Abstract
Free full text

Two functional alpha-tubulin genes of the yeast Saccharomyces cerevisiae encode divergent proteins.
Abstract
Two alpha-tubulin genes from the budding yeast Saccharomyces cerevisiae were identified and cloned by cross-species DNA homology. Nucleotide sequencing studies revealed that the two genes, named TUB1 and TUB3, encoded gene products of 447 and 445 amino acids, respectively, that are highly homologous to alpha-tubulins from other species. Comparison of the sequences of the two genes revealed a 19% divergence between the nucleotide sequences and a 10% divergence between the amino acid sequences. Each gene had a single intervening sequence, located at an identical position in codon 9. Cell fractionation studies showed that both gene products were present in yeast microtubules. These two genes, along with the TUB2 beta-tubulin gene, probably encode the entire complement of tubulin in budding yeast cells.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. [Europe PMC free article] [Abstract] [Google Scholar]
- Arce CA, Hallak ME, Rodriguez JA, Barra HS, Caputto R. Capability of tubulin and microtubules to incorporate and to release tyrosine and phenylalanine and the effect of the incorporation of these amino acids on tubulin assembly. J Neurochem. 1978 Jul;31(1):205–210. [Abstract] [Google Scholar]
- Argaraña CE, Barra HS, Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978 Feb 24;19(1):17–21. [Abstract] [Google Scholar]
- Barra HS, Arcce CA, Rodriguez JA, Caputto R. Uncorporation of phenylalanine as a single unit into rat brain protein: reciprocal inhibition by phenylalanine and tyrosine of their respective incorporations. J Neurochem. 1973 Nov;21(5):1241–1251. [Abstract] [Google Scholar]
- Barra HS, Arce CA, Rodríguez JA, Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1384–1390. [Abstract] [Google Scholar]
- Barra HS, Rodriguez JA, Arce CA, Caputto R. A soluble preparation from rat brain that incorporates into its own proteins ( 14 C)arginine by a ribonuclease-sensitive system and ( 14 C)tyrosine by a ribonuclease-insensitive system. J Neurochem. 1973 Jan;20(1):97–108. [Abstract] [Google Scholar]
- Benton WD, Davis RW. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. [Abstract] [Google Scholar]
- Blattner FR, Williams BG, Blechl AE, Denniston-Thompson K, Faber HE, Furlong L, Grunwald DJ, Kiefer DO, Moore DD, Schumm JW, et al. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. [Abstract] [Google Scholar]
- Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [Abstract] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. [Abstract] [Google Scholar]
- Carlson M, Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. [Abstract] [Google Scholar]
- Cleveland DW, Sullivan KF. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. [Abstract] [Google Scholar]
- Davidse LC, Flach W. Differential binding of methyl benzimidazol-2-yl carbamate to fungal tubulin as a mechanism of resistance to this antimitotic agent in mutant strains of Aspergillus nidulans. J Cell Biol. 1977 Jan;72(1):174–193. [Europe PMC free article] [Abstract] [Google Scholar]
- Deanin GG, Gordon MW. The distribution of tyrosyltubulin ligase in brain and other tissues. Biochem Biophys Res Commun. 1976 Jul 26;71(2):676–683. [Abstract] [Google Scholar]
- Deanin GG, Preston SF, Hanson RK, Gordon MW. On the mechanism of turnover of the carboxy-terminal tyrosine of the alpha chain of tubulin. Eur J Biochem. 1980 Aug;109(1):207–216. [Abstract] [Google Scholar]
- Delgado MA, Conde J. Benomyl prevents nuclear fusion in Saccharomyces cerevisiae. Mol Gen Genet. 1984;193(1):188–189. [Abstract] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. [Abstract] [Google Scholar]
- Hanahan D, Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. [Abstract] [Google Scholar]
- Hartwell LH. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. [Europe PMC free article] [Abstract] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984 Dec;39(2 Pt 1):349–358. [Abstract] [Google Scholar]
- Kilmartin JV. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981 Jun 9;20(12):3629–3633. [Abstract] [Google Scholar]
- Kumar N, Flavin M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem. 1981 Jul 25;256(14):7678–7686. [Abstract] [Google Scholar]
- Kuo CL, Campbell JL. Cloning of Saccharomyces cerevisiae DNA replication genes: isolation of the CDC8 gene and two genes that compensate for the cdc8-1 mutation. Mol Cell Biol. 1983 Oct;3(10):1730–1737. [Europe PMC free article] [Abstract] [Google Scholar]
- Langford CJ, Gallwitz D. Evidence for an intron-contained sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983 Jun;33(2):519–527. [Abstract] [Google Scholar]
- Langford CJ, Klinz FJ, Donath C, Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. [Abstract] [Google Scholar]
- Moens PB, Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. [Europe PMC free article] [Abstract] [Google Scholar]
- Nasmyth K, Nurse P. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1981;182(1):119–124. [Abstract] [Google Scholar]
- Neff NF, Thomas JH, Grisafi P, Botstein D. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell. 1983 May;33(1):211–219. [Abstract] [Google Scholar]
- Newman AJ, Lin RJ, Cheng SC, Abelson J. Molecular consequences of specific intron mutations on yeast mRNA splicing in vivo and in vitro. Cell. 1985 Aug;42(1):335–344. [Abstract] [Google Scholar]
- Nurse P, Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980 Nov;96(3):627–637. [Europe PMC free article] [Abstract] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. [Abstract] [Google Scholar]
- Oakley BR, Morris NR. Nuclear movement is beta--tubulin-dependent in Aspergillus nidulans. Cell. 1980 Jan;19(1):255–262. [Abstract] [Google Scholar]
- Parker R, Guthrie C. A point mutation in the conserved hexanucleotide at a yeast 5' splice junction uncouples recognition, cleavage, and ligation. Cell. 1985 May;41(1):107–118. [Abstract] [Google Scholar]
- Peterson JB, Ris H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J Cell Sci. 1976 Nov;22(2):219–242. [Abstract] [Google Scholar]
- Pikielny CW, Teem JL, Rosbash M. Evidence for the biochemical role of an internal sequence in yeast nuclear mRNA introns: implications for U1 RNA and metazoan mRNA splicing. Cell. 1983 Sep;34(2):395–403. [Abstract] [Google Scholar]
- Pillus L, Solomon F. Components of microtubular structures in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2468–2472. [Europe PMC free article] [Abstract] [Google Scholar]
- Ponstingl H, Krauhs E, Little M, Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. [Europe PMC free article] [Abstract] [Google Scholar]
- Preston SF, Deanin GG, Hanson RK, Gordon MW. The phylogenetic distribution of tubulin:tyrosine ligase. J Mol Evol. 1979 Oct;13(3):233–244. [Abstract] [Google Scholar]
- Quinlan RA, Pogson CI, Gull K. The influence of the microtubule inhibitor, methyl benzimidazol-2-yl-carbamate (MBC) on nuclear division and the cell cycle in Saccharomyces cerevisiae. J Cell Sci. 1980 Dec;46:341–352. [Abstract] [Google Scholar]
- Raff EC. Genetics of microtubule systems. J Cell Biol. 1984 Jul;99(1 Pt 1):1–10. [Europe PMC free article] [Abstract] [Google Scholar]
- Raybin D, Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977 May 17;16(10):2189–2194. [Abstract] [Google Scholar]
- Rigby PW, Dieckmann M, Rhodes C, Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. [Abstract] [Google Scholar]
- Rodriguez JA, Borisy GG. Tyrosination state of free tubulin subunits and tubulin disassembled from microtubules of rat brain tissue. Biochem Biophys Res Commun. 1979 Aug 13;89(3):893–899. [Abstract] [Google Scholar]
- Rosbash M, Harris PK, Woolford JL, Jr, Teem JL. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. [Abstract] [Google Scholar]
- Rose M, Grisafi P, Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. [Abstract] [Google Scholar]
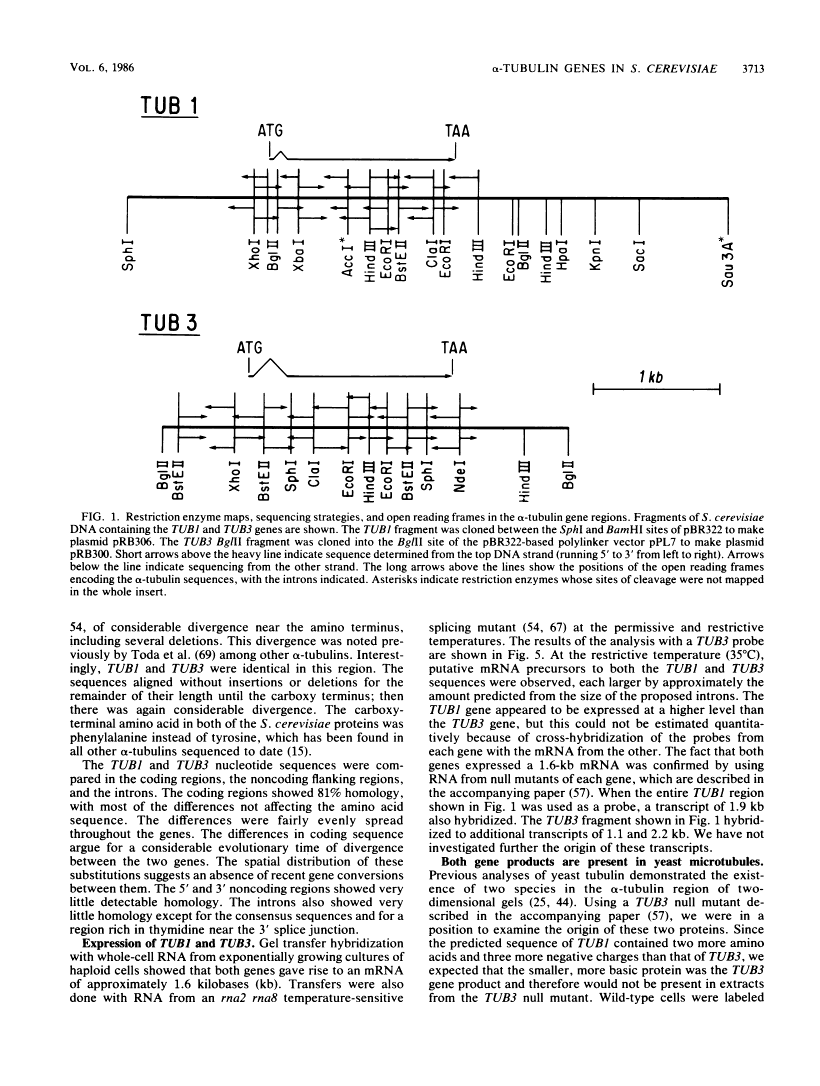
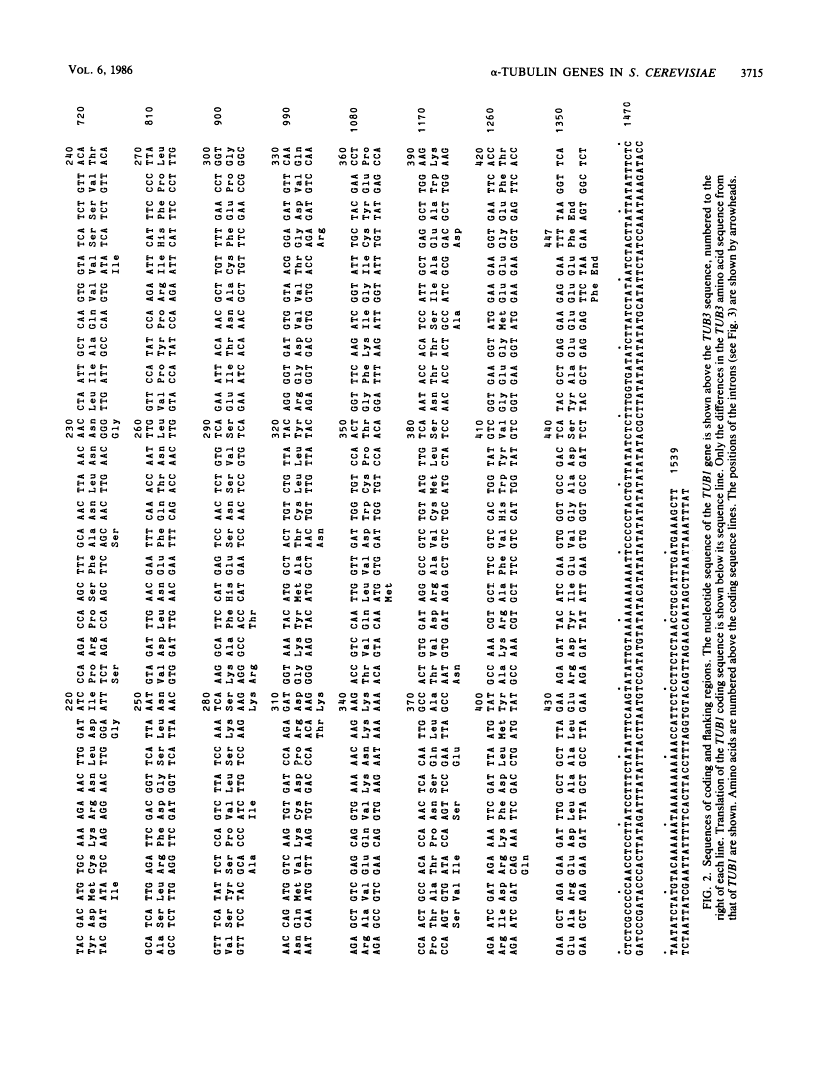
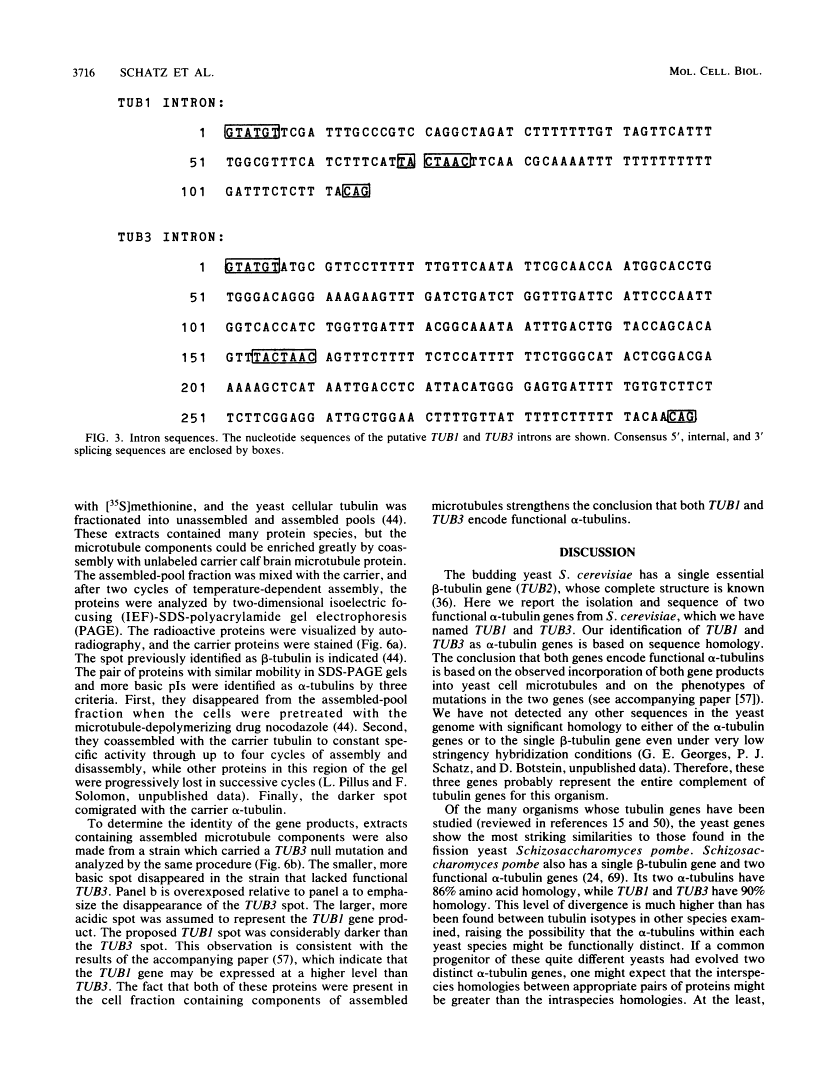
- Schatz PJ, Solomon F, Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986 Nov;6(11):3722–3733. [Europe PMC free article] [Abstract] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. [Europe PMC free article] [Abstract] [Google Scholar]
- Sheir-Neiss G, Lai MH, Morris NR. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. [Abstract] [Google Scholar]
- Shortle D, Novick P, Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. [Europe PMC free article] [Abstract] [Google Scholar]
- Silflow CD, Chisholm RL, Conner TW, Ranum LP. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985 Sep;5(9):2389–2398. [Europe PMC free article] [Abstract] [Google Scholar]
- Snyder JA, McIntosh JR. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- St John TP, Davis RW. The organization and transcription of the galactose gene cluster of Saccharomyces. J Mol Biol. 1981 Oct 25;152(2):285–315. [Abstract] [Google Scholar]
- Teem JL, Abovich N, Kaufer NF, Schwindinger WF, Warner JR, Levy A, Woolford J, Leer RJ, van Raamsdonk-Duin MM, Mager WH, et al. A comparison of yeast ribosomal protein gene DNA sequences. Nucleic Acids Res. 1984 Nov 26;12(22):8295–8312. [Europe PMC free article] [Abstract] [Google Scholar]
- Teem JL, Rodriguez JR, Tung L, Rosbash M. The rna2 mutation of yeast affects the processing of actin mRNA as well as ribosomal protein mRNAs. Mol Gen Genet. 1983;192(1-2):101–103. [Abstract] [Google Scholar]
- Thomas JH, Neff NF, Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. [Europe PMC free article] [Abstract] [Google Scholar]
- Toda T, Adachi Y, Hiraoka Y, Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell. 1984 May;37(1):233–242. [Abstract] [Google Scholar]
- Toda T, Umesono K, Hirata A, Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol. 1983 Aug 5;168(2):251–270. [Abstract] [Google Scholar]
- Umesono K, Toda T, Hayashi S, Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983 Aug 5;168(2):271–284. [Abstract] [Google Scholar]
- Wahl GM, Stern M, Stark GR. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. [Europe PMC free article] [Abstract] [Google Scholar]
- Wood JS. Genetic effects of methyl benzimidazole-2-yl-carbamate on Saccharomyces cerevisiae. Mol Cell Biol. 1982 Sep;2(9):1064–1079. [Europe PMC free article] [Abstract] [Google Scholar]
- Wood JS, Hartwell LH. A dependent pathway of gene functions leading to chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1982 Sep;94(3):718–726. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.6.11.3711-3721.1986
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/6/11/3711
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/6/11/3711
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/124520590
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/mcb.6.11.3711-3721.1986
Article citations
Balancing Act: Tubulin Glutamylation and Microtubule Dynamics in Toxoplasma gondii.
Microorganisms, 12(3):488, 28 Feb 2024
Cited by: 1 article | PMID: 38543539 | PMCID: PMC10974701
Review Free full text in Europe PMC
α-tubulin regulation by 5' introns in Saccharomyces cerevisiae.
Genetics, 225(4):iyad163, 01 Dec 2023
Cited by: 1 article | PMID: 37675603 | PMCID: PMC10697811
Insights on the Role of α- and β-Tubulin Isotypes in Early Brain Development.
Mol Neurobiol, 60(7):3803-3823, 21 Mar 2023
Cited by: 7 articles | PMID: 36943622
Review
Microtubules in Microorganisms: How Tubulin Isotypes Contribute to Diverse Cytoskeletal Functions.
Front Cell Dev Biol, 10:913809, 05 Jul 2022
Cited by: 5 articles | PMID: 35865635 | PMCID: PMC9294176
Review Free full text in Europe PMC
α1A and α1C form microtubules to display distinct properties mainly mediated by their C-terminal tails.
J Mol Cell Biol, 13(12):864-875, 01 Jan 2022
Cited by: 10 articles | PMID: 34609491 | PMCID: PMC8800519
Go to all (130) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Characterization of two divergent beta-tubulin genes from Colletotrichum graminicola.
Gene, 86(2):163-170, 01 Feb 1990
Cited by: 36 articles | PMID: 2323570
Isolation and characterization of a beta-tubulin gene from Candida albicans.
Gene, 63(1):53-63, 01 Jan 1988
Cited by: 46 articles | PMID: 3290053
The beta-tubulin gene family of pea: primary structures, genomic organization and intron-dependent evolution of genes.
Plant Mol Biol, 18(4):639-651, 01 Feb 1992
Cited by: 36 articles | PMID: 1558942
Comparative analysis of tubulin sequences.
Comp Biochem Physiol B, 90(4):655-670, 01 Jan 1988
Cited by: 53 articles | PMID: 3073909
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P01-CA26712
NIGMS NIH HHS (2)
Grant ID: GM-18973
Grant ID: GM-21253