Abstract
Purpose
Add systemic bevacizumab (Bev) to adjuvant hepatic arterial infusion (HAI) plus systemic therapy after liver resection to increase recurrence-free survival (RFS).Patients and methods
Patients were randomly assigned to HAI plus systemic therapy with or without Bev. If 1-year RFS of ≥ 80% was obtained in Bev arm, then the regimen would be studied further. HAI with fluorodeoxyuridine plus dexamethasone was given on days 1 to 14 of a 5-week cycle. Systemic therapy and Bev 5 mg/kg was delivered on days 15 and 29: oxaliplatin 85 mg/m², leucovorin 400 mg/m², and fluorouracil 2,000 mg/m² infusion for 2 days (if patients received prior oxaliplatin, then irinotecan 150 mg/m² was used). RFS and survival were estimated by using the Kaplan-Meier method and compared by using the log-rank test.Results
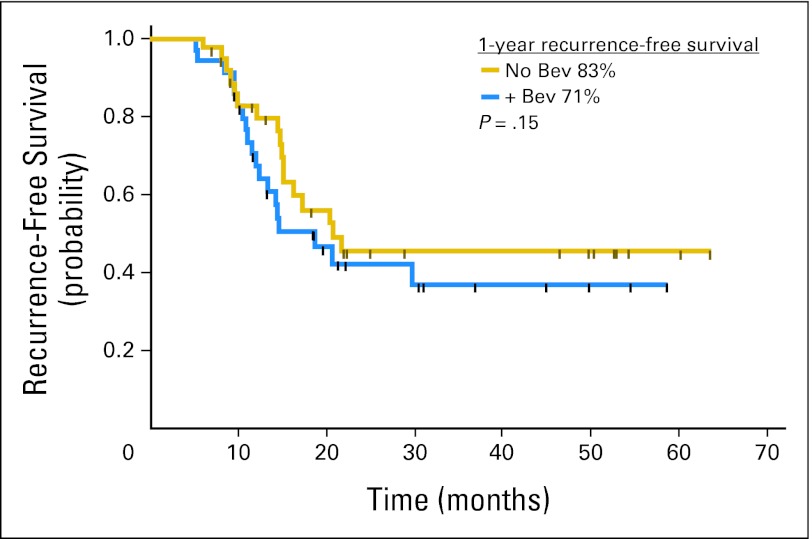
The two arms had similar characteristics: synchronous disease (66% v 63%), more than one metastasis (84% v 74%), and clinical risk score ≥ 3 (50% v 46%) for no Bev versus Bev arms, respectively. With a median follow-up of 30 months, 4-year survival was 85% and 81% (P = .5), and 4-year RFS was 46% versus 37%; 1-year RFS was 83% and 71% (P = .4) for no Bev versus Bev arms. Bilirubin > 3 mg/dL was seen in zero of 38 versus five of 35 patients (P = .02) and biliary stents were placed in zero versus four patients (P = .05) in no Bev versus Bev arms.Conclusion
The addition of Bev to adjuvant HAI plus systemic therapy after liver resection did not seem to increase RFS or survival but appeared to increase biliary toxicity. Four-year survival was 85% and 81% for no Bev and Bev arms, respectively.Free full text

Randomized Phase II Trial of Adjuvant Hepatic Arterial Infusion and Systemic Chemotherapy With or Without Bevacizumab in Patients With Resected Hepatic Metastases From Colorectal Cancer
Abstract
Purpose
Add systemic bevacizumab (Bev) to adjuvant hepatic arterial infusion (HAI) plus systemic therapy after liver resection to increase recurrence-free survival (RFS).
Patients and Methods
Patients were randomly assigned to HAI plus systemic therapy with or without Bev. If 1-year RFS of ≥ 80% was obtained in Bev arm, then the regimen would be studied further. HAI with fluorodeoxyuridine plus dexamethasone was given on days 1 to 14 of a 5-week cycle. Systemic therapy and Bev 5 mg/kg was delivered on days 15 and 29: oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and fluorouracil 2,000 mg/m2 infusion for 2 days (if patients received prior oxaliplatin, then irinotecan 150 mg/m2 was used). RFS and survival were estimated by using the Kaplan-Meier method and compared by using the log-rank test.
Results
The two arms had similar characteristics: synchronous disease (66% v 63%), more than one metastasis (84% v 74%), and clinical risk score ≥ 3 (50% v 46%) for no Bev versus Bev arms, respectively. With a median follow-up of 30 months, 4-year survival was 85% and 81% (P = .5), and 4-year RFS was 46% versus 37%; 1-year RFS was 83% and 71% (P = .4) for no Bev versus Bev arms. Bilirubin > 3 mg/dL was seen in zero of 38 versus five of 35 patients (P = .02) and biliary stents were placed in zero versus four patients (P = .05) in no Bev versus Bev arms.
Conclusion
The addition of Bev to adjuvant HAI plus systemic therapy after liver resection did not seem to increase RFS or survival but appeared to increase biliary toxicity. Four-year survival was 85% and 81% for no Bev and Bev arms, respectively.
INTRODUCTION
Each year, 149,000 new cases of colorectal cancer are diagnosed.1 Fifteen percent of the patients will have hepatic metastases at diagnosis, and 60% of the patients with metastatic disease will develop liver metastases.2 Patients with hepatic metastases who undergo complete liver resection have a 5-year survival of 30% to 50%3,4; however, up to 70% of these patients will have disease recurrence.5
Systemic therapy alone (in the adjuvant setting after liver resection) has been evaluated in several studies.6 A meta-analysis showed an increase in disease-free survival (DFS) with the use of systemic fluorouracil (FU) and leucovorin (LV) only after adjustment for poor prognostic factors.7 Systemic FU/LV/oxaliplatin (FOLFOX) both before and after liver resection demonstrated a significant increase in progression-free survival (PFS) when compared with no systemic treatment in resected patients,8 although the difference in PFS was not significant in the intention-to-treat population of the study. Hepatic arterial infusion (HAI) with or without systemic therapy significantly increased DFS when compared with systemic therapy alone (or with no further therapy) in three of four randomized studies.9–13 Since bevacizumab (Bev) had demonstrated increased survival in patients with metastatic disease,14 this study was designed to address whether adding Bev to HAI plus systemic chemotherapy would increase recurrence-free survival (RFS) after hepatic resection.
PATIENTS AND METHODS
Eligible patients had histologically confirmed colorectal adenocarcinoma with fully resected liver metastases. Exclusion factors were extrahepatic disease, prior hepatic radiation, infection, history of stroke or transient ischemic attack, history of serious systemic illness, Karnofsky performance score < 60, other malignancy (within 5 years before study entry), WBC count ≤ 3,000/μL, absolute neutrophil count < 1,500/μL, platelet count ≤ 75,000/μL, and total bilirubin > 2.0 mg/dL. Prior chemotherapy was permitted provided the last dose was given ≥ 3 weeks before study entry. Computed tomography (CT) scans of the chest, abdomen, and pelvis were required within 6 weeks before protocol registration. All patients provided signed informed consent; the protocol and informed consent were approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) institutional review board.
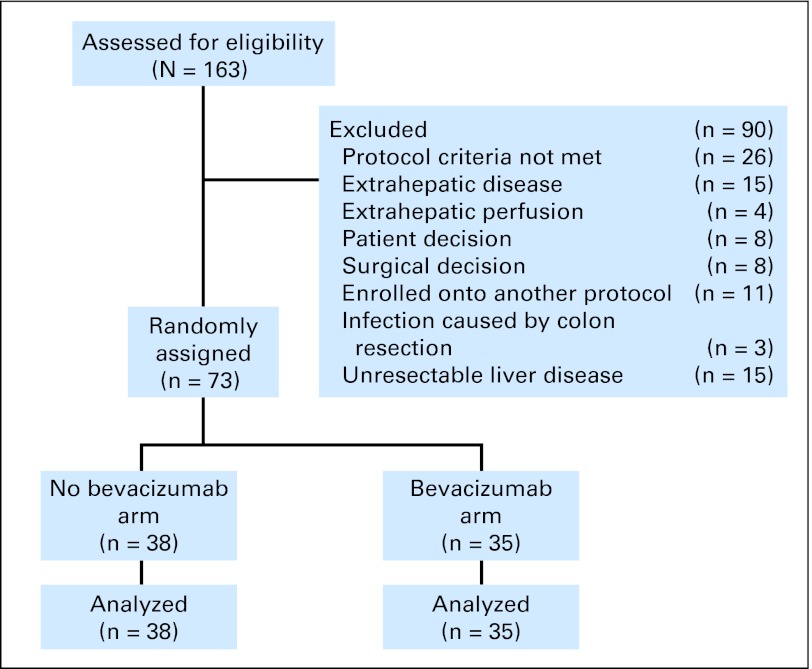
Patient assessment for protocol entry is shown in the CONSORT diagram (Fig 1). Pretreatment evaluation included complete medical and surgical histories, physical examinations, and appropriate laboratory studies. To determine adequate hepatic arterial anatomy, all patients underwent preoperative hepatic CT angiograms with visualization of the celiac and superior mesenteric arteries. Guidelines for pump placement have previously been reported.15 An intraoperative injection of methylene blue dye and a postoperative 99mTc (metastable nuclear isomer of technetium-99) macroaggregated albumin perfusion study via the pump's sideport were done to confirm hepatic confinement of the pump effluent and adequate perfusion of the liver remnant.
Chemotherapy Administration and Toxicity Evaluation
Patients commenced chemotherapy 4 to 5 weeks postsurgery. On day 1 of a 5-week cycle, HAI with fluorodeoxyuridine (FUDR, Bedford Laboratories, Bedford, OH) was infused at a dose of 0.12 mg × kg × pump volume (30 mL) divided by pump flow rate (supplied by the pump manufacturer; Codman and Schurtleff, Raynham, MA). For patients who were ≥ 25% above ideal weight, the actual dose of fluorodeoxyuridine was calculated by using a weight that averaged the patient's actual weight and their ideal weight. During fluorodeoxyuridine administration, dexamethasone was administered concurrently at the rate of 1.0 mg × kg × 30 mL divided by pump flow rate and combined with 30,000 units of unfractionated heparin and normal saline in a quantity sufficient to fill the pump reservoir to 30 mL. On days 15 and 29 of each cycle, the residual was removed from the pump and the pump was refilled with 30 mL heparinized saline. On days 15 and 29, systemic therapy was also administered (Table 1 ). Patients who were chemonaive or who received chemotherapy regimens without oxaliplatin, received oxaliplatin 85 mg/m2 (2-hour infusion) concurrently with LV 400 mg/m2 via a Y-connection, followed by FU 2,000 mg/m2 delivered via external pump for a 48-hour infusion. Patients previously treated with oxaliplatin received irinotecan (over 30 minutes) 150 mg/m2 concurrently with the same doses of FU and LV. Patients received Bev 5 mg/kg on days 15 and 29 since systemic chemotherapy was administered on those days. Six cycles of therapy were to be administered. All toxicities were graded according to the National Cancer Institute Common Toxicity Criteria 3.0 (CTC 3.0).16 Liver function blood tests were evaluated on days 1, 15, and 29 of each cycle. Changes in doses of fluorodeoxyuridine were calculated according to the changes in liver function tests. Because patients entered the study with various degrees of hepatic enzyme abnormalities, hepatic toxicity from treatment was defined as a significant increase over individual baseline values (twofold or greater for alkaline phosphatase, threefold or greater for AST, and 1.5-fold or greater increase in bilirubin). Doses were held or lowered as outlined previously.17 Epigastric pain or severe abdominal pain during HAI treatment resulted in emptying of drug from the pump and evaluation of pain (including a repeat flow scan to rule out extrahepatic perfusion).
Table 1.
HAI and Systemic Therapy
| Day 1 | Day 15 | Day 29 | Day 36 (day 1 of next cycle) |
|---|---|---|---|
| HAI with fluorodeoxyuridine + dexamethasone* | Systemic chemotherapy† with or without Bev‡ | Systemic chemotherapy† with or without Bev‡ | HAI with fluorodeoxyuridine + dexamethasone* |
Abbreviations: HAI, hepatic arterial infusion; Bev, bevacizumab.
Patients were required to have an absolute neutrophil count > 1,000/μL, platelet count ≥ 75,000/μL, and creatinine ≤ 1.8 mg/dL for subsequent cycles of systemic chemotherapy to be administered. If counts were below these levels on the scheduled day, treatment was delayed for 1 week. After treatment completion, CT scans of the chest, abdomen, and pelvis, carcinoembryonic antigen, and liver function tests were performed every 3 months for the first 2 years, every 4 months for the next 2 years, every 6 months after 4 years, and yearly after 5 years.
Study Design and Statistical Analysis
The primary objective was to determine whether intravenous Bev added to adjuvant HAI and systemic chemotherapy after liver resection increased RFS. Secondary end points were evaluation of overall survival and toxicity.
After stratification by clinical risk score and previous chemotherapy, 80 patients with resected liver metastases were to be randomly assigned to HAI plus systemic therapy with or without Bev. If the 1-year RFS in the Bev arm was 80% or higher, that would be considered a sign of potentially improved efficacy and an encouragement for a larger comparative study. The decision to initiate such a study would depend on other factors as well as tolerability of the regimen. RFS was defined as time from liver resection to any recurrence or death, whichever occurred first. Patients alive without recurrence were censored at their last follow-up date. Overall survival was defined as time from liver resection to death or last follow-up. Survival curves were estimated by using the Kaplan-Meier method and compared by using the log-rank test. Associations between categoric variables were assessed by using Fisher's exact test. The study was terminated early with 73 patients because of signs of increased biliary toxicity in the Bev arm; therefore, only these 73 are included in the statistical analysis. To ensure that the distribution was relatively balanced between the two treatment arms, randomization was stratified by using two variables: prior chemotherapy (oxaliplatin or no oxaliplatin) and clinical risk score (CRS). CRS uses a point system, awarding one point for each of the following factors: node-positive primary, disease-free interval of < 12 months, more than one metastasis, metastasis size > 5 cm, and carcinoembryonic antigen > 200 ng/mL.17 Patients were stratified by a CRS of 0 to 2 points versus 3 or more. The random assignment took place after complete resection of liver metastases.
RESULTS
Patient Characteristics
Seventy three patients who underwent liver resection were entered into the study. Patient characteristics are listed in Table 2 . Established poor prognostic indicators8,18 in each arm include stage III primary (53% and 60%), < 12-month interval from primary resection (84% and 74%), positive margins (11% and 8%), more than one metastatic lesion (84% and 77%), at least four metastases (61% and 49%), prior liver surgery or radiofrequency ablation (5% and 17%), and lesion size > 5 cm (18% and 17%), in the no Bev and Bev arms, respectively. The type of surgery was similar in both arms with major hepatectomy completed in 18 of 38 versus 13 of 35 patients in the no Bev and Bev arms, respectively.
Table 2.
Patient Characteristics
| Characteristic | No Bev (%) (n = 38) | Bev (%) (n = 35) | Total No. of Patients (N = 73) |
|---|---|---|---|
| Age ≥ 60 years | 29 | 29 | 29 |
| Karnofsky performance score ≥ 80 | 95 | 91 | 93 |
| Sex, female | 42 | 54 | 48 |
| Clinical risk score ≥ 3 | 50 | 46 | 48 |
    Lymph nodes positive at primary Lymph nodes positive at primary | 53 | 60 | 55 |
    Disease-free interval < 12 months Disease-free interval < 12 months | 84 | 74 | 79 |
    More than one liver metastasis More than one liver metastasis | 84 | 77 | 81 |
    Largest diameter ≥ 5 cm Largest diameter ≥ 5 cm | 18 | 17 | 18 |
| Synchronous | 66 | 63 | 64 |
| Four or more liver metastases | 61 | 49 | 55 |
| Margins < 1 cm | 68 | 69 | 68 |
| Positive margins or < 0.1 mm | 11 | 8 | 10 |
| Bilobar | 63 | 57 | 60 |
| Colon/rectum | 68/32 | 77/23 | 72/28 |
| KRAS mutated | 28 | 21 | 25 |
| Preoperative CEA > 30 | 21 | 26 | 23 |
| Postoperative CEA > 2.5 | 53 | 63 | 58 |
| Previous chemotherapy | 92 | 80 | 86 |
    Prior to colon resection Prior to colon resection | 26 | 31 | 29 |
    Adjuvant Adjuvant | 18 | 23 | 21 |
    Metastatic Metastatic | 58 | 46 | 52 |
| Previous oxaliplatin prior to entry | 79 | 69 | 74 |
| Prior liver surgery/radiofrequency ablation | 5 | 17 | 11 |
Abbreviations: Bev, bevacizumab; CEA, carcinoembryonic antigen.
Toxicity
Toxicities during treatment are listed in Table 3. Zero versus five patients (14%) in the no Bev and Bev arms developed a bilirubin elevation > 3 mg/dL (P = .02). Biliary stents were placed in zero versus four patients (11%) in the no Bev versus Bev arms (P = .05). Over the course of the protocol, 14 patients required hospital admissions: five (13%) in the no Bev arm (one for abdominal pain, two for allergic reactions to chemotherapy, and two for fever), and nine (26%) in the Bev arm (three for diarrhea, three for abdominal pain, two for infection, and one for thrombosis). Overall complications from surgery were similar in both arms and included three abscesses, two pulmonary emboli, five wound infections or breakdowns, one infectious colitis, and four paralytic ileuses.
Table 3.
Toxicity
| Adverse Event | No Bev (n = 38) | Bev (n = 35) | P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Abdominal pain | 3 | 8 | 1 | 3 | .6 |
| Thrombosis | 0 | 3 | 9* | .1 | |
| Diarrhea | 3 | 8 | 6 | 17 | .3 |
| Alkaline phosphatase twofold or higher increase | 6 | 16 | 11 | 31 | .3 |
| Bilirubin > 3 mg/dL | 0 | 5 | 14 | .02 | |
| Biliary stents† | 0 | 4 | 11 | .05 | |
Abbreviation: Bev, bevacizumab.
We calculated the dose each patient should receive in six cycles of therapy and divided by the actual dose they received. We then calculated the average dose received per study arm. The average dose delivered was 88.8% and 81.5% for irinotecan, 55.6% and 56% for oxaliplatin, and 45% and 38% for fluorodeoxyuridine in the no Bev and Bev arms, respectively. The average Bev dose was 70.9%. The majority of patients received all six cycles: 34 of 38 and 23 of 35 in the no Bev and Bev arms, respectively. The reasons for not completing therapy were the development of metastases (two and four patients), complications such as portal thrombosis, catheter bleed, ulcer, nasal perforation (one and four patients), refusal of therapy (one and two patients), and placement of biliary stents (zero and four patients) in the no Bev and Bev arms, respectively. Systemic treatment was held within the first three cycles because of diarrhea, mucositis, low blood counts, abdominal pain, malaise, or hospital admission in 28% of patients, with the majority held 1 to 2 weeks. The study was terminated early because of increased biliary toxicity in the Bev arm.
Progression and Survival
With a median follow-up of 30 months, the 1-year RFS was 83% (95% CI, 66% to 92%) and 71% (95% CI, 52% to 83%), and 4-year RFS was 46% and 37% (P = .4) for the no Bev and Bev arms, respectively (Fig 2). Thus, the Bev arm did not meet the criterion specified by the study design of a 1-year RFS > 80%. A futility analysis demonstrated that adding five more patients to the Bev arm to reach the proposed sample size would have not changed the outcome of this study. In the best-case scenario (which assumes the additional five patients would have been recurrence free at 1 year), the 1-year RFS would have been estimated at 74%.
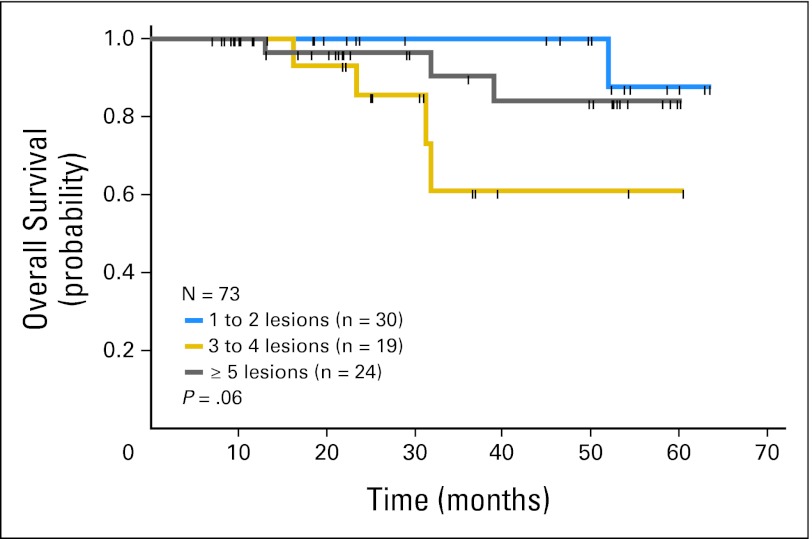
The 4-year survival is 85% (95% CI, 60% to 95%) in the no Bev arm and 81% (95% CI, 56% to 93%) in the Bev arm (log-rank test P = .5; Figure 3). Overall survival by number of metastases is depicted in Figure 4. In the no Bev arm, 15 patients (39%) recurred: seven (18%) in the liver and 10 (26%) in extrahepatic sites (some patients in both sites). Of those who recurred, five received salvage therapy via re-operation or radiofrequency ablation; thus, 74% are currently free of disease. In the Bev arm, 19 (54%) recurred: nine (26%) in the liver and 16 (49%) in extrahepatic sites. Four patients received salvage therapy, so 46% are currently free of disease.
DISCUSSION
Liver metastases cause significant morbidity for patients with colorectal cancer. Resection of hepatic metastases increases survival, but the relapse rates are high and occur most commonly in the liver. These relapses may be related to the persistence of undetected micrometastases or missing lesions due to clinical responses from preoperative chemotherapy.19 Therefore adjuvant therapy should be considered post resection for most patients. Systemic FU + LV has not produced a significant increase in survival in the adjuvant setting after hepatic resection, but a meta-analysis showed an increase in DFS after adjustment for poor prognostic factors.7 Systemic irinotecan, folinic acid, and fluorouracil (FOLFIRI) in the adjuvant setting after liver resection showed no increase in DFS versus FU + LV.20 In the European Organisation for Research and Treatment of Cancer (EORTC) study,8 perioperative therapy with FOLFOX increased PFS versus no chemotherapy (with a PFS for the treated arm who underwent liver resection of 42% and 28% at 3 and 5 years, respectively). In the EORTC study, 94% of patients had one to three metastases (none had more than four). In this HAI adjuvant study, as the number of metastases increased, overall survival (Fig 4) and RFS decreased.
Metastases that may remain after hepatic resection are probably in the range of 2 to 3 mm and are therefore not detectable by the ultrasound scanning technique.21 These metastases derive most of their blood supply from the arterial circulation.22 Therefore, adjuvant chemotherapy via the arterial circulation may be useful.
Several studies have randomly assigned patients to HAI versus no further therapy or systemic therapy alone after liver resection. The Eastern Cooperative Oncology Group (ECOG) study12 achieved its primary end point of an increase in DFS with HAI plus systemic therapy versus no further therapy (46% v 25%, respectively) at 4 years (P = .04). At MSKCC, patients were randomly assigned to HAI and systemic therapy versus systemic therapy alone with 2-year survival of 86% versus 72%, respectively (P = .03), which was the primary end point. Updated analyses reveal a 10-year survival of 41% and 27% and a median PFS of 31 and 17 months (P = .02), respectively.9,13 A Greek study10 produced a significant improvement in 5-year DFS (58% v 34%; P = .002) and survival (73% v 60%; P = .05) for adjuvant HAI plus systemic chemoimmunotherapy versus systemic therapy alone. A German trial11 showed no improvement in median PFS (14.2 v 13.7 months) or survival (34.5 v 40.8 months) after liver resection for HAI plus systemic therapy versus no further therapy, respectively. Several patients were not treated or resected; analysis of treated patients demonstrated median survivals of 44.8 and 39.7 months for HAI versus control groups. Two phase I studies17,23 conducted at MSKCC after liver resection were reported. HAI with oxaliplatin plus FU + LV yielded a 5-year survival of 88% and a 4-year PFS of 50%.17 A National Surgical Adjuvant Breast and Bowel Project (NSABP) study24 of systemic capecitabine and oxaliplatin alternating with HAI with fluorodeoxyuridine produced a 2-year survival of 88% with no hepatic recurrence in 80% of the patients.
When this trial was initiated, adjuvant trials using Bev for the treatment of colon cancer had not been published. Recently, an adjuvant trial (NSABP C-08)25 of FOLFOX with or without Bev in patients with stage II and III colon cancer showed no difference in DFS. The AVANT BO1792026 trial confirmed these results in a randomized trial of chemotherapy with or without Bev which did not increase DFS in stage III colon cancer. In both trials,26 Bev was administered for 6 months. Bev was administered every 2 weeks in the first trial and every 3 weeks in the second trial, in which Bev was administered at a higher dose (7.5 mg/kg). In this study, Bev 5 mg/kg was given twice within a 5-week schedule so that it would coincide with systemic chemotherapy. The dose used was a lower dose than that usually administered. In addition, several patients (34%) could not complete 6 months of treatment because of toxicity and/or recurrence.
RFS (PFS, DFS) is now considered an important end point in the treatment of metastatic colorectal carcinoma, especially when considering adjuvant treatment.27 However, RFS after liver resection may not completely relate to overall survival. Some patients who progress after liver resection exhibit one small lung metastasis which can be resected; therefore, the survival is still excellent. Conversely, recurrence with multiple liver metastases or intra-abdominal metastases will decrease survival. In new studies evaluating treatment after liver resection, it is important to consider overall survival as well.
The primary objective of this study was to determine whether intravenous Bev added to adjuvant HAI and systemic chemotherapy after liver resection would increase RFS. The study was terminated early because of signs of increased biliary toxicity in the Bev arm, including the need for biliary stenting in 11% of patients while no stents were required in the no Bev arm. In previous adjuvant HAI studies,9,13,16 the need for biliary stents was also much lower (3% to 5%). The decision to close the study was also based on increased biliary toxicity in two other studies at MSKCC28 that used Bev concurrently (targeting primary liver cancer and unresectable colon cancer).
The question of why Bev increases biliary toxicity in this setting needs to be addressed. The usual toxicities from Bev include hypertension, proteinuria, thrombosis, and hemorrhage. There are a few rare toxicities reported with anti–vascular endothelial growth factor agents including thrombotic microangiopathy,29 osteonecrosis of the jaw,30 nasal perforation,31 and tracheoesophageal fistulas.32 Constant irritation may cause mucosal abrasions, and along with impaired neovascularization from anti–vascular endothelial growth factor agents, may impair wound healing. For example, nasal perforation from Bev may be due to nose blowing from chemotherapy (ie, FU), leading to vulnerable avascular nasal cartilage which cannot heal during Bev therapy.31 Osteonecrosis of the jaw may arise from Bev compromising microvascular integrity at the jaw, leading to clinical compromise of the ostium.30
Physiologic angiogenesis is observed in wound healing.33 Therefore blocking angiogenesis may limit wound healing. Hepatic artery perfusion allows the study drug to access the tumor, but it also perfuses the bile ducts causing damage to the endothelium of the bile duct. This normally heals during drug rest periods, but healing may be prevented by Bev.
This phase II study demonstrated a good 4-year survival after liver resection of colorectal metastases (85% and 81% for the no Bev and Bev arms, respectively), although 48% of the patients had a clinical risk score ≥ 3 and 81% of the patients had more than one metastasis. The study cannot provide definitive results because of its size, but it does suggest that the addition of Bev to adjuvant HAI and systemic therapy after liver resection did not increase RFS or survival and appeared to increase biliary toxicity.
Footnotes
Supported in part by Genentech.
Presented as a poster at the 46th Annual Meeting of the American Society of Clinical Oncology, June 4-8, 2010, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00200200.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Yuman Fong, Genentech (C), Ethicon/Johnson & Johnson (C) Stock Ownership: None Honoraria: None Research Funding: Nancy E. Kemeny, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Nancy E. Kemeny, William R. Jarnagin, Yuman Fong, Ronald P. DeMatteo, Michael I. D'Angelica
Provision of study materials or patients: William R. Jarnagin, Yuman Fong, Ronald P. DeMatteo, Michael I. D'Angelica
Collection and assembly of data: Marinela Capanu, Alexandra N. Gewirtz
Data analysis and interpretation: Nancy E. Kemeny, William R. Jarnagin, Marinela Capanu, Alexandra N. Gewirtz, Michael I. D'Angelica
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2010.32.5977
Read article for free, from open access legal sources, via Unpaywall:
https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2010.32.5977?role=tab
Subscription required at intl.jco.org
http://intl.jco.org/cgi/content/full/29/7/884
Subscription required at intl.jco.org
http://intl.jco.org/cgi/reprint/29/7/884.pdf
Free to read at intl.jco.org
http://intl.jco.org/cgi/content/abstract/29/7/884
Citations & impact
Impact metrics
Citations of article over time
Article citations
PLOD3 facilitated T cell activation in the colorectal tumor microenvironment and liver metastasis by the TNF-α/ NF-κB pathway.
J Transl Med, 22(1):30, 06 Jan 2024
Cited by: 2 articles | PMID: 38184566 | PMCID: PMC10771005
Gemcitabine with Cisplatin Versus Hepatic Arterial Infusion Pump Chemotherapy for Liver-Confined Unresectable Intrahepatic Cholangiocarcinoma.
Ann Surg Oncol, 31(1):115-124, 09 Oct 2023
Cited by: 3 articles | PMID: 37814188 | PMCID: PMC10695893
Risks of hypertension and thromboembolism in patients receiving bevacizumab with chemotherapy for colorectal cancer: A systematic review and meta-analysis.
Cancer Med, 12(24):21579-21591, 08 Dec 2023
Cited by: 2 articles | PMID: 38069531 | PMCID: PMC10757147
Review Free full text in Europe PMC
Clusterin Expression in Colorectal Carcinomas.
Int J Mol Sci, 24(19):14641, 27 Sep 2023
Cited by: 2 articles | PMID: 37834086 | PMCID: PMC10572822
Review Free full text in Europe PMC
Hepatic arterial infusion in combination with systemic chemotherapy in patients with hepatic metastasis from colorectal cancer: a randomized phase II study - (NCT05103020) - study protocol.
BMC Cancer, 23(1):691, 22 Jul 2023
Cited by: 1 article | PMID: 37481515 | PMCID: PMC10363309
Go to all (64) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00200200
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes.
Ann Surg, 261(2):353-360, 01 Feb 2015
Cited by: 76 articles | PMID: 24646562 | PMCID: PMC4578807
Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases - a randomized phase II/III trial - PACHA-01 (NCT02494973).
BMC Cancer, 18(1):787, 06 Aug 2018
Cited by: 15 articles | PMID: 30081865 | PMCID: PMC6080555
Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer.
Ann Oncol, 20(7):1236-1241, 20 Feb 2009
Cited by: 56 articles | PMID: 19233901 | PMCID: PMC9724731