Abstract
Background
Stromal cell-derived factor-1alpha (SDF-1alpha) binding to its cognate receptor, CXCR4, regulates a variety of cellular functions such as stem cell homing, trafficking, and differentiation. However, the role of the SDF-1alpha-CXCR4 axis in modulating myocardial ischemia/reperfusion injury is unknown.Methods and results
In mice subjected to ischemic preconditioning, myocardial SDF-1alpha mRNA was found to be increased 3 hours later (P<0.05). Myocardial SDF-1alpha and CXCR4 mRNA and protein were found to be expressed in both cardiac myocytes and fibroblasts. SDF-1alpha production increased significantly after 1 or 4 hours of hypoxia and 18 hours of reoxygenation in cultured myocytes (P<0.05) but did not change in fibroblast cultures. In isolated myocytes, CXCR4 activation by SDF-1alpha resulted in increased phosphorylation of both ERK 1/2 and AKT and decreased phosphorylation of JNK and p38. Cultured myocytes pretreated with SDF-1alpha were resistant to hypoxia/reoxygenation damage, exhibiting less lactate dehydrogenase release, trypan blue uptake, and apoptotic cell death (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay) (P<0.05). This protective effect was blocked by the CXCR4 selective antagonist AMD3100. In vivo, administration of SDF-1alpha before 30 minutes of coronary occlusion followed by 4 hours of reperfusion decreased infarct size (P<0.05). The decrease in infarct size with SDF-1alpha administration also was blocked by AMD3100.Conclusions
We conclude that SDF-1alpha and its receptor, CXCR4, constitute a paracrine or autocrine axis in cardiac myocytes that is activated in response to preconditioning and hypoxic stimuli, recruiting the antiapoptotic kinases ERK and AKT and promoting an antiapoptotic program that confers protection against ischemia/reperfusion damage.Free full text

Stromal Cell–Derived Factor-1α Confers Protection Against Myocardial Ischemia/Reperfusion Injury
Abstract
Background
Stromal cell–derived factor-1α (SDF-1α) binding to its cognate receptor, CXCR4, regulates a variety of cellular functions such as stem cell homing, trafficking, and differentiation. However, the role of the SDF-1α–CXCR4 axis in modulating myocardial ischemia/reperfusion injury is unknown.
Methods and Results
In mice subjected to ischemic preconditioning, myocardial SDF-1α mRNA was found to be increased 3 hours later (P<0.05). Myocardial SDF-1α and CXCR4 mRNA and protein were found to be expressed in both cardiac myocytes and fibroblasts. SDF-1α production increased significantly after 1 or 4 hours of hypoxia and 18 hours of reoxygenation in cultured myocytes (P<0.05) but did not change in fibroblast cultures. In isolated myocytes, CXCR4 activation by SDF-1α resulted in increased phosphorylation of both ERK 1/2 and AKT and decreased phosphorylation of JNK and p38. Cultured myocytes pretreated with SDF-1α were resistant to hypoxia/reoxygenation damage, exhibiting less lactate dehydrogenase release, trypan blue uptake, and apoptotic cell death (terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling assay) (P<0.05). This protective effect was blocked by the CXCR4 selective antagonist AMD3100. In vivo, administration of SDF-1α before 30 minutes of coronary occlusion followed by 4 hours of reperfusion decreased infarct size (P<0.05). The decrease in infarct size with SDF-1α administration also was blocked by AMD3100.
Conclusions
We conclude that SDF-1α and its receptor, CXCR4, constitute a paracrine or autocrine axis in cardiac myocytes that is activated in response to preconditioning and hypoxic stimuli, recruiting the antiapoptotic kinases ERK and AKT and promoting an antiapoptotic program that confers protection against ischemia/reperfusion damage.
Stromal cell–derived factor-1α (SDF-1α) is a CXC chemokine known to play a critical role in the trafficking of hematopoietic and lymphopoietic cells and stem cell progenitors and in maintaining hematopoietic stem cell niches in bone marrow.1 SDF-1α also has been shown to increase cell survival during serum withdrawal,2 development, and differentiation.2–4 SDF-1α produces these effects as the unique ligand for its receptor, CXCR4.5 Studies performed primarily in hematopoietic cells demonstrate that SDF-1α–CXCR4 signaling regulates diverse cell functions via a Gαi-dependent mechanism and activation of PI3K, MAPK, PKC, and JAK/STAT signaling.6 SDF-1α and CXCR4 are widely expressed and are essential for development. SDF-1α and CXCR4 knockout mice have demonstrated a fundamental developmental role for this receptor-ligand axis5,7; deletion of the gene encoding CXCR4 is lethal in utero, whereas deletion of the gene encoding SDF-1α is lethal soon after birth, with several organs in both knockouts, including the heart, displaying abnormal development.
SDF-1α expression has been shown to increase under hypoxic conditions8 and thus may serve to attract stem cells to sites of tissue injury. SDF-1α expression is significantly upregulated in experimental rat and mouse models of infarction9,10 and in plasma and cardiac tissue of patients with myocardial infarction.11–13 Increased SDF-1α expression may be relevant to tissue regeneration because transplantation of fibroblasts overexpressing SDF-1α in a rat infarct model results in SDF-1α overexpression in the peri-infarct zone and increased infiltration of hematopoietic stem cells.14,15 These findings suggest that local SDF-1α expression may serve as a key factor for stem cell homing to the site of myocardial infarction. However, the source of cardiac SDF-1α and its effects on cardiac viability and signaling have not been characterized in these studies and are currently unknown. Specifically, the role of SDF-1α in myocardial ischemia/reperfusion injury remains unknown. Furthermore, although SDF-1α has been demonstrated in the heart, it is unknown whether its receptor (CXCR4) is present in cardiac tissue and, if so, what role it plays in cardiac biology. The present study was designed to address these issues in isolated murine myocytes and in intact mice subjected to coronary artery occlusion and reperfusion in vivo. The results demonstrate for the first time that the SDF-1α receptor CXCR4 is expressed in the myocardium and that administration of SDF-1α is cardioprotective both in vitro and in vivo.
Methods
Materials
Cell culture reagents and fetal bovine serum were products of Invitrogen (Carlsbad, Calif). Medium M1018, lactate dehydrogenase (LDH) assay reagents, and the CXCR4 receptor antagonist AMD3100 were purchased from Sigma (St Louis, Mo). Synthetic SDF-1α was purchased from Upstate Biotechnology (Lake Placid, NY). Laminin was purchased from BD Bioscience (San Jose, Calif). Protease inhibitor cocktail was from Roche Applied Science (Indianapolis, Ind). Antibodies for phospho-ERK, phospho-AKT, phospho-p38, and phospho-JNK and those for total-ERK, total-AKT, total-p38, total-JNK, and tubulin were purchased from Cell Signaling Technology (Beverly, Mass). Antibodies against SDF-1α and CXCR4 were from eBioscience (San Diego, Calif) and Torrey Pines Biolabs (Houston, Tex), respectively. The SDF-1α ELISA kit was purchased from R&D Systems (Minneapolis, Minn). The terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay kit was from Roche Applied Science. C57BL/6 mice were housed in accordance with guidelines from the American Association for Laboratory Animal Care.
Cardiac Myocyte and Fibroblast Isolation
Ventricular cardiomyocytes and fibroblasts were isolated from adult male C57BL/6 mice. In brief, mice were heparinized (100 U IP) and anesthetized with sodium pentobarbital (100 mg/kg IP). The heart was quickly removed, and the aorta was cannulated and perfused retrogradely by the Langendorff method at a constant flow rate of 3 mL/min for 5 minutes with a Ca2+-free bicarbonate-based buffer containing (in mmol/L) 113 NaCl, 4.7 KCl, 1.2 MgSO4, 0.6 KH2PO4, 0.6 Na2HPO4, 5.5 glucose, 22 NaHCO3, 10 HEPES, 10 2,3-butanedione monoxime, and 30 taurine continuously gassed with 95% O2 plus 5% CO2. Myocyte isolation by enzymatic digestion was started by perfusing with liberase blendzyme 1 (0.25 mg/mL; Roche) and trypsin (0.14 mg/mL) in perfusion buffer containing 12.5 μmol/L Ca2+ for 8 to 10 minutes. The digested ventricular tissue was cut into pieces and gently triturated with a transfer pipette. The cell pellet was finally resuspended in perfusion buffer containing 500 μmol/L Ca2+ for experiments. Fibroblasts were expanded and used in experiments at passages 3 through 6 in culture in Dulbecco modified Eagle medium with 5% fetal bovine serum. Fibroblasts from neonatal rat hearts were isolated and cultured as described previously.16
Real-Time Reverse-Transcription Polymerase Chain Reaction and Reverse-Transcription Polymerase Chain Reaction Analysis of the SDF-1α and CXCR4 Transcripts
Total RNA was prepared from isolated mouse cardiac myocytes and cardiac fibroblasts using TriZol reagent (Invitrogen). For real-time reverse-transcription polymerase chain reaction (RT-PCR) and RT-PCR analysis, 3 μg of total RNA was reverse transcribed and used as template in PCR with cDNA-specific primers spanning the exon/intron junction. GAPDH served as a control for all reactions. The sequence of primers for RT-PCR was as follows: SDF-1α: unlabeled sense, 5′-CTC GGT GTC CTC TTG CTG TCC-3′; FAM-labeled antisense, 5′-CGG TAT CAG GCT GAC TGG TTT ACC G-3′; and GAPDH: unlabeled sense, 5′-CAC AAT GGG TCT GGG ATG GAA -3′; FAM-labeled antisense, 5′-CCA GCA AGG ACA CTG AGC AAG-3′; and the sequence of primers for standard PCR was as follows: SDF-1α: sense, 5′-ATG GAC GCC AAG GTC GTC GCC-3′; antisense, 5′-TTA CTT GTT TAA AGC TTT CTC -3′; CXCR4: sense, 5′-ATG GAA CCG ATC GTG AG-3′; anti-sense, 5′-CAG CTG AGG ATC ACG GCT AG-3′; and GAPDH: sense, 5′-CAC AAT GGG TCT GGG ATG GAA-3′; anti-sense 5′-CCA GCA AGG ACA CTG AGC AAG-3′. For quantitative real-time PCR after RT, 2 μL cDNA from RT reactions was used in reactions conducted with the Roche Lightcycler; denaturation 5 minutes at 94°C followed by 45 cycles of 5 seconds at 94°C, 10 seconds at 58°C, and 15 seconds at 72°C. For PCR after RT, 2 μL cDNA from RT reactions was denatured for 5 minutes at 94°C followed by 35 cycles of 1 minute at 94°C, 1 minute at 57°C, and 1 minute at 72°C, terminating with a 5-minute extension at 72°C. Copy number in RT-PCR experiments was calculated from SDF-1α and GAPDH cDNA standard curves.
SDF-1α and CXCR4 Immunoprecipitation
Control and treated myocytes and COS7 cells overexpressing SDF-1α and CXCR4 were harvested in lysis buffer and incubated with protein G agarose beads to preclear lysates. Lysates were then incubated for 4 hours with SDF-1α and CXCR4 antibodies and control rabbit IgG at 4°C and then with protein G agarose beads overnight at 4°C. Immunoprecipitated proteins were centrifuged and pellets washed twice with lysis buffer. Washed SDF-1α and CXCR4 antibody complexes were resolved by PAGE and transferred to polyvinylidene difluoride. SDF-1α and CXCR4 were then identified by Western analysis and enhanced chemiluminescence.
Hypoxia/Reoxygenation Studies in Cardiac Myocytes and Fibroblasts
Isolated mouse cardiac myocytes and fibroblasts were cultured at 106 per 60-mm dish precoated with laminin (10 μg/mL) for 1 hour in culture medium M1018 containing 2.5% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. To demonstrate CXCR4-specific function, cells were treated with the CXCR4-specific antagonist AMD3100 (40 μmol/L) for 4 hours before and during SDF-1α (25 nmol/L) treatment for 10 minutes. The culture medium was then immediately changed to ischemic buffer without SDF-1α containing (in mmol/L) 118 NaCl, 24 NaHCO3, 1.0 NaH2PO4, 2.5 CaCl2-2H2O, 1.2 MgCl2, 20 sodium lactate, 16 KCl, 10 2-deoxyglucose (pH adjusted to 6.2) under hypoxia conditions, 1% O2, 5% CO2, and 94% N2 at 37°C in an airtight Plexiglas chamber (Billups-Rothenberg, Del Mar, Calif) for 1, 4, and 16 hours. Control cells were cultured under normoxic conditions with 5% CO2 at 37°C. Cells were reoxygenated by replacing ischemic buffer with culture medium at 5% CO2 and 37°C for another 18 hours.
Cell viability was assessed by trypan blue staining, and the fraction of blue cells was quantified by light microscopy. In each dish, at least 200 cells were counted in 10 random fields. The extent of cellular injury also was monitored by measuring lactate LDH released into culture medium. LDH activity was measured at λ = 492 nm. LDH release was defined as the ratio of LDH activity in the medium to the LDH activity observed after total cell death according to the manufacturer’s protocol and was expressed as percent total LDH activity. Three independent experiments (each from a different heart) were performed in duplicate.
TUNEL Staining
Cardiomyocyte apoptotic cell death was detected by measuring nuclear DNA fragmentation by fluorescent TUNEL assay. In brief, myocytes in 2-chamber slides were fixed with 4% paraformaldehyde after treatment and stained for DNA fragmentation by TUNEL assay according to the manufacturer’s protocol. The slides were then mounted and counterstained with Vectashield mounting medium (Vector Laboratories, Burlingame, Calif). In each group, at least 200 cells were counted in 10 fields per experiment.
Western Blot Analysis
Isolated cardiomyocytes (2 to 3×105) were treated with 25 nmol/L SDF-1α and incubated for indicated time periods at 37°C. Treatments were terminated by the addition of excess cold PBS. Cells were homogenized immediately in lysis buffer containing (in mmol/L) 10 Tris, 150 NaCl (pH 7.5), 1 EDTA, 60 β-Gly PO4, 10 NaF, 1 phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Roche Applied Science). Total cell lysates were cleared by centrifugation at 9200g for 10 minutes. Samples were resolved by 12% SDS-PAGE and transferred to polyvinylidene difluoride membrane for Western analysis. Blots were incubated with primary antibody overnight at 4°C. All antibodies were used as recommended by the manufacturer. Proteins were detected using horseradish peroxidase–conjugated secondary antibody and enhanced chemiluminescence (ECL Plus Western blotting detection system, GE Healthcare Biosciences, Piscataway NJ) and quantified by densitometry.
SDF-1α ELISA
Cardiomyocyte and fibroblast SDF-1α was measured using the mouse SDF-1α Quantikine kit according to the manufacturer’s recommendation. Cell lysates were homogenized in lysis buffer as in the Western blot assays. Extracts were centrifuged at 9200g for 1 hour at 4°C. Supernatants were collected for ELISA assay. Optical density was measured at 450 nm with wavelength correction at 570 nm. Results were calculated from a standard curve generated by dilutions of a known amount of recombinant SDF-1α protein. Levels of SDF-1α were normalized to total protein and are presented as mean±SEM (in pg/100 μg protein). The SDF-1α concentration in each sample is the average of 3 independent experiments, each from a different heart.
In Vivo Infarct Studies
The murine model of myocardial ischemia/reperfusion has been described previously.17 Briefly, male C57BL/6 mice were anesthetized with sodium pentobarbital (60 mg/kg IP), intubated, and ventilated with room air supplemented with oxygen at a rate of 105 strokes per minute and a tidal volume of 0.3 mL. Body temperature was monitored carefully with a rectal probe and maintained at 37.0°C. To prevent a drop in blood pressure, blood from a donor mouse was given intravenously at 40 mL/kg in 3 equal boluses. After administration of antibiotics, the chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the mid left anterior descending coronary artery with an 8-0 nylon suture. The left anterior descending coronary artery was then occluded for 30 minutes followed by reperfusion. The chest was closed in layers, and the mice were allowed to recover. After 4 hours of reperfusion, the mouse was anesthetized with sodium pentobarbital (80 mg/kg IP), and the heart was removed and perfused retrogradely through an aortic cannula with Krebs-Henseleit buffer. The infarcted myocardium was delineated by perfusing the heart with 1% triphenyltetrazolium chloride in phosphate buffer for 5 minutes to stain viable myocardium. The region at risk was then delineated by tying the coronary artery at the site of the previous occlusion and perfusing the aortic root with 10% phthalo blue dye. Transverse left ventricle sections were fixed in 10% neutral buffered formaldehyde, weighed, and photographed under a stereomicroscope to measure the infarct region and the region at risk by computerized videoplanimetry. Infarct size was calculated as a percentage of the region at risk.
Statistical Analysis
All data are represented as mean±SEM. Differences between groups were compared by the Mann-Whitney test, Kruskal-Wallis statistic followed by Dunn’s multiple-comparison test, 1-way ANOVA followed by Bonferroni’s multiple-comparison test, and 2-way repeated-measures ANOVA (Graphpad, San Diego, Calif). A value of P<0.05 was considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
SDF-1α and CXCR4 Are Expressed in Murine Cardiac Myocytes and Fibroblasts
We identified SDF-1α as a potential cardioprotective factor in expression profiling studies using DNA microarrays. We found that SDF-1α mRNA increased 4.5-fold 3 hours after in vivo ischemic preconditioning (PC) (6 cycles of 4 minutes of coronary occlusion and 4 minutes of reperfusion) in mice (Figure 1), suggesting that this protein may play an important role in PC. Because SDF-1α has not been characterized in the normal myocardium, we determined the cell type–specific expression of SDF-1α and CXCR4 in myocytes and fibroblasts isolated from the adult mouse heart. RT-PCR for SDF-1α and CXCR4 demonstrated the presence of both mRNAs in myocytes and fibroblasts (Figure 2A). Both SDF-1α protein and CXCR4 protein were detected by immunoprecipitation and Western analysis from myocyte and fibroblast homogenates (Figure 2B). The finding that myocytes and fibroblasts express both SDF-1α and CXCR4 demonstrates that an SDF-1α–CXCR4 axis resides in the heart.

Induction of myocardial SDF-1α with ischemic PC. SDF-1α mRNA levels were determined in murine myocardium 3 hours after in vivo ischemic PC as described in Methods. SDF-1α mRNA was quantified by real time RT-PCR with normalization to GAPDH mRNA using Lux primers (Invitrogen). Values are mean±SEM. n = 6. *P<0.05 vs sham.

SDF-1α and CXCR4 expression in mouse cardiomyocytes and fibroblasts. Expression of SDF-1α and CXCR4 mRNA and protein was determined in cardiac myocytes and fibroblasts isolated from adult mouse hearts. A, mRNA was detected by RT-PCR using primers described in Methods. B, Protein was detected by immunoprecipitation and immunoblotting with antibodies against SDF-1α and CXCR4 (in which lentiviral overexpression of both SDF-1α and CXCR4 in 293FT cells served as positive control).
Hypoxia Induces SDF-1α Expression in Myocytes
Although SDF-1α expression has been shown to increase in response to hypoxia in noncardiac tissue,8,18 the response of SDF-1α to hypoxia in myocytes is unknown. SDF-1α mRNA and protein were measured in myocytes cultured under hypoxic conditions (Figure 3). After 4 hours of hypoxia, SDF-1α mRNA was upregulated 7.2-fold compared with normoxic levels (Figure 3A). Using immunoprecipitation, we found that SDF-1α protein also was upregulated after 4 hours of hypoxia (Figure 3B). In addition, increased SDF-1α protein was observed by ELISA in myocytes after 1 or 4 hours of hypoxia followed by 15 hours of reoxygenation (245.9±69.6 and 245.9±33.3 pg/100 μg protein, respectively [P<0.05] versus normoxia 122.4±18.7 pg/100 μg protein; Figure 3C). SDF-1α expression in cultured myocytes was punctate and localized to the cytoplasm (Figure IIA and IIB in the online Data Supplement). Hypoxia did not change overall SDF-1α localization but did increase immunostaining. The effect of hypoxia on SDF-1α also was determined in cardiac fibroblasts. Unlike myocytes, SDF-1α protein was not induced in cardiac fibroblasts, although basal levels were higher than in myocytes (Figure 3D). Thus, although both myocytes and fibroblasts express SDF-1α, only myocytes exhibit a significant response to hypoxia with an increase in SDF-1α.
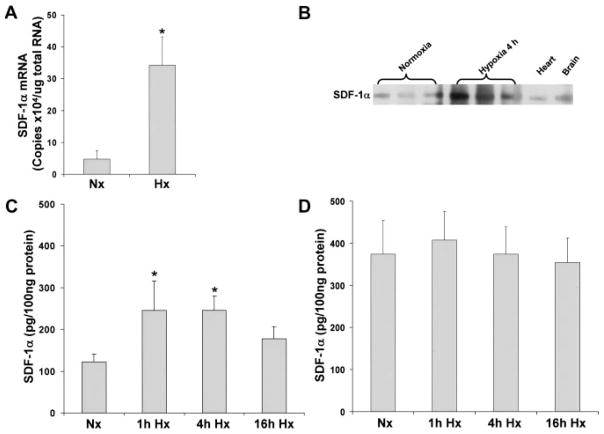
SDF-1α is induced with hypoxia/reoxygenation in myocytes but not in fibroblasts. Cultured murine myocytes and murine fibroblasts were exposed to hypoxia (Hx) followed by reoxygenation or normoxia (Nx) as described in Methods. SDF-1α mRNA was measured by real time RT-PCR (A). SDF-1α protein was determined by immunoprecipitation (B); ELISA after 1, 4, and 16 hours of hypoxia and 16 hours of reoxygenation in myocytes (C) and fibroblasts (D). Values are mean±SEM. n = 3. *P<0.05 vs normoxia.
The SDF-1α-CXCR4 Axis Activates MAPK and AKT Signaling in Myocytes
To determine whether myocyte CXCR4 is functional and regulates signaling, phosphorylation of ERK was measured in isolated myocytes exposed to increasing concentrations of SDF-1α for 5 minutes. Incubation with SDF-1α resulted in an increase in ERK phosphorylation that was evident at 6 nmol/L and maximal at 25 nmol/L (Figure 4). Total ERK remained unchanged (data not shown). These results demonstrate that CXCR4 is present in myocytes and is functionally coupled to downstream signaling.

SDF-1α stimulates ERK phosphorylation in a dose-dependent manner. Myocytes were treated with increasing SDF-1α concentrations for 5 minutes. A, Phospho-ERK (p-ERK) was measured by Western analysis with anti–phospho-p42/44 (top) and anti-tubulin antibodies (bottom). B, Summary of Western analysis after densitometry. n = 3. Data are mean±SEM. *P<0.05 vs vehicle.
Signaling studies in myocytes were expanded to include the MAPKs, JNK, p38, and AKT. Using the optimal dose of SDF-1α identified in the ERK studies (25 nmol/L), we incubated myocytes for 5 or 30 minutes with SDF1-1α (Figure 5). ERK phosphorylation was increased at 5 minutes (P<0.05), returning to baseline levels by 30 minutes (Figure 5A). AKT also was activated rapidly, with increased phosphorylation at 5 minutes (P<0.05), also returning to baseline levels by 30 minutes. Conversely, both JNK and p38 phosphorylation was dramatically decreased at the early 5-minute time point (P<0.01), returning to baseline levels by 30 minutes. Overall, the SDF-1α action on myocyte signaling was rapid, with changes occurring by 5 minutes and then disappearing by 30 minutes, and with a selective increase in ERK and AKT phosphorylation and a decrease in JNK and p38 phosphorylation (Figure 5B).
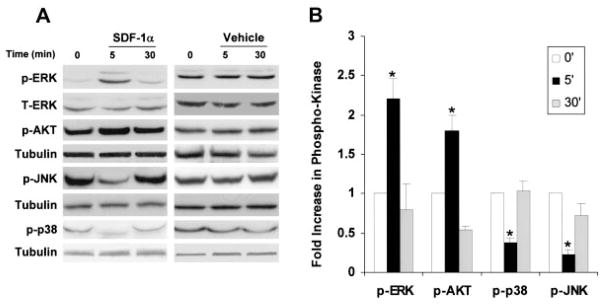
SDF-1α regulation of MAPK and AKT signaling in cardiomyocytes. A, Representative Western analysis of myocyte phospho-ERK, -AKT, -p-38, -JNK, and tubulin in the presence or absence of 25 nmol/L SDF-1α for 5 and 30 minutes. B, Summary and quantification of myocyte phospho-ERK, -AKT, -p38, and -JNK Western analyses normalized to total protein. Values are mean±SEM of phospho-ERK, -AKT, -p38, and -JNK normalized to total protein. n = 3.*P<0.05. p-ERK indicates phospho-ERK; p-AKT, phospho-AKT; -p-p38, phospho-p38; and p-JNK, phospho-JNK.
The SDF-1α-CXCR4 Axis Promotes Protection Against Hypoxia/Reoxygenation Cell Death
To determine whether SDF-1α has the potential to promote cardiomyocyte survival and serve a cardioprotective capacity, cultured adult murine myocytes were treated with SDF-1α or vehicle and then subjected to hypoxia and reoxygenation in the absence of SDF-1α. Myocyte survival was evaluated by 2 independent methods: trypan blue exclusion and LDH release (indexes of cell membrane integrity) and apoptotic cell death, as assessed by TUNEL staining.
After SDF-1α (25 nmol/L) treatment, myocytes were incubated in ischemic buffer under hypoxic conditions for 1 hour followed by normoxic reoxygenation for 18 hours. Hypoxia/reoxygenation significantly increased trypan blue staining in untreated myocytes (Figure 6A). SDF-1α–treated myocytes were more resistant to damage and exhibited less uptake of trypan blue. Consistent with the changes in trypan blue uptake, LDH release was increased with hypoxia (Figure 6B), and this increase in LDH was attenuated by SDF-1α treatment before hypoxia. To determine whether the increased myocyte survival resulted from a specific SDF-1α–CXCR4 interaction, we used the CXCR4-specific antagonist AMD3100.19 AMD3100 binds competitively to CXCR4, effectively blocking >90% of SDF-1α binding within 30 minutes with slower-release kinetics.20 Pretreatment of myocytes with AMD3100 before exposure to SDF-1α (Figure 6A and 6C) blocked the actions of SDF-1α on myocyte survival, increasing the number of trypan blue–positive myocytes and the level of LDH release back to those observed with hypoxia/reoxygenation alone. A causative relationship between SDF-1α–CXCR4 activation, ERK and AKT activation, and myocyte survival was tested using inhibitors of ERK and AKT, PD98059 and LY290042, respectively. Pretreatment of myocytes with PD98059 or LY290042 before 5 minutes of SDF-1α treatment effectively blocked SDF-1α–mediated ERK and AKT phosphorylation (online Data Supplement). Pretreatment of myocytes with PD98059 and LY290042 before SDF-1α treatment and hypoxia reoxygenation effectively blocked the increase in myocyte viability, increasing trypan blue staining (Figure 6B) and LDH release (Figure 6D) and demonstrating ERK and AKT are required for SDF-1α–mediated myocyte survival after hypoxia reoxygenation. Collectively, these data indicate that the SDF-1α–CXCR4 axis in cardiac myocytes is cardioprotective.

SDF-1α protects myocytes from hypoxia/reoxygenation-induced cell death. Cultured myocytes were treated with 25 nmol/L SDF-1α or vehicle before exposure to 1 hour of hypoxia followed by 18 hours of reoxygenation. Myocytes also were treated with 40 μmol/L AMD3100 for 4 hours before the addition of SDF-1α. Cell death was assessed by trypan blue exclusion and LDH release. A, Trypan blue exclusion assay demonstrated increased cell death in hypoxia, which was prevented by SDF-1α (25 nmol/L); AMD3100 blocked the effects of SDF-1α. B, ERK and AKT inhibition by 20 μmol/L PD98059 and 20 μmol/L LY294002 blocks SDF-1α protection from hypoxia/reoxygenation, decreasing trypan blue cell exclusion. C, LDH release was highest in the hypoxia group; this was prevented by SDF-1α, and the effect was reversed by AMD3100. D, ERK and AKT inhibition by 20 μmol/L PD98059 and 20 μmol/L LY294002 blocks SDF-1α protection from hypoxia/reoxygenation, increasing LDH release. Values are mean±SEM. *P<0.05 vs normoxia (Nx), **P<0.01 vs Nx, †P<0.05 vs hypoxia (Hx). n = 3.
The increased ERK and AKT phosphorylation after SDF-1α suggests that apoptotic signaling may be attenuated. Accordingly, apoptosis was evaluated in myocytes exposed to hypoxia/reoxygenation with and without SDF-1α pretreatment in the presence and absence of AMD3100. Hypoxia/reoxygenation increased TUNEL-positive nuclei, indicating apoptotic cell death (Figure 7). SDF-1α pretreatment decreased the number of TUNEL-positive nuclei to normoxic levels, and this antiapoptotic action of SDF-1α was blocked by AMD3100. Thus, the SDF-1α–CXCR4 axis promotes myocyte survival through the recruitment of an antiapoptotic signaling program.
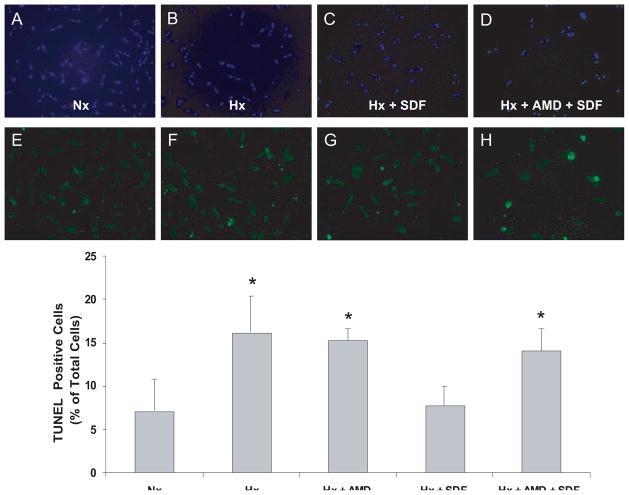
SDF-1α inhibits apoptosis in cardiomyocytes. Myocytes pretreated with 40 μmol/L AMD3100 or vehicle for 40 minutes were stimulated with 25 nmol/L SDF-1α for 10 minutes followed by 1 hour of hypoxia (Hx) and 18 hours of reoxygenation. Apoptotic nuclei were determined by TUNEL assay. A and E, Normoxic (Nx) control; B and F, 1 hour of Hx and 18 hours of reoxygenation; C and G, 25 nmol/L SDF-1α before 1 hour of Hx and 18 hours of reoxygenation; D and H, 40 μmol/L AMD pretreatment with 25 nmol/L SDF-1α before 1 hour of Hx and 18 hours of reoxygenation; A through D, total nuclei (DAPI staining); E through H, TUNEL-positive myocyte nuclei (green fluorescent). Bottom, Summary of quantitative data from 3 independent experiments performed in duplicate. n = 3. *P<0.05 vs Nx.
SDF-1α Protects the Myocardium From Ischemia/Reperfusion Damage In Vivo
The in vitro signaling and functional studies summarized above demonstrate that SDF-1α binding to its receptor, CXCR4, activates antiapoptotic signaling pathways capable of protecting cardiac myocytes from hypoxia/reoxygenation damage. Next, we determined whether SDF-1α–CXCR4 can exert this protective function in vivo. Preliminary studies were conducted to identify the dose of SDF-1α that would be capable of reducing infarct size. An SDF-1α dose of 14 μg · kg−1 · min−1, estimated to result in plasma levels similar to the SDF-1α concentration used in in vitro experiments, produced a small decrease in infarct size. Thus, we selected a higher dose (35 μg · kg−1 · min−1). SDF-1α was infused at 35 μg · kg−1 · min−1 into the left ventricular cavity of male C57BL/6 mice for 5 minutes followed by 10 minutes of washout before a 30-minute left anterior descending coronary artery occlusion and 4-hour reperfusion.
The risk region was similar among the control (group 1), early PC (group 2), AMD 3100 (group 3), SDF-1α (group 4), and AMD3100+SDF-1α (group 5) mice (Table 1). No differences existed in heart rate or temperature among the 5 groups (Table 2), except that heart rate in the SDF-1α group was slightly lower. Early ischemic PC (6 cycles of 4 minutes of coronary artery occlusion followed by 4 minutes of reperfusion) significantly decreased infarct size after 4 hours of reperfusion (Table 1 and Figure 8). SDF-1α pretreatment significantly reduced infarct size compared with control (29.4±3.9% versus 61.5±3.0%, respectively, of the risk region; P<0.05; Table 1 and Figure 8); the reduction was similar to that induced by ischemic PC. Infusion of AMD3100 (10 μg/kg IV) 30 minutes before SDF-1α treatment blocked the reduction in infarct size with SDF-1α (53.8±5.1% versus 29.4±3.9%, respectively, of the risk region; Table 1 and Figure 8). Thus, in vivo administration of SDF-1α protects the myocardium from ischemia/reperfusion damage, in keeping with the protective actions observed in cultured myocytes.

SDF-1α reduces infarct size in vivo. Mice were preconditioned either with 6 cycles of 4 minutes of coronary occlusion/reperfusion (group 2) or SDF-1α administered into the LV cavity at 35 μg · kg−1 · min−1 for 5 minutes (group 4) ending 10 minutes before a 30-minute coronary artery occlusion followed by 4 hours of reperfusion. The CXCR4 antagonist AMD3100 (10 μg/kg IV) was infused in control (group 3) and 30 minutes before SDF-1α administration (group 5). These mice were compared with mice that underwent sham surgery and a 30-minute occlusion followed by 4 hours of reperfusion (group 1). Infarct size is expressed as percent of the region at risk. MI indicates myocardial infarction. *P<0.05 vs group 1.
TABLE 1
Size of Left Ventricle, Risk Region, and Infarct
| Group | Age, wk | Body Wt, g | Heart Wt, mg | Heart Wt/Body Wt | LV Wt, mg | Risk Region Wt, mg | Infarct Wt, mg | Risk Region, % of LV | Infarct, % of Risk Region | Infarct, %of LV |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 (AMI; n = 10) | 18±2 | 30.0 ±1.0 | 157.7±7.9 | 0.53±0.02 | 117.3±7.0 | 49.6±4.1 | 30.0 ±2.4 | 42.8 ±3.5 | 61.5±3.0 | 26.1±2.2 |
| Group 2 (EPC, AMI; n = 7) | 20±1 | 31.4±0.7 | 161.0±7.8 | 0.51±0.02 | 125.4±5.5 | 49.1±2.1 | 9.9 ±1.7* | 39.5 ±2.1 | 19.8±2.6* | 7.8±1.2* |
| Group 3 (AMD, AMI; n = 9) | 20±3 | 27.5±1.5 | 125.0±8.0 | 0.50±0.0 | 92.9±6.0 | 33.2±2.6 | 17.0 ±1.9 | 36.0 ±2.1 | 51.1±3.5 | 18.3±1.5 |
| Group 4 (SDF-1α, AMI; n = 8) | 17±3 | 28.6 ±2.1 | 121.9±4.7 | 0.44±0.02 | 91.0±4.2 | 32.9±1.9 | 9.7 ±1.3* | 36.1 ±1.2 | 29.4±3.9* | 10.6±1.5* |
| Group 5 (AMD+SDF-1α, AMI; n = 6) | 12±1 | 26.1 ±0.6 | 120.0±4.1 | 0.46±0.02 | 90.3±2.6 | 34.7±1.5 | 18.6 ±1.7 | 38.7 ±2.2 | 53.8±5.1 | 20.4±1.3 |
Wt indicates weight; Heart Wt, total heart weight (ventricles and atria); LV, left ventricle; AMI, acute myocardial infarction; and EPC, early PC. Data are mean±SEM.
TABLE 2
Rectal Temperature and Heart Rate on the Day of the 30-Minute Coronary Occlusion
| Preocclusion | Occlusion
| Reperfusion
| |||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature, °C | |||||||
 Group 1 (AMI) Group 1 (AMI) | 37.0±0.1 | 37.1±0.1 | 37.0±0.0 | 37.1±0.0 | 37.0±0.1 | 37.1±0.1 | 37.1±0.2 |
 Group 2 (EPC) Group 2 (EPC) | 37.0±0.1 | 37.0±0.1 | 36.9±0.1 | 36.9±0.1 | 37.0±0.1 | 37.0±0.1 | 36.9±0.1 |
 Group 3 (AMD) Group 3 (AMD) | 37.1±0.1 | 37.2±0.0 | 37.0±0.0 | 37.1±0.1 | 37.1±0.0 | 37.1±0.1 | 36.9±0.1 |
 Group 4 (SDF-1α–induced EPC) Group 4 (SDF-1α–induced EPC) | 37.1±0.1 | 37.0±0.1 | 36.9±0.1 | 36.9±0.1 | 36.9±0.1 | 37.0±0.1 | 36.9±0.1 |
 Group 5 (AMD+SDF-1α–induced EPC) Group 5 (AMD+SDF-1α–induced EPC) | 37.1±0.1 | 37.1±0.1 | 37.1±0.1 | 37.2±0.0 | 37.0±0.1 | 36.9±0.1 | 36.8±0.1 |
| Heart rate, bpm | |||||||
 Group 1 (AMI) Group 1 (AMI) | 522±23 | 543±21 | 537±22 | 553±16 | 559±22 | 552±17 | 558±25 |
 Group 2 (early PC) Group 2 (early PC) | 533±9 | 542±13 | 534±10 | 535±8 | 543±13 | 544±14 | 556±21 |
 Group 3 (AMD) Group 3 (AMD) | 527±13 | 563±20 | 534±18 | 530±18 | 532±16 | 542±18 | 551±19 |
 Group 4 (SDF-1α–induced EPC) Group 4 (SDF-1α–induced EPC) | 505±21 | 484±15 | 466±15 | 478±13 | 496±18 | 495±18 | 556±23 |
 Group 5 (AMD+SDF1-α–induced EPC) Group 5 (AMD+SDF1-α–induced EPC) | 565±17 | 557±13 | 556±10 | 559±17 | 561±15 | 533±20 | 536±15 |
AMI indicates acute myocardial infarction; EPC, early PC. Data are mean±SEM. Measurements of rectal temperature and heart rate were taken before the 30-minute coronary occlusion (preocclusion); at 5, 15, and 30 minutes into the 30-minute occlusion; and at 5, 15, and 30 minutes after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text. The experimental protocols for the 4 groups of mice are specified in the text.
Discussion
The chemokine SDF-1α and its receptor, CXCR4, have attracted considerable attention as a result of their involvement in hematopoietic regulation and metastatic cancers. Recently, SDF-1α has been found to increase after myocardial infarction and in heart failure,11–13 and its chemoattractant properties have been demonstrated to enhance the recruitment of circulating hematopoietic stem cells to the infarcted myocardium.14,15 Although these actions of SDF-1α are well known, the direct actions of SDF-1α on the myocardium have not been studied. Because our earlier gene expression studies with microarrays demonstrated that SDF-1α was markedly upregulated after ischemic PC in mouse hearts, we sought to examine the effect of this chemokine on myocardial ischemia/reperfusion injury. Our results demonstrate that SDF-1α is expressed in adult cardiomyocytes, is induced with hypoxia, and acts through its cognate receptor, CXCR4. This cardiac ligand-receptor axis, SDF-1α–CXCR4, is functional and couples to downstream MAPK and AKT signaling. Its activation results in protection not only against hypoxia/reoxygenation damage in vitro but also against myocardial ischemia/reperfusion injury in vivo. To the best of our knowledge, this is the first report demonstrating the existence of a cardiac SDF-1α–CXCR4 autocrine/paracrine signaling module that confers protection against ischemia/reperfusion damage.
The SDF-1α–CXCR4 axis has been shown to promote survival of myeloid progenitor cells4; neural, oligodendrocyte, and trophoblast precursors3,21; and glioma cells2 in response to several stimuli associated primarily with serum deprivation or apoptotic cell death during development or differentiation. The present study reveals a new function of SDF-1α–CXCR4 activation, protection against ischemia/reperfusion injury. In vitro and in vivo studies with the CXCR4 antagonist AMD3100 confirmed that the actions of SDF-1α occurred through the activation of CXCR4. Furthermore, experiments performed in isolated adult mouse cardiac myocytes demonstrate that the protective action was by direct activation of myocyte CXCR4.
SDF-1α–mediated survival in cardiac myocytes was associated with increased activation of ERK and AKT and decreased activation of p38 and JNK. SDF-1α–mediated survival in CD4 T cells and glioma cells also has been associated with activation of ERK and AKT.2,22 ERK and AKT signaling pathways are thought to be protective through inhibition of several proapoptotic factors23,24; in the present study, their antiapoptotic actions were demonstrated by decreased TUNEL staining in hypoxia/reoxygenation experiments in vitro. In addition to activation of ERK and AKT, SDF-1α decreased phosphorylation of both JNK and p38. The actions of JNK and p38 on cell survival have been ambiguous, and these kinases have been reported to modulate apoptosis both negatively and positively.22,25 Thus, decreased activation in adult mouse myocytes may serve to decrease apoptotic pressure. SDF-1α has been reported to activate the phosphatase SHP-1, resulting in CXCR4 interaction26 and negative regulation of MAPK signaling,27 which may account for decreased JNK and p38 signaling. We found that SDF-1α modulation of signaling was rapidly attenuated after the initial activation at 5 minutes. Studies have reported the involvement of GRK6 and RGS 16 in downregulation of CXCR4 signaling, limiting functional coupling.28,29 The functional consequences of downregulation/desensitization of SDF-1α–CXCR4 signaling with respect to myocardial protection are unknown.
To determine whether the SDF-1α–CXCR4 axis exists in the heart as a paracrine or autocrine system, we examined the induction of SDF-1α in response to one of its known stimuli, hypoxia. Isolated adult myocytes exposed to increasing durations of hypoxia exhibited increased production of SDF-1α mRNA and protein. Increased SDF-1α production in response to hypoxia/ischemia has been described in several tissues, including infarcted myocardium,9,14,15 and has recently been reported to depend on activation of the hypoxia-inducible transcription factor-1.8 The induction of SDF-1α production in adult myocytes suggests that it may play an important role in myocardial protection. The postnatal and in utero lethality of SDF-1α and CXCR4 knockouts,5,7 respectively, demonstrates the importance of the SDF-1α–CXCR4 axis in development. The presence of a cardiac SDF-1α–CXCR4 axis and the induction of SDF-1α with hypoxia also may play an important role in the attraction of CXCR4pos cardiac30 and noncardiac31 stem cells. Indeed, upregulation of SDF-1α in the heart by transplantation of SDF-1α–overexpressing fibroblasts14 or by viral infection15 has been shown to increase stem cell infiltration. Whether the cardiac SDF-1α–CXCR4 axis plays a role in modulating endogenous stem cell function is currently unknown.
Conclusions
The present study demonstrates for the first time the presence of a cardiac SDF-1α–CXCR4 axis that is capable of providing protection against ischemia/reperfusion-induced damage. SDF-1α and CXCR4 were found to reside on both myocytes and fibroblasts. The induction of SDF-1α with hypoxia in vitro and ischemic PC in vivo suggests that this chemokine is important for myocardial protection through either a paracrine or an autocrine mechanism. SDF-1α–CXCR4 –induced cardioprotection is associated with MAPK and AKT activation and decreased apoptosis. These findings provide new insights into the role of SDF-1α–CXCR4 in cardiac biology and may open new avenues to elucidate the molecular mechanism of myocardial repair and tissue regeneration.
Supplementary Material
1
2
3
4
5
Acknowledgments
Sources of Funding
The present study was supported by National Institutes of Health grants HL-071896, HL-55757, HL-70897, HL-76794, and HL-78825.
Footnotes
The online-only Data Supplement, consisting of Methods and figures, is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.106.672451/DC1.
Disclosures
None.
References
Full text links
Read article at publisher's site: https://doi.org/10.1161/circulationaha.106.672451
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.106.672451
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Role of HIF-1α-Activated IL-22/IL-22R1/Bmi1 Signaling Modulates the Self-Renewal of Cardiac Stem Cells in Acute Myocardial Ischemia.
Stem Cell Rev Rep, 20(8):2194-2214, 12 Sep 2024
Cited by: 0 articles | PMID: 39264501 | PMCID: PMC11554697
Repair of the Infarcted Heart: Cellular Effectors, Molecular Mechanisms and Therapeutic Opportunities.
Circ Res, 134(12):1718-1751, 06 Jun 2024
Cited by: 3 articles | PMID: 38843294
Review
Elevated levels of plasma inactive stromal cell derived factor-1α predict poor long-term outcomes in diabetic patients following percutaneous coronary intervention.
Cardiovasc Diabetol, 23(1):114, 30 Mar 2024
Cited by: 0 articles | PMID: 38555431 | PMCID: PMC10981820
ALK1 Deficiency Impairs the Wound-Healing Process and Increases Mortality in Murine Model of Myocardial Infarction.
J Cardiovasc Transl Res, 17(3):496-504, 08 Dec 2023
Cited by: 1 article | PMID: 38064044
Vagus nerve stimulation-induced stromal cell-derived factor-l alpha participates in angiogenesis and repair of infarcted hearts.
ESC Heart Fail, 10(6):3311-3329, 29 Aug 2023
Cited by: 3 articles | PMID: 37641543 | PMCID: PMC10682864
Go to all (220) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human coronary microvascular effects of cardioplegia-induced stromal-derived factor-1alpha.
Ann Thorac Surg, 82(2):657-663, 01 Aug 2006
Cited by: 3 articles | PMID: 16863781
SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury.
Am J Physiol Heart Circ Physiol, 301(4):H1496-505, 05 Aug 2011
Cited by: 57 articles | PMID: 21821779 | PMCID: PMC3197365
Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells.
Cancer Res, 62(21):6304-6311, 01 Nov 2002
Cited by: 165 articles | PMID: 12414661
The SDF-1/CXCR4 axis in stem cell preconditioning.
Cardiovasc Res, 94(3):400-407, 26 Mar 2012
Cited by: 87 articles | PMID: 22451511
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (11)
Grant ID: P01 HL078825
Grant ID: HL-071896
Grant ID: HL-55757
Grant ID: R01 HL071896
Grant ID: R37 HL055757
Grant ID: HL-76794
Grant ID: HL-78825
Grant ID: HL-70897
Grant ID: R01 HL076794
Grant ID: R01 HL055757
Grant ID: R01 HL070897





