Abstract
Background
Leukocytosis has been associated with thrombosis and mortality in cancer patients. We explored the association of leukocytosis with venous thromboembolism (VTE) and early mortality in cancer patients initiating chemotherapy.Methods
Data from a prospective, multicenter observational study of treatment-related complications in 4,405 ambulatory cancer patients initiating chemotherapy was used for this analysis. The association of leukocytosis, VTE and mortality during the course of chemotherapy was evaluated in univariate and multivariate analysis.Results
Ninety-three patients (2.1%) developed VTE and 134 (3%) died over a median follow up of 75 days (range 0-384). Of 4391 patients with available baseline white blood cell (WBC) count, 561 (12.8%) had elevated pretreatment leukocyte counts, defined as WBC > 11 x 10(9) cells/L. VTE occurred in 25 of 561 patients (4.5%) with baseline leukocytosis compared to 68 of 3830 (1.8%) with WBC <or= 11 x 10(9) cells/L (P < 0.0001). Baseline leukocytosis was associated with VTE by multivariate analysis as well (HR 2.1, 95% confidence interval 1.3-3.4, p = 0.003). Forty one patients (7.3%) with leukocytosis died compared to 92 (2.4%) with WBC <or= 11 x 10(9) cells/L (P < 0.0001). Baseline leukocytosis was associated with early mortality by multivariate analysis as well (HR 2.2, 95% confidence interval 1.5-3.3, p < 0.0001). Mortality was greatest in patients with both leukocytosis and VTE. In multivariate analysis several factors were predictive of leukocytosis.Conclusions
Elevated WBC, particularly neutrophils, is strongly associated with increased risk of VTE and mortality in cancer patients receiving systemic chemotherapy. Further studies are needed to elicit the mechanisms involved.Free full text

Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy
Abstract
Background
Leukocytosis has been associated with thrombosis and mortality in cancer patients. We explored the association of leukocytosis with venous thromboembolism (VTE) and early mortality in cancer patients initiating chemotherapy.
Methods
Data from a prospective, multicenter observational study of treatment-related complications in 4,405 ambulatory cancer patients initiating chemotherapy was used for this analysis. The association of leukocytosis, VTE and mortality during the course of chemotherapy was evaluated in univariate and multivariate analysis.
Results
Ninety-three patients (2.1%) developed VTE and 134 (3%) died over a median follow up of 75 days (range 0–384). Of 4391 patients with available baseline white blood cell (WBC) count, 561 (12.8%) had elevated pretreatment leukocyte counts, defined as WBC > 11×109 cells/L. VTE occurred in 25 of 561 patients (4.5%) with baseline leukocytosis compared to 68 of 3830 (1.8%) with WBC ≤ 11×109 cells/L (P<0.0001). Baseline leukocytosis was associated with VTE by multivariate analysis as well (HR 2.1, 95% confidence interval 1.3–3.4, p=0.003). Forty one patients (7.3%) with leukocytosis died compared to 92 (2.4%) with WBC ≤ 11×109 cells/L (P<0.0001). Baseline leukocytosis was associated with early mortality by multivariate analysis as well (HR 2.2, 95% confidence interval 1.5–3.3, p<0.0001). Mortality was greatest in patients with both leukocytosis and VTE. In multivariate analysis several factors were predictive of leukocytosis.
Conclusions
Elevated WBC, particularly neutrophils, is strongly associated with increased risk of VTE and mortality in cancer patients receiving systemic chemotherapy. Further studies are needed to elicit the mechanisms involved.
Introduction
Leukocytosis has been shown to be associated with increased risk of both arterial and venous thrombosis in a number of clinical settings including ischemic heart disease[1], cerebrovascular disease[2], and myeloproliferative disorders [3–5]. An elevated leukocyte count prior to initiation of chemotherapy was recently identified as one of five clinical factors predictive of increased risk for venous thromboembolism (VTE) in cancer patients[6]. In addition, leukocytosis has been demonstrated to be associated with increased mortality in several subgroups of cancer patients [7–11].
Cancer-associated thrombosis is an important problem. Cancer patients are at 2–7 fold increased risk of developing VTE compared to patients without cancer [12,13]. Thrombosis is the second leading cause of death in cancer patients receiving chemotherapy second only to disease progression [14], and is associated with other significant morbidities including increased risk of bleeding and recurrent VTE [15–17].
The objective of the current analysis was to characterize the relationship between leukocytosis, thrombosis, and early mortality in a large prospective observational study of cancer patients initiating chemotherapy. A better understanding of the role leukocytes play in cancer progression and thrombosis may guide approaches such as targeted thromboprophylaxis which could potentially reduce the incidence of cancer-associated thrombosis.
Methods
This analysis was conducted on data collected as part of the Awareness of Neutropenia in Chemotherapy Study Group Registry, an observational multicenter study designed to evaluate febrile neutropenia and other chemotherapy-related complications in cancer patients. Patients were enrolled at the start of a new chemotherapy regimen and followed prospectively during the first four cycles of the treatment. The study was conducted at 115 sites within the United States with patients enrolled between March 2002 and October of 2005. The study was approved by a central institutional review board (IRB) as well as the University of Rochester IRB.
Patients were required to have a histologically confirmed diagnosis of cancer, with targeted enrollment of specific tumor types including breast, lung, ovarian, sarcoma, colon, and lymphomas. Patients were also required to be at least 18 years of age and capable of providing informed consent. Exclusions included a diagnosis of acute leukemia or myeloma, concurrent cytotoxic, biologic, or immunologic therapy for other conditions, previous stem cell transplantation, pregnancy or lactation, active infection requiring antibiotics, or participation in a double-blind study.
Baseline information including current medications, recent surgery, comorbidities, and the planned chemotherapy regimen and dosing schedule were collected at time of study enrollment. Data regarding changes made to the planned chemotherapy regimen such as dose reductions or discontinuation of therapy were collected prospectively at the start of each new cycle and at mid-cycle visits. A complete blood count was obtained at baseline prior to initiation of chemotherapy, at nadir during mid-cycle visits, and at the beginning of each cycle of chemotherapy. Symptomatic VTE events were diagnosed by the appropriate treating physician by routine diagnostic testing and these events were recorded at new-cycle and mid-cycle visits depending on the timing of the event. Screening for asymptomatic VTE was not conducted. Mortality during chemotherapy treatment was recorded. Deaths reported during study follow up or within 60 days of the last visit were considered as early mortality events.
Leukocytosis was defined as a white blood cell count (WBC) >11×109 cells/L based on the upper limit of normal from a central reference laboratory. Likewise the upper limits of normal for leukocyte subtype from the central reference laboratory were used to define absolute lymphocytosis (>4.4×109 cells/L), absolute monocytosis (>1.2×109 cells/L), and absolute neutrophilia (>7.7×109 cells/L). Liver abnormality was defined as having at least one of the following: bilirubin > 1 mg/dl, aspartate aminotransferase (AST)> 35 units/L, alanine aminotransferase (ALT)>35 units/L, or alkaline phosphates >120 units/L or a recorded medical history of liver disease.
The association of pre-chemotherapy leukocytosis, defined at start of chemotherapy, and clinical variables was evaluated in univariate analysis using chi-square test. Each variable was evaluated for missing values. Overall percentage of missing values was low, less then 1% for majority of covariates, reaching a maximum of 3.4% for some baseline chemistry measurements such as bilirubin, albumin and glucose). Multivariate logistic regression analysis was performed using the forward stepwise method with criteria set to P=0.10 for inclusion or elimination of covariates. The pool of variables for the selection process consisted of factors associated with leukocytosis in the univariate analysis (P <.10). Cancer type, disease stage and performance status was required to be included in the model a priori at each step. Colorectal cancer was used as the reference category for cancer type in the multivariate analysis because colorectal cancer was not associated with leukocytosis and had relatively low rates of VTE. Clinically important interactions were checked and none were found to be statistically significant.
Proportional hazards regression multivariate analysis was employed to study the association of elevated pre-chemotherapy leukocyte count with development of VTE. Proportionality assumption of the model was evaluated using logarithm of negative logarithm of survival distribution function versus logarithm of time plots. First order interactions among model covariates were checked, and no statistically significant interactions were determined.
For trend of VTE rates related to increasing WBC count analyses the population was divided into quartiles based on the pre-chemotherapy leukocyte count and the quartile with the highest leukocyte count was further divided to delineate the group with leukocytosis.
A t-test was used to compare means for leukocyte counts between groups. Chi-square test was used to compare binary outcomes for categorical variables. The Cochran-Armitage test was used to determine trends across ordered categories. Mortality was estimated by the method of Kaplan and Meier and differences between groups were compared using log-rank test. All reported p-values are two sided and tests with P<0.05 were considered statistically significant. Statistical analysis was conducted using SAS statistical software (SAS Institute, Cary, NC).
Results
Patient Characteristics
The study population comprised 4,405 patients with solid tumors or lymphoma enrolled between March 2002 and October 2005. The average age of the population was 60 years (range 18–97, SD 13) and 40% of patients were 65 years of age or older (Table 1). Breast cancer was the most common solid malignancy, followed by lung and colorectal cancer. Non-Hodgkin lymphoma was the most common hematologic malignancy. A majority of the patients were female because of targeted enrollment of breast cancer patients. Over one-third of the study population had metastatic disease. Of the 4391 patients with available baseline white blood cell counts, 461 (12.8%) had elevated an leukocyte counts.
Table 1
Characteristics of Study Population.
| Categoryv
| No. (%)
|
|---|---|
| All Patients | 4405* (100) |
| Age | |
 < 65 yrs < 65 yrs | 2631 (59.7) |
 ≥65 yrs ≥65 yrs | 1774 (40.3) |
| Gender | |
 Male Male | 1470 (33.4) |
 Female Female | 2935 (66.6) |
| Ethnicity | |
 White White | 3727 (84.6) |
 Black Black | 454 (10.3) |
 Other/unknown Other/unknown | 224 (5.1) |
| Stage | |
 1 to 3 1 to 3 | 2668 (60.6) |
 4 4 | 1671 (37.9) |
 Unknown Unknown | 66 (1.5) |
| Performance status, ECOG | |
 0 to 1 0 to 1 | 3983 (90.5) |
 2–4 2–4 | 420 (9.5) |
| Primary site of cancer | |
 Breast Breast | 1473 (33.4) |
 Colorectal Colorectal | 521 (11.8) |
 Lung Lung | 907 (20.6) |
 Gynecologic Gynecologic | 436 (9.9) |
 Gastric and pancreatic Gastric and pancreatic | 90 (2.0) |
 Lymphoma Lymphoma | 547 (12.4) |
 Other solid tumors Other solid tumors | 431 (9.8) |
| Comorbidities | |
 Cerebrovascular disease Cerebrovascular disease | 84 (1.9) |
 Moderate or severe renal disease Moderate or severe renal disease | 49 (1.1) |
 Chronic pulmonary disease Chronic pulmonary disease | 364 (8.3) |
 Diabetes mellitus Diabetes mellitus | 533 (12.1) |
 Body mass index≥35 kg/m2 Body mass index≥35 kg/m2 | 532 (12.1) |
 Liver complications Liver complications | 1308 (29.7) |
| Baseline laboratory values | |
 WBC > 11 × 109/L WBC > 11 × 109/L | 561 (12.8) |
 Platelet count≥350 × 109/L Platelet count≥350 × 109/L | 993 (22.6) |
 Hemoglobin < 10 g/dL Hemoglobin < 10 g/dL | 271 (6.2) |
 Bilirubin > 1 mg/dL Bilirubin > 1 mg/dL | 194 (4.6) |
 Albumin<3.5 g/dL Albumin<3.5 g/dL | 927 (21.8) |
 Creatinine > 1.5 mg/dL Creatinine > 1.5 mg/dL | 146 (3.4) |
 Glucose > 120 mg/dL Glucose > 120 mg/dL | 1292 (30.4) |
| Type of regimen | |
 Anthracycline-containing Anthracycline-containing | 1542 (35.1) |
 Platinum-containing Platinum-containing | 1586 (36.1) |
 Taxane-containing Taxane-containing | 1360 (31.0) |
| Prior cancer treatments | |
 Prior chemotherapy Prior chemotherapy | 1049 (23.8) |
 Surgery within past month Surgery within past month | 1401 (31.8) |
| Growth factor/steroid use after chemotherapy initiation | |
 Prophylactic Myeloid growth factor Prophylactic Myeloid growth factor | 889 (20.2) |
 Erythropoiesis-stimulating factor started by cycle 2 Erythropoiesis-stimulating factor started by cycle 2 | 1137 (25.8) |
Leukocytosis and VTE
VTE developed in 93 patients (2.1%) over a median follow-up of 75 days (range 0–384 days). Approximately two-thirds of the events were deep vein thromboses and one-third were pulmonary emboli. The median time from initiation of chemotherapy to development of VTE was 38 days (0–123 days). Sixty eight of 3830 (1.8%) patients with normal pre-chemotherapy leukocyte count developed VTE compared to 25 of 561 (4.5%) with elevated pre-chemotherapy leukocyte count. The incidence of VTE was highest in those patients with the highest pre-chemotherapy leukocyte counts (Fig. 1). In addition pre-chemotherapy leukocytosis was associated with increased VTE when adjusted for in a multivariate model including type of malignancy, stage, pre-chemotherapy platelet count, pre-chemotherapy hemoglobin level, use of erythropoietic stimulating agents, and BMI (Hazard Ratio (HR) 2.1, 95% confidence interval (CI) 1.3–3.4, p=0.003) (Table 2). The increased risk of VTE with higher leukocyte count persisted while patients were on chemotherapy. After excluding the patients who developed VTE or died during the first cycle, the VTE rate in patients who had persistent leukocytosis after the first cycle of chemotherapy (3.0%) is significantly higher than the VTE rate in patients whose baseline leukocytosis resolves (1.7%) and patients with normal leukocyte counts throughout (1.2%) (P=0.03 for trend). The mean baseline leukocyte count in patients who did not develop VTE prior to initiation of chemotherapy was 8.0×109 cells/L compared to 9.6×109 cells/L in those that did develop VTE (P=0.001), and this significant difference remained prior to subsequent cycles of chemotherapy. (Fig. 2) Elevation of both absolute neutrophil count (ANC) (>7.7×109 cells/L) and absolute monocyte count (AMC) (>1.2×109 cells/L) prior to initiation of chemotherapy was associated (P<0.0001 for each) with increased risk of VTE although baseline lymphocytosis (>4.8×109 cells/L) was not (Fig. 3).
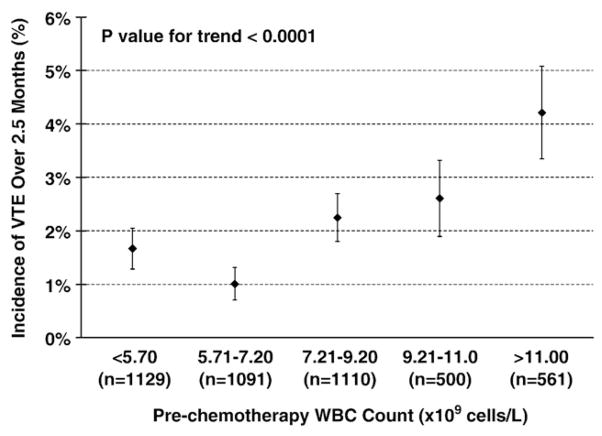
Incidence of venous thromboembolic events (VTE) by pre-chemotherapy white blood cell count (WBC). Legend: Elevated pre-chemotherapy leukocyte count and the risk of VTE during chemotherapy. The frequency of VTE is shown for the study population when divided into quartiles by pre-chemotherapy leukocyte count with the highest quartile further divided to delineate the subgroup with pre-chemotherapy leukocytosis (>11×109 cells/L). The highest VTE rate is seen in patients with leukocytosis (P for trend <0.001). Error bars represent standard errors.
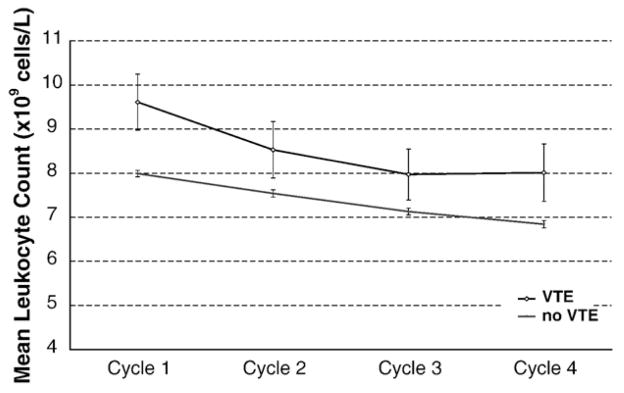
Mean Leukocyte count prior to each cycle of chemotherapy in patients with and without VTE. Legend: Mean leukocytes at the initiation of chemotherapy were higher among the patients that developed VTE (P=.001) and remained elevated during the entire course of treatment (p=0.0005). The mean leukocyte values by cycle and standard errors are presented.
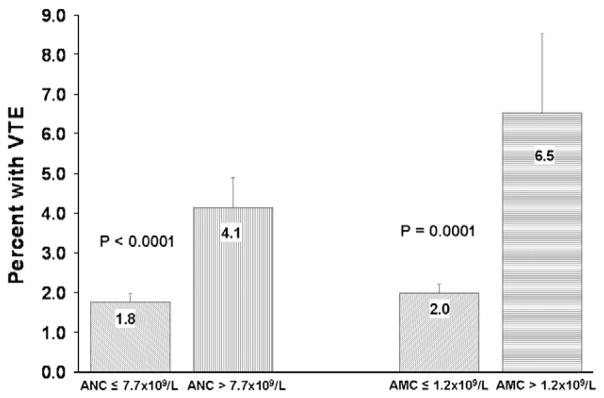
Venous thromboembolic event (VTE) rates by type of leukocyte. Legend: VTE rates were higher in patients with elevation of absolute neutrophil count (ANC) defined as >7.7×109 cells/L compared to patients with normal baseline absolute neutrophil count (P<0.0001). VTE rates were also higher in patients with elevation of absolute monocyte count (AMC) defined as >1.2×109 cells/L compared to patients with normal baseline absolute monocyte count (P=0.0001). Error bars represent standard errors.
Table 2
Clinical variables associated with VTE by multivariate analysis.
| Patient Characteristics | HR | P-value | 95 % CI |
|---|---|---|---|
| Site of cancer | |||
 Very high risk (stomach, pancreas) Very high risk (stomach, pancreas) | 3.84 | 0.006 | 1.47–10.03 |
 High risk (lung, lymphoma, gynecologic, genitourinary excluding prostate) High risk (lung, lymphoma, gynecologic, genitourinary excluding prostate) | 1.58 | 0.043 | 1.02–2.46 |
 Low risk (breast, colorectal, head and neck) Low risk (breast, colorectal, head and neck) | 1.00 | (Reference) | |
| Prechemotherapy platelet count≥350×109/L | 1.83 | 0.006 | 1.19–2.83 |
| Hemoglobin level < 100 g/L or use of red cell growth factors | 2.31 | 0.000 | 1.52–3.51 |
| Prechemotherapy leukocyte count >11×109/L | 2.10 | 0.003 | 1.30–3.40 |
| BMI≥35 kg/m2 | 1.83 | 0.026 | 1.07–3.13 |
BMI; body mass index, HR;-hazard ratio, CI; confidence interval.
Leukocytosis and Mortality
One hundred and thirty three patients died during the study follow-up for an early mortality rate of 3.0%. The median time to death was 38 days (range 4–169 days). Early mortality rate was significantly higher in patients with pre-chemotherapy leukocytosis compared to patients with normal baseline leukocyte count. The 150 day mortality rate as estimated by the Kaplan-Meier method in patients with baseline leukocytosis was 14.0% (95% CI 8.9–21.6%) compared to 4.4% (95% CI 3.2–6.1%) in patients with normal leukocyte count (P<0.0001) (Fig. 4). Elevation of both absolute neutrophil count and absolute monocyte count prior to initiation of chemotherapy was associated with increased risk of early mortality, but elevation of absolute lymphocyte count was not (Fig. 4). The mortality rate in patients who have persistent leukocytosis after the first cycle of chemotherapy (4.2%) is significantly higher than the mortality rate in patients whose baseline leukocytosis resolves (3.8%) and patients with normal leukocyte counts (1.6%) (P=0.003 for trend). Pre-chemotherapy leukocytosis was associated with early mortality in a multivariate model after adjusting for other clinical variables including age, gender, type of malignancy, stage, Eastern Cooperative Group erformance status, VTE pre-chemotherapy platelet count, pre-chemotherapy hemoglobin level, use of erythropoietic stimulating agents, and BMI (HR 2.2, 95% CI 1.5–3.3 p-value<0.0001).
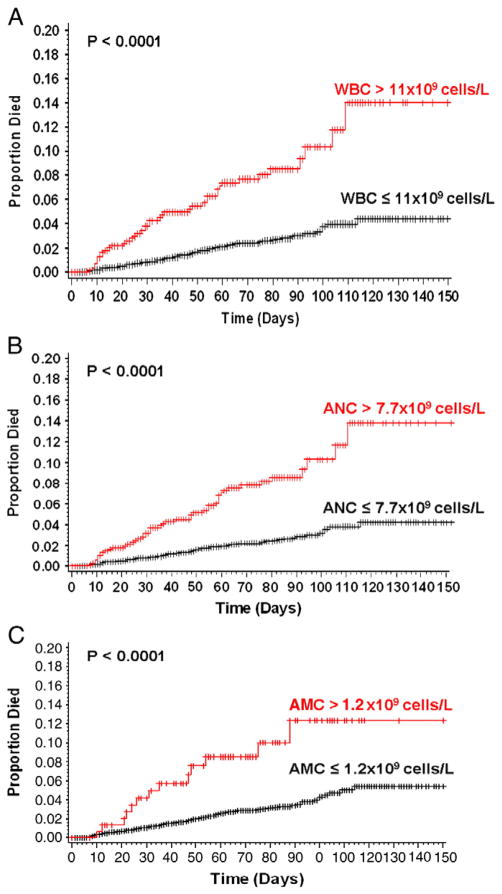
Early mortality rate by pre-chemotherapy white blood cell. Legend: Early mortality rates defined as mortality occurring during study follow-up was significantly higher in patients with baseline leukocytosis. (A) The 150 day mortality as estimated by the Kaplan Meier method is 14.0% in patients baseline leukocytosis compared to 4.4% in patients with normal baseline total leukocyte count (P<0.0001). (B) The 150 day mortality as estimated by the Kaplan-Meier method is 13.8% in patients baseline elevation of absolute neutrophil count (>7.7×109 cells/L) compared to 4.3% in patients with normal baseline absolute neutrophil count (P<0.0001). (C) The 150 day mortality as estimated by the Kaplan-Meier method is 12.4% in patients baseline elevation of absolute monocyte count (>1.2×109 cells/L) compared to 5.4% in patients with normal baseline absolute monocyte count (P<0.0001).
Leukocytosis, Thrombosis and Mortality
The highest pre-chemotherapy leukocyte counts were found in patients who developed VTE and died during study follow-up (Fig. 5). Also the highest early mortality rate was observed in the group of patients with pre-chemotherapy leukocytosis who also developed VTE during study (Fig. 5). The mortality rate of 20% in this group was significantly higher than the early mortality rate in the other three groups (P<0.0001).
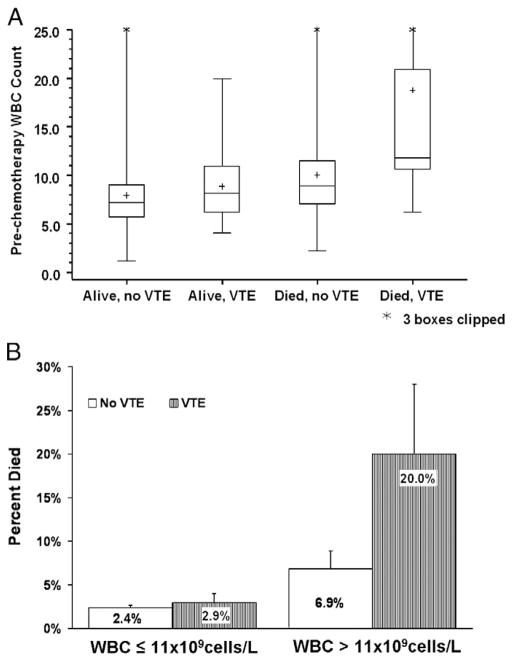
Pre-chemotherapy leukocytosis, venous thromboembolic events (VTE) and early mortality. Legend: (A) Box plot of the distribution of pre-chemotherapy white blood cells (WBC) by mortality and VTE events. The mean is represented by the +. The horizontal bars in the rectangle represent the 25th percentile, median, and 75% percentile of the various groups. The upper and lowers bars represent the maximum and minimum respectively. (B) Bar graph of the mortality rate by prechemotherapy WBC and VTE events with standard errors. The highest mortality rate (20.0%) was seen in patients with prechemotherapy leukocytosis who also developed VTE during study (P<0.0005).
Variables Associated with Pre-chemotherapy Leukocytosis
Multiple clinical variables were associated with pre-chemotherapy leukocytosis in univariate analysis including type of cancer, male gender, poor performance status, metastatic disease, chronic pulmonary disease, liver abnormality and obesity (P<0.05 for each). In addition, several laboratory findings were associated with pre-chemotherapy leukocytosis by univariate analysis including thrombocytosis (platelet count≥350×109/L), anemia (hemoglobin <10 g/dl), elevated bilirubin (>1 mg/dl), low albumin (<3.5 g/dl) and hyperglycemia (glucose >120 mg/dl) (P<0.05 for each). In multivariate analysis, after adjusting for peformance status, the type of malignancy, advanced stage (OR 1.3), neutrophil percentage>70%(OR 4.3), elevated baseline platelet count (OR 2.8), low albumin (OR 1.5), liver disease (OR 1.3), and hyperglycemia (OR 1.3) were associated with pre-chemotherapy leukocytosis (Table 3).
Table 3
Clinical factors associated with pre-chemotherapy leukocytosis by multivariate analysis (n=4391).
| Covariate | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Cancer type | – | <.0001 |
| Colorectal (reference) | 1.00 | – |
| Gynecologic | 1.21 (0.75–1.95) | 0.4473 |
| Breast | 1.42 (0.94–2.14) | 0.0986 |
| Other solid tumors | 1.43 (0.91–2.25) | 0.1222 |
| Gastric and pancreatic | 1.99 (1.02–3.9) | 0.0449 |
| Lung | 2.07 (1.4–3.04) | 0.0002 |
| Lymphoma | 2.35 (1.53–3.61) | <.0001 |
| Other baseline clinical variables | ||
| Stage IV | 1.28 (1.03–1.59) | 0.0273 |
| ECOG performance status | 1.16 (0.87–1.56) | 0.3111 |
| Glucose>120 mg/dL | 1.29 (1.05–1.59) | 0.0151 |
| Liver disease | 1.30 (1.05–1.61) | 0.0153 |
| Albumin <3.5 g/dL | 1.50 (1.2–1.88) | 0.0004 |
| Platelets≥350 × 109/L | 2.80 (2.27–3.45) | <.0001 |
| Neutrophils>70% of leukocytes | 4.28 (3.4–5.38) | <.0001 |
ECOG-Eastern Cooperative Oncology Group.
Discussion
Thrombosis is an important cause of morbidity and mortality in cancer patients [14,16]. This study demonstrates a strong association between leukocytosis and increased risk of both thrombosis and early mortality in a large cohort of cancer patients initiating chemotherapy. Previous analyses of the same cohort of patients have identified pre-chemotherapy leukocytosis as one of five clinical factors predicting increased risk of both VTE [6] and early mortality [18] when adjusting for multiple clinical covariates in multivariate models. Several other groups have confirmed the Khorana risk model predicts for VTE risk in cancer patients [19,20].
The current study provides a more in-depth analysis of the relationship between leukocytosis and cancer patient outcomes, and includes several novel findings. Thorough prospective collection of data allowed for multivariate analysis identifying several clinical factors which are associated with pre-therapy leukocytosis in cancer patients. We also demonstrate that elevation of neutrophil and monocyte counts, but not lymphocyte counts, are important. These findings allow for speculation on potential mechanisms involved. We also establish a temporal association between leukocytosis and VTE risk by showing leukocytosis correlates with thrombosis risk throughout treatment course thus supporting a causative role for leukocytes in mediating VTE in cancer patients. Lastly, to our knowledge this is the only study to demonstrate an association between leukocytosis and both thrombosis and mortality in the same cohort of cancer patients.
The mechanisms responsible for the associations demonstrated by this analysis are unclear. Leukocytosis may be a marker of an underlying process such as more aggressive malignancy, more significant co-morbidities, or inflammation, or leukocytes may be actively involved in disease progression and cancer-associated thrombosis. The association of leukocytosis with advanced stage and type of malignancy in this study may suggest that leukocytosis is identifying patients with more aggressive disease or increased disease burden not captured by other means. This study also shows that leukocytosis is associated with thrombocytosis, a known negative prognostic indicator in patients with cancer [21,22]. In this cohort baseline leukocytosis correlates with several clinical findings known to be associated with underlying inflammation including thrombocytosis [23], hyperglycemia [24,25], hypoalbuminemia [26,27], and transaminitis [28,29].
Although when adjusting for many of these clinical covariates in multivariate models leukocytosis remained a significant predictor of both VTE [6] and early mortality[18] suggesting that an independent mechanism exists. A recent analysis of thrombosis in patients with myeloproliferative neoplasms (MPN) offers a compelling argument that leukocytes have a causative role in MPN-associated thrombosis [30]. A similar argument could be made for leukocytosis and cancer patient outcomes, and several mechanisms may be responsible.
Leukocytes may directly contribute to thrombus formation and disease progression through release of tissue factor and vascular endothelial growth factor (VEGF). Tissue factor and VEGF levels in leukocytes from patients with cancer are many fold higher than in leukocytes from normal controls [31–34], and a recent prospective study in patients with pancreatic cancer demonstrated an association between leukocyte count and plasma tissue factor activity [20]. Leukocyte interactions with platelets and endothelium may also be important. An elevated level of P-selectin, a protein expressed on activated platelets and involved in platelet leukocyte interactions, is a biomarker for increased risk of cancer-associated thrombosis[35]. It is also known that leukocytes produce several cytotoxic mediators like TNF-alpha, IL-1, and interferons which are capable of tumor destruction [36]. Leukocyte products may also promote a tumor microenvironment which is supportive of thrombus generation, tumor growth, metastasis, and chemotherapy resistance [37–40].
There are several limitations to the current study. The ANC registry was designed to study complications resulting from chemotherapy induced neutropenia. VTE and mortality were not intended primary endpoints of the original study. However data regarding mortality and VTE were prospectively collected and the study size is adequate for an analysis of this nature. Secondly, only symptomatic VTE events were recorded and studies suggest that asymptomatic VTE rates are significantly higher [41]. Some subgroups of cancer patients such as those with brain or prostate cancer are under-represented, but this cohort of patients is significantly more diverse than other studies looking at the significance of leukocytosis in cancer patients where most cohorts consist of only one type of cancer. Lastly, due to the nature and magnitude of this study biologic samples were not collected and stored to allow for more detailed analysis of the mechanisms responsible for the observed associations. However this powerful prospective analysis in a large cohort of patients will certainly serve to formulate hypotheses regarding mechanisms and will help direct future studies.
In conclusion, clinicians should be aware that pre-chemotherapy leukocytosis is associated with significantly increased rates of VTE and early mortality in cancer patients initiating chemotherapy. A better understanding of the role leukocytes play in cancer-associated VTE and cancer progression may allow for interventions such as targeted thromboprophylaxis which could significantly impact cancer patient outcomes. It is also conceivable that further opportunities for intervention will emerge as we gain a better understanding of the mechanisms involved, and as our ability to manipulate these pathways progresses.
Acknowledgments
Dr. Connolly received the 2009 Sanofi-Aventis/ISTH Fellowship for Clinical Research. Dr. Khorana is supported by grants from the National Cancer Institute K23 CA120587-01, the National Heart, Lung and Blood Institute 1R01HL095109-01 and the V Foundation. Dr. Lyman is supported by a grant from the National Heart, Lung and Blood Institute 1R01HL095109-01. Dr. Francis is supported by a grant from the National Heart, Lung and Blood Institute 1R01HL095109-01.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.thromres.2010.05.012
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3635827?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.thromres.2010.05.012
Article citations
Haemostatic Gene Expression in Cancer-Related Immunothrombosis: Contribution for Venous Thromboembolism and Ovarian Tumour Behaviour.
Cancers (Basel), 16(13):2356, 27 Jun 2024
Cited by: 1 article | PMID: 39001418
Combining KRAS gene status with preoperative D‑dimer levels as a predictive marker of venous thromboembolism risk in patients with resectable colorectal cancer: A prospective cohort study.
Biomed Rep, 20(6):96, 22 Apr 2024
Cited by: 0 articles | PMID: 38765860 | PMCID: PMC11099602
Assessing the risk of venous thromboembolism in patients with haematological cancers using three prediction models.
J Cancer Res Clin Oncol, 149(20):17771-17780, 07 Nov 2023
Cited by: 5 articles | PMID: 37935936
The impact of sarcopenia on esophagectomy for cancer: a systematic review and meta-analysis.
BMC Surg, 23(1):240, 17 Aug 2023
Cited by: 5 articles | PMID: 37592262 | PMCID: PMC10433615
Review Free full text in Europe PMC
The Saudi Consensus for the Management of Cancer-Associated Thromboembolism: A Modified Delphi-Based Study.
TH Open, 7(1):e14-e29, 07 Jan 2023
Cited by: 1 article | PMID: 36751300 | PMCID: PMC9825204
Go to all (71) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
White blood cell count measured prior to cancer development is associated with future risk of venous thromboembolism--the Tromsø study.
PLoS One, 8(9):e73447, 04 Sep 2013
Cited by: 29 articles | PMID: 24023876 | PMCID: PMC3762748
Elevated white blood cell count and outcome in cancer patients with venous thromboembolism. Findings from the RIETE Registry.
Thromb Haemost, 100(5):905-911, 01 Nov 2008
Cited by: 35 articles | PMID: 18989537
A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality.
Oncologist, 21(7):861-867, 28 Apr 2016
Cited by: 40 articles | PMID: 27125754 | PMCID: PMC4943384
Effect of testing for cancer on cancer- and venous thromboembolism (VTE)-related mortality and morbidity in people with unprovoked VTE.
Cochrane Database Syst Rev, 8:CD010837, 23 Aug 2017
Cited by: 7 articles | PMID: 28832905 | PMCID: PMC6483606
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: K23 CA120587-01
Grant ID: K23 CA120587
Grant ID: UC2 CA148041
NHLBI NIH HHS (2)
Grant ID: 1R01HL095109-01
Grant ID: R01 HL095109





