ions and peroxides. The biochemical downstream effects are the accumulation of acidic metabolites and the amplification of inflammatory substances such as cytokines (Fig. 2).
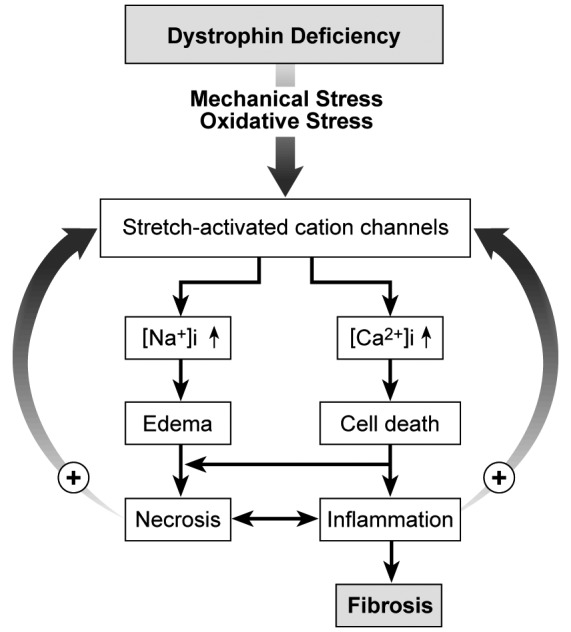
Flowchart: from dystrophin deficiency to fibrosis.
Membranes lacking the dystroglycan complex are intrinsically vulnerable to mechanical and oxidative stress. Overstrain and overuse lead to activation of stretch-activated cation channels. Influx of sodium and calcium cannot be compensated for by pumps regulating electrolyte balance. Cellular oedema and calcium overload cause depletion of energy supply. Calcium acts as a second messenger and activates a cascade of inflammatory processes. In terms of a vicious cycle, further stress on muscle cell membranes and activation of ion channels is inevitable. Finally, the cellular integrity is unsustainable. Myofibre necrosis and inflammation lead to fibrotic tissue remodelling.
Lactic acid was considered the key element in acidosis- induced tissue damage until the 1970s. However, lactic acid is more than 99% dissociated into La– and H+ at physiological pH. La– increases collagen promoter activity leading to an increase of procollagen messenger RNA production and finally collagen synthesis. Furthermore, La– enhances angiogenesis via stimulation of vascular endothelial growth factor (VEGF) production in macrophages (12).
Chemotaxis and mechanical stimuli initiate the production of fibrous tissue. It is composed of extracellular fluid containing fibronectin, glycosaminoglycans and proteoglycans. These molecules can bind to water and thereby influence the mechanic properties of the tissue. The ECM also contains collagen fibres, collagen sheets and to a lesser extent elastin. The cells found in fibrous tissue are mainly fibrocytes and myofibroblasts. Both cell types are in smooth transition depending on the amount of contractile filaments. The contractile properties are mainly based on α-smooth muscle actin (α-SMA). Precursor cells differentiate into these cell types through various stimulators. One of them is the transforming growth factor-β1 (TGF-β1), which also promotes the build-up of ground substance as well as regulating expression of catabolic enzymes and other mediators (13). Recently, it has been shown in an animal model that down-regulation of TGF-β1 is preventive for fibrosis (14, 15).
Interestingly the direction of strain on (myo)fibroblasts is decisive for the excretion of humoral and chemotactic substances. Significant higher release of interleukin-6 (IL6) and macrophage derived chemokine (MDC) were found in fibroblasts which have been strained heterobiaxially in comparison to non-strained and/or equibiaxially challenged cells. IL6 does not directly modulate collagenase activity, but it does induce the synthesis of a tissue inhibitor of metalloproteinases. Under inflammatory conditions these proteinases are up-regulated in connective tissue. In other words, irregular strain such as in injury leads to IL6 production, which balances the connective tissue degrading enzymes. In heterobiaxially strained cells, there is also a trend towards increased production of nitric oxide (NO), which is an important neurotransmitter and vasodilator (16).
Insulin-like growth factor (IGF) is a key element in controlling tissue activity not only during childhood growth but also in tissue repair and diseases like neoplastic cell growth. IGFs bind to cell surface receptors and to IGF-binding proteins, which themselves are powerful regulators of myofibroblast and satellite cell proliferation. Connective tissue growth factor (CTGF) also binds to IGF-binding proteins as well as to members of the TGFβ family such as fibronectin and probably also myostatin. CTGF is associated with virtually all fibrotic remodelling. CTGF activity correlates with fibrotic activity in several tissues. Endothelin-1 induces CTGF expression in (myo)fibroblasts (17).
Chronic tissue contracture is generated by a combination of cellular contraction and collagen fibre remodelling (Fig. 3). Myofibroblasts actively contract via a calcium- dependent phosphorylation of the myosin light chain. Myofibroblasts exhibit spontaneous calcium oscillations, which are linked to mechanical force transmission. There is a second mode of contraction, which is based on a rhokinase- mediated inhibition of myosin dephosphorylation. This pathway is calcium-independent and accounts for long-lasting contractures. In summary, these cells can produce either smooth muscle cell like contractions in the range of several minutes to hours, but also long lasting and even irreversible contractures. In terms of a lockstep mechanism, the contracture is highly energy efficient (18).
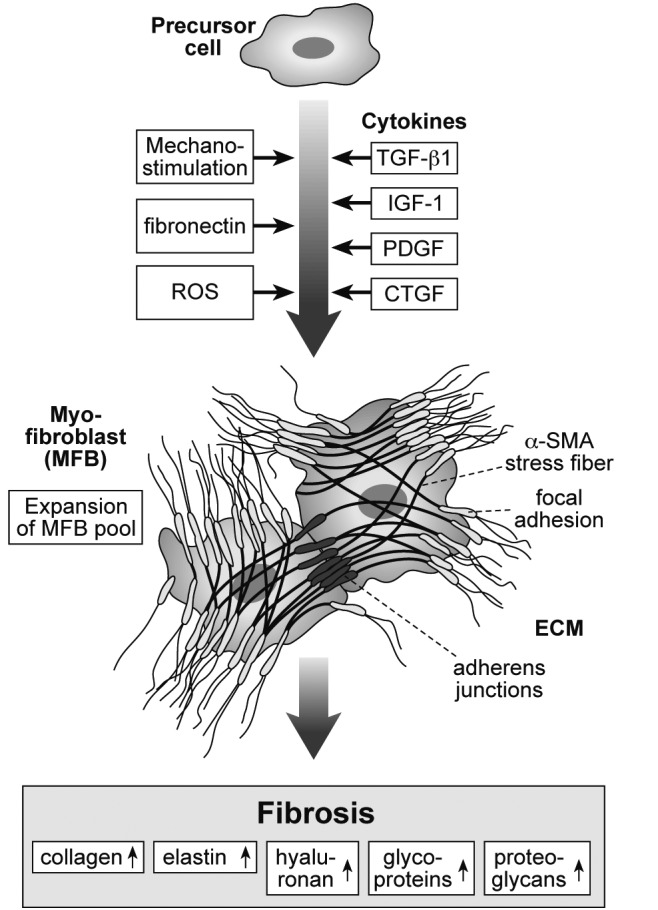
Fibroblasts as the major mechanoresponsive cells in connective tissue.
Mechanostimulation, fibronectin and/or chemical stimuli like reactive oxygen species (ROS) activate fibroblast differentiation from precursor cells, protein expression and transition to mobile fibrocytes and myofibroblasts which are characterised by contractile filaments (α-smooth muscle actin, α-SMA stress fibres). Mechanical stability is generated by focal adhesions which anchor the fibres to cell membrane. Several growth factors which stimulate synthesis of collagen and concomitant compounds are also expressed in response to mechanical loading. The most important examples are transforming growth factor-ß-1 (TGFß-1), insulin-like growth factor-I (IGF-I), platelet-derived growth factor (PDGF) and connective tissue growth factor (CTGF). These factors also induce proliferation of fibroblasts and myofibroblasts which connect via irregular extensions and also adherent junctions. The contractile properties are substantial for fibrotic tissue shrinkage. These mechanisms finally result in increased production of collagen, elastin, hyaluronan, glycoproteins and proteoglycans. Remodelling of the extracellular matrix (ECM) leads to fibrotic conversion of connective tissue and later fatty involution.
Fibrocytes and myofibroblasts usually are present for a time during tissue repair, such as in wound healing. In the case of DMD, activation of myofibroblasts is persistent due to constant myofibre breakdown. This results in an altered production of ECM. The provisional ECM in fibrosis is different in composition from the ECM in normal tissue, and its components originate mainly from myofibroblasts. In the early stage of fibrosis, the relative content of fibronectin and hyaluronan is high in comparison to non-injured tissue. This microenvironment creates a very hydrated matrix and facilitates cell migration. Later this provisional matrix is replaced with an ECM containing a more dense ultrastructure (19).
Muscle oedema in DMD and its reduction with eplerenone
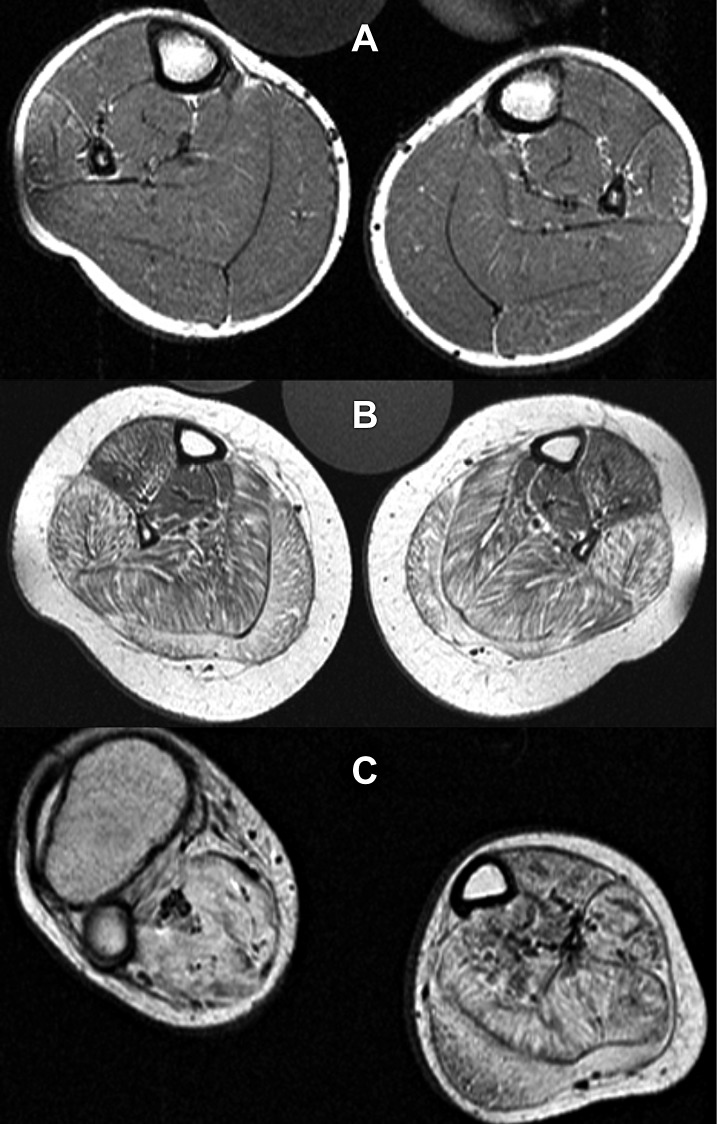
Previously muscle oedema was reported for DMD that was widely attributed to an interstitial inflammation (20). Recently oedema was regularly observed in all DMD boys as long as muscle tissue has not been completely replaced by fat and fibrosis (21). The oedema was already markedly visible at an age at which fatty degeneration is still absent (Fig. 4). Some of us showed that the oedema persisted at follow-up (22) and was mainly caused by an elevated cytoplasmic Na+ concentration. Therefore the oedema seems mainly to be of osmotic origin and may contribute to fibre necrosis and finally to fibrosis.

Intracellular water and sodium accumulation in DMD muscle.
Calves of a 5 year old DMD boy (left panels) and a 5 year old healthy volunteer (right): conventional T1-weighted 1H-MR images (upper panels); short tau inversion recovery (STIR). 1H-MR images (middle panels), and 23Na inversion recovery MR images (Na-IR, lower panels). Neither the DMD boy nor the healthy volunteer showed fatty degeneration of the triceps surae muscles. However, an oedema is visible in the calf muscles of the DMD boy that is pronounced in the soleus muscles. The 23Na IR showed an elevated signal in the calf muscles of the DMD boy compared to the volunteer. Note that the signal of the reference tube containing free 51.3 mM Na+ solution (asterisks) is suppressed in the 23Na IR sequence, while the contralateral reference tube in which 51.3 mM Na+ is trapped in 5% agarose gel (circle) is quite visible. Modified after Weber et al. (22).
The ligands of the mineralocorticoid receptor have been known for a long time to regulate sodium-potassium homeostasis by transcriptional and translational effects on genes encoding the Na+/K+ ATPase (23) and the epithelial sodium channel, ENaC. Recently additional nongenomic effects of the ligands of the mineralocorticoid receptor in skeletal muscle via kinases have been reported (24). Similarly to the heart muscle (25), the mechanism to reduce sodium overload in skeletal muscle might be the sodium proton exchanger (NHE).
This regulation contributes to the beneficial effects of the aldosterone antagonist spironolactone, which preserved cardiac and skeletal muscle function in mdx mice (26). As the more specific aldosterone antagonist eplerenone shows a reduced affinity to progesterone and androgen receptors, this drug might be more appropriate as a DMD treatment. Indeed administration to a severely affected female DMD patient resulted in a reduction in the strikingly increased cytoplasmic sodium and water signals as well as increased strength and mobility (27).
Therapeutic avenues
Antifibrotic drugs
Several avenues appear promising in antifibrotic therapy. The potential use of anti-inflammatory drugs – such as TNFα drugs, inhibitors of NF-κB, steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) as well as broader anti-inflammatories like flavocoxid – has recently been well reviewed by De Luca (28) and previously by Zhou (29). The same applies to the potential use of antioxidants such as antagonist of the effects of angiotensin-II (example: losartan) and substances like Nacetyl- cystein, epigallocatechin gallate (found in green tea), idebenone, resveratrol and BN82270. In terms of further antifibrotic effects, drugs countering myostatin such as formeterol are promising candidates as well as the collagen type 1 synthesis inhibitor halofunginone. In addition, substances that enhance NO signalling such as sildenafil or poloxamer 188 are currently being investigated as potential future antifibrotic medications (28, 30-32). Activation of cyclic AMP via forskolin has recently been indicated as a promising antifibrotic avenue (33). Application of this approach to DMD remains to be explored. Suramin, a TGF β1 blocker, effectively prevents muscle fibrosis in mdx mice (14).
Eplerenone
According to GeneNote, mineralocorticoid aldosterone receptor expression is not specific to the kidney but is instead found in many tissues including cardiac, skeletal, and smooth muscle. Additionally, aldosterone appears to not only be synthesised in the adrenal gland but also locally in other organs such as the heart (34). In the heart, it upregulates proinflammatory molecules such as growth factor TGF-β, adhesion molecules, and plasminogen activator inhibitors through mechanisms that are both dependent on and independent from the mineralocorticoid receptor, e.g. by osteopontin upregulation or through NF-κB activation (reviewed in 35). Additionally, aldosterone increases reactive oxygen species through NADPH oxidase activation (36, 37). Taken together, aldosterone induces inflammation and oxidative stress. Finally, aldosterone can induce fibrosis, an effect that can be inhibited by blocking the mineralocorticoid receptor with spironolactone (reviewed in 38). In skeletal muscle, a combination of an angiotensine-converting enzyme (ACE) inhibitor and spironolactone prevented fibrosis in a phenotypically very mild mouse model of Duchenne dystrophy (26). However, the many hormone side effects make it illadapted for the treatment of children. Eplerenone as a specific mineralcorticoid antagonist without the side effects of spironolactone has been shown to also inhibit fibrosis in tissues such as blood vessels, the heart and other internal organs (39, 40). Its use in Duchenne dystrophy is appealing and the single Duchenne patient in a very advanced disease stage that has been treated with eplerenone to date indeed showed increased mobility and strength (27).
Nutrition
While epigallocatechin gallate (green tea extract) seems to have promising antifibrotic effects, much higher concentrations than are usually used for food supplements seem to be required (40). Whether an anti-inflammatory diet – often involving omega-3 fatty acids and curcumin – may be able to provide significant effects on fibrosis remains to be clarified (41). Supplementation with L-arginine has been shown to induce anti-inflammatory effects in mdx mice (42). However, there are indications that a combination of anti-inflammatory nutrition and appropriately calibrated exercise may trigger stronger effects than predicted by the summation of both components (43).
Temperature
It is possible that temperature modifications could be incorporated as part of future antifibrotic therapy with DMD patients. A recent investigation of the effects of temperature on fatigability showed that application of hyperthermia (35 °C) resulted in increased muscle fatigability in mdx mice (compared with controls), while no difference in fatigability between mdx and wild type mice was found at 20 °C (44). This finding appears to be in congruence with common clinical recommendations for DMD patients to avoid hyperthermia during muscular activity.
Whereas the effects of temperature on inflammatory dynamics have been more extensively explored, additional studies have demonstrated significant effects of temperature changes on wound healing (45, 46). It seems relevant – particularly in relation to the above-explained cytokine dynamics involved in fibrosis – that application of hypothermia has been shown to exert inhibitory effects on wound healing in rats, and that these changes are associated with a delayed expression of TGF-β1 by macrophages (47).
As is often reported in the public media, application of hypothermia is currently being explored as a promising intervention to support recovery from overuse injuries in sports as well as from other types of tissue challenges (48-50). In this respect the findings by Frink et al. (50), who explored the application of hypothermia after various trauma models, provide important insights. It reports that the application of hypothermia resulted in a decrease in pro-inflammatory cytokines as well as chemokines and adhesion molecules, in addition to an increased anti-inflammatory cytokine response.
It would be of interest to clarify whether some of these effects could be utilised for a hypothermia-enhanced treatment of fibrosis. In fact, cryotherapy has already been successfully applied in the treatment of hypertrophic scars (51). Further studies are therefore recommended to explore the potential antifibrotic effects of hypothermia in muscular dystrophies.
Exercise
While vigorous exercise can induce detrimental effects on muscle fibres in DMD patients (52), there are several indications, which suggest that moderate, and adequately tailored exercise may be beneficial. Call et al. (43) showed that daily voluntary running enhanced endurance capacity in mdx mice and that this improvement was associated with an attenuation of oxidative stress. Interestingly the addition of antioxidant green tea extract had similar beneficial effects, while a combination of running and green tee extract had even larger effects. Ambrosio et al. (53) reported an antifibrotic effect of treadmill running in normal mice recovering from muscle damage. Extensive research with healthy humans has shown that adequately calibrated exercise can increase the release of IGF-1 and decrease myostatin levels (54, 55). As explained earlier, both of these cytokine modifications would tend to foster antifibrotic effects.
Further studies are needed to explore the potential application of exercise in human DMD patients. Concerning the choice and adequate calibration of potential exercise loading, the study by Kern et al. (56) may be of interest which indicated that vibrational-proprioceptive exercise tends to induce a much stronger increase of IGF-1 and myostatin inhibition when compared with isokinetic exercise. Another recent study showed that uphill running induced adaptive effects on collagen tissues, consisting mainly of an increase of IGF-1 and decrease of TGF-β1 expression, while avoiding pathological changes often associated with regular or downhill running (57). While this effect was explained by the dominantly concentric muscular contraction mode, treadmill running may not always be appropriate for moderate and advanced stages of DMD. Use of concentric cycle ergometers, however, could probably achieve similar effects (58, 59).
Molecular microdialysis investigations suggest that exercise loading in healthy athletes is accompanied with an increased likelihood of overuse injury in the affected connective tissues if the same type of loading is repeated on a daily basis. However, if the exercise regimen is performed with two or three 'recovery days' in between, healthier collagen remodelling can be observed (60). If a similar response pattern can also be confirmed in DMD patients, then a helpful model could be provided for the prescription of exercise regimens in these patients.
The authors agree with Markert et al. (61) that if exercise is advised for DMD patients, it should be confirmed that antioxidant activity levels remain elevated afterwards. It would therefore be useful to determine which of the available measurement technologies is most practical and affordable while still sufficiently specific for this assessment (62-64).
Additional mechanostimulatory modalities
Massage treatments have long been used in the treatment of hypertrophic scars, however no substantial evidence had been provided about their effectiveness (65). A recent animal study has now convincingly demonstrated that myofascial massage is able to effectively decrease and prevent post-surgical visceral adhesions in rats (66). This finding appears to be in congruence with the cell culture studies from Standley et al. (67), which indicate that a gentle massage-like stimulation of fibroblasts elicits an anti-inflammatory cytokine expression. Additional in vitro examinations from the same group (68) showed that a massage-like treatment, applied after a repetitive strain overloading challenge, induced a significant reduction in cellular apoptosis accompanied with a decrease in deathrelated protein kinase (DAPK-2).
Can these encouraging findings be extrapolated to massage-like treatments with humans? A new clinical massage study – using for the first time biopsies from healthy human patients – suggests a positive answer: a 10 minute massage treatment applied to 11 young males, performed after exercise induced muscle damage, was shown to activate the mechanotransduction signalling pathways focal adhesion kinase (FAK) and extracellular signal-regulated kinase ½ (ERK1/2). It potentiated mitochondrial biogenesis signalling and altered the behaviour of NF-κB, causing less of this key inflammatory mediator to accumulate in the nucleus. Consequently, the NF-κBregulated heat shock proteins and immune cytokines IL-6 and TNF-α were less active, a sign of less cellular stress and inflammation (69).
While massage has commonly been advised for other purposes in the field of musculoskeletal medicine, these newly reported anti-inflammatory effects suggest that massage therapy could possibly be used as an alternative to NSAIDs in some circumstances. Further studies are nevertheless needed for clarification as to whether such massage-induced anti-inflammatory effects can be utilised in the treatment of fibrotic pathologies such as in muscular dystrophies.
If mechanostimulation provides an important access point for influencing fibrosis development, then mechanical traction, such as in therapeutic stretching, could possibly be used as well. A rodent study using in vivo as well as ex vivo examination of a 10 minute static stretch application showed that this stretch attenuated the increase in both soluble TGF-β1 (ex vivo) and type-1 procollagen (in vivo) following tissue injury. The investigators interpreted this as an indication for a potential anti-fibrotic effect of static stretching (70). A subsequent in vivo study by the same group, also conducted with rodents, showed that a yogalike stretch application of 10 minutes – conducted twice a day over a period of 12 days – clearly decreased the inflammatory responses induced by previous injection of carragean into the subcutaneous connective tissues. Congruently this response included a reduction of macrophage expression in the respective soft connective tissues (71).
Further research is required to clarify to what extend (if at all) the reported anti-inflammatory and/or antifibrotic effects of massage or stretching can be applied in DMD patients. Nevertheless, for potential future clinical application the following considerations can already be made concerning the adequate sequencing of these modalities, based on the above reported insights regarding general physiological dynamics around thermostimulation and mechanostimulation. While active exercise, such as with a bicycle ergometer or with a vibrational-proprioceptive tool, may accordingly be applied every second or third day only, we suggest that this could be preceded with a few minutes of slow and gentle yoga-like static stretching immediately before, with the added benefit of a temporarily increased mobility due to viscoelastic deformation (72). Hypothermia may then be applied immediately after exercise, while high dosages of anti-inflammatory nutrients such as green tea extract may be taken as additional support on these exercise days. If available, massage treatments could then be added on 'non exercise days' in order to complement the overall antifibrotic treatment. However, any exercise regimen should be accompanied by regular assessment of antioxidant stress levels as well as the on-going development of fibrotic tissue changes (see below).
General considerations for clinical treatment
Fibrosis in DMD is a complex cascade involving mechanical, humoral and cellular factors. Originating from wounded myofibres, muscle cell necrosis and inflammatory processes are present in DMD. Muscular recovery is limited due to the limited number and capacity of satellite cells. Hence, a proactive and multimodal approach is necessary in order to activate protective mechanisms and to hinder catabolic and tissue degrading pathways.
Fibrotic changes in muscle are not confined to DMD muscle. Back in the 1980s, Michelsson developed an animal model to study the effects that develop after forceful exercise of immobilised limbs. He concluded that autoinflammatory processes lead to myositis and secondarily to fibrosis and even calcification with heterotopic bone formation (73). Two main points can be extracted for DMD. First of all, immobilisation is not advisable and second forceful overstrain should be avoided.
Corticosteroids are frequently used in myofascial inflammation. Corticosteroids generally reduce gene expression and inhibit the proliferation and activity of myofibroblasts, which mainly leads to suppression of collagen production. Additionally myofibroblast migration, which is fundamental in fibrosis, is delayed after corticosteroid injection. These corticoid-associated disturbances on tendon cell metabolism may affect the structural integrity of the tendon and weaken its mechanical properties. There are many side effects of corticosteroids, which include a risk of diabetes, disturbance of hormonal glands and metabolism, suppression of angioneogenesis, immune function and coagulation. Hence, therapeutic protocols that lower the use of corticosteroids are desirable.
Stiffening of pulmonary and pericardial connective tissues
Mortality in DMD patients is often due to respiratory or cardiac problems. In both body areas – the pulmonary and the pericardial connective tissues – the fibrotic changes in muscular dystrophy tend to be very severely expressed and they tend to influence strongly muscular function (74, 75). An increased stiffening of related tissues can impair muscular function. Pulmonary fibrosis, for example, can strongly impair respiratory functioning by increasing stiffness and decreasing the elasticity of bronchial connective tissues (76). Development of antifibrotic therapies should therefore place a special focus on their efficacy in relation to pulmonary and pericardial fibrosis. The reported successful application of sildenafil on reversing cardiac dysfunction in mdx mice (29) is certainly encouraging, although extrapolation of these findings to human muscular dystrophies needs to be further investigated. Of similar interest are the recent investigations by Nelson et al. (77) in which the application of 1D11 (a neutralising antibody to all three isoforms of TGF-β) significantly improved respiratory function in mdx mice.
While results from mdx mice are not always representative of the usually more severely developed dystrophic pathology in DMD, these preliminary findings suggest that an antifibrotic therapy could be helpful in enhancing life expectancy in human patients, in particular if they result in an attenuation of the fibrotic stiffening of pulmonary and pericardial connective tissues.
It may be tempting to explore therapeutic use of cold air inhalation, in which the air is enriched with antifibrotic agents – possibly during exercise and/or during sleep. However, the exploration of this direction should take into account that inhalation of air that is excessively cold can also lead to bronchoconstriction, particularly if the air used is low in humidity (78).
Use of adequate assessment technologies
MR imaging
Conventional hydrogen magnetic resonance imaging (1H-MRI) is a non-invasive in-vivo method without ionising radiation. Dependent on the sequences, it has the ability to resolve muscle, fat, connective tissue and bone. Lying still for 15 minutes is required, which most children after the age of 5 are capable of. According to our experience, children are not afraid of the examination if their head and chest can remain outside the MRI tube (21).
T1-weighted images detect structural alterations and provide a qualitative assessment of skeletal muscle, but cannot differentiate between fat and water. A visual fourpoint semi-quantitative scale rates the intramuscular fat using the intensity of subcutaneous fat as reference for lipomatous degeneration (79). Implementation of a fatsuppression procedure during a spin echo sequence (e.g., short-tau inversion recovery imaging) suppresses the signal from adipose tissue, and thus the resulting signal is more sensitive to increased extracellular or intracellular water content, either consistent with an inflammatory oedema or with an osmotic oedema. The scale can be expanded with the evaluation of STIR MR images as follows (21):
Grade 1 – homogeneous, hypointense signal, contrasting sharply with subcutaneous and intermuscular fat (normal muscle, no oedema);
Grade 2 – slightly hyperintense, patchy intramuscular signal changes on STIR (< 50% of muscle crosssectional area);
Grade 3 – markedly hyperintense, patchy, but widespread intramuscular signal changes on STIR (> 50% of muscle cross-sectional area);
Grade 4 – homogeneous hyperintense signal in whole muscle on STIR (100% of muscle cross-sectional area).
Overall, skeletal muscle MRI is a powerful and sensitive technique in the evaluation of muscle disease, and its use as a biomarker for disease progression or therapeutic response in clinical trials deserves further study.
Bioelectric Impedance
In some circumstances measurement of electric impedance may be a suitable tool for the assessment of changes in extracellular or intracellular fluid in muscular tissues. Impedantometry has many advantages over radioisotopic methods as it is inexpensive, noninvasive, fast and portable. The electrical impedance of a given tissue is highly responsive to changes in water content, given that the amount of other conducting elements in the tissue remains constant. Besides the amount of water, also the location of water (extracellular or intracellular) influences the conductivity, which is then reflected in the electrical impedance (80, 81). While low frequency current passes mainly through extracellular tissue, higher frequency current penetrates cell membranes and tissue interfaces and passes through both intracellular and extracellular tissues. A comparison between both modalities can then permit assessment of respective changes in extracellular and intracellular water content (81, 82).
Such multifrequency impedance measurement has been shown to be sufficiently accurate when conducted under standardized clinical conditions and with eu-hydrated persons. However, as pointed out by O'Brien (83), changes in fluid and electrolyte content can independently affect electrical conductivity. Since some hydration changes may involve concomitant changes in fluid and in electrolyte content, the interpretation of a change in impedance could be confounded. To our knowledge the use of impedantometry with DMD patients has not yet been systematically evaluated. If it proves to be similar in accuracy to when conducted with eu-hydrated patients under standardised clinical conditions, then a future application in the assessment of the efficacy of administration of eplerenone (or similar substances that aim to alter intracellular water content) may become an appealing prospect.
Elastography
The development of fibrosis can be assessed via elastography. Here information about the stiffness of tissue is obtained by assessing the propagation of mechanical shear waves through the tissue with either ultrasound or magnetic resonance technology. The assessment involves three basic steps: (a) generating shear waves in the tissue, (b) acquiring MR or ultrasound imaging representations of the propagation of the induced shear waves, and (c) processing the images of the shear waves to generate quantitative maps of tissue stiffness, called elastograms. While MR elastography has been successfully applied in the assessment of liver fibrosis, additional applications for assessment of fibrotic changes in other tissues such as in bronchial connective tissues, kidney, breast, skeletal muscle and brain tissue are currently being investigated (84).
A major disadvantage of MR elastography is the high costs of this technology. Ultrasound elastography, a novel development for assessing tissue stiffness, is considerably more affordable. This technology demonstrates a high applicability for the assessment of muscular tissues located within a few centimetres of the skin (85). While a skilled physician with excellent palpatory skills may be able to detect and assess regions of increased tissue stiffness as well, this technology could be used to document the progression of muscular fibrosis (e.g. when conducted once per year) in a quantifiable manner (86). However, for assessment of the important fibrotic changes in bronchial and pericardic tissues in DMD patients, ultrasound elastography is not sufficient. For these purposes, MR elastography seems more suitable.
Myography
The development of passive tissue stiffness can also be assessed by biomechanical measurement of the tissue response to a calibrated indentation. Several portable tools are available that claim to measure muscle viscoelastic properties by simulated palpation through the skin (87). The newly developed MyotonPRO seems most promising, as it contains a highly sensitive triaxial accelerometer and its basic technology has been successfully tested in conditions such as Parkinson's disease and stroke (88, 89). While a previous model (Myoton-2) had been examined as a reliable tool for measuring muscle viscoelastic stiffness in healthy adults (90), the reliability of this improved version still needs to be properly examined, particularly with a wider range of patients and tissue properties, including DMD patients with different disease severities. Only tissues close to the skin can be examined; deeper connective tissues such as from deeper muscles or around the lungs and heart cannot be reached with this tool. Advantages of this technology include its portability, affordability and ease of application.
Conclusions
While muscular dystrophies have mostly been approached as a dysfunction of skeletal myofibres, the accompanying changes in the connective tissue deserve specific interest as well. An increased understanding of the molecular dynamics underlying inflammation, myofibroblast proliferation, tissue contracture and fibrosis will help in the development of future antifibrotic therapy approaches. A proactive early intervention is recommended, with particular emphasis being given to the pulmonary and pericardial connective tissues. In order to monitor the fibrotic tissue changes, the investigation and further development of appropriate assessment tools seems essential.
Acknowledgements
We thank Dr. Reinhardt Rüdel for his active involvement in the generation of this review und Dr. Heike Jäger for discussions. Frank Lehmann-Horn is endowed Senior Research Professor for Neurosciences of the non-profit Hertie Foundation.
References
Articles from Acta Myologica are provided here courtesy of Pacini Editore
Citations & impact
Impact metrics
Article citations
Magnetic Resonance Imaging Biomarkers of Muscle.
Tomography, 10(9):1411-1438, 02 Sep 2024
Cited by: 0 articles | PMID: 39330752 | PMCID: PMC11436019
Review Free full text in Europe PMC
Benfotiamine improves dystrophic pathology and exercise capacity in mdx mice by reducing inflammation and fibrosis.
Hum Mol Genet, 33(15):1339-1355, 01 Jul 2024
Cited by: 0 articles | PMID: 38710523 | PMCID: PMC11262745
The Role of MicroRNA in the Pathogenesis of Duchenne Muscular Dystrophy.
Int J Mol Sci, 25(11):6108, 01 Jun 2024
Cited by: 2 articles | PMID: 38892293 | PMCID: PMC11172814
Review Free full text in Europe PMC
Studying intramuscular fat deposition and muscle regeneration: insights from a comparative analysis of mouse strains, injury models, and sex differences.
Skelet Muscle, 14(1):12, 29 May 2024
Cited by: 1 article | PMID: 38812056 | PMCID: PMC11134715
Cell-type specific effects of mineralocorticoid receptor gene expression suggest intercellular communication regulating fibrosis in skeletal muscle disease.
Front Physiol, 15:1322729, 26 Apr 2024
Cited by: 0 articles | PMID: 38737833 | PMCID: PMC11082420
Go to all (134) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis.
Cell Tissue Res, 364(3):647-660, 07 Jan 2016
Cited by: 77 articles | PMID: 26742767
A new model of experimental fibrosis in hindlimb skeletal muscle of adult mdx mouse mimicking muscular dystrophy.
Muscle Nerve, 45(6):803-814, 01 Jun 2012
Cited by: 27 articles | PMID: 22581532
Abnormal NFAT5 Physiology in Duchenne Muscular Dystrophy Fibroblasts as a Putative Explanation for the Permanent Fibrosis Formation in Duchenne Muscular Dystrophy.
Int J Mol Sci, 21(21):E7888, 24 Oct 2020
Cited by: 5 articles | PMID: 33114289 | PMCID: PMC7660673
Targeting fibrosis in Duchenne muscular dystrophy.
J Neuropathol Exp Neurol, 69(8):771-776, 01 Aug 2010
Cited by: 89 articles | PMID: 20613637 | PMCID: PMC2916968
Review Free full text in Europe PMC