| PMC full text: | Published online 2013 Apr 2. doi: 10.4161/cc.24479
|
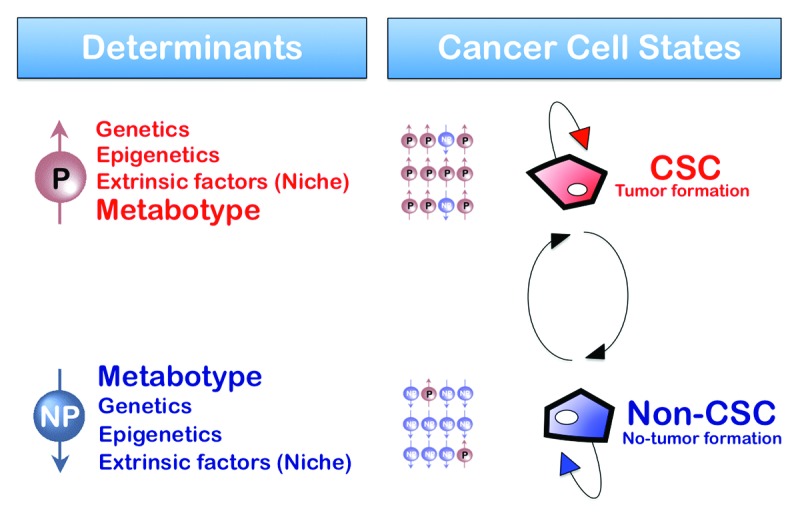
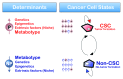
Figure 2. Metabolic reprogramming: A new parameter in the plasticity of the tumor cell differentiation-state that dictates the transition rates between non-CSC and CSC cellular states. Bidirectional interconversion exists between non-CSC and CSC cellular states. Any convergence toward phenotypic equilibrium in cancer cell state proportions likely occurs due to specific cancer cell state interconversion within tumors. Although the ultimate mechanisms stabilizing phenotypic proportions remain unclear, the transition rates between cell states, which vary across distinct cancer cell populations, have been suggested to largely depend on the intrinsic degree of differentiation-state plasticity dictated by genetic, epigenetic and/or microenvironmental (cell niche) parameters. Indeed, current descriptions of cancer cell states, i.e., the set of characteristics that are informative for the transition between two cell states of interest, consist mostly of the levels of gene expression or the levels of epigenetic methylation or acetylation of DNA or chromatin. The final dimension of the non-CSC/CSC transitions is therefore mostly “hard-wired” by the genome. Our current model hypothesizes that the metabotype is an additional characteristic that, in addition to genetic determinants, epigenetics and extrinsic factors (niche), causally determines either “permissive” (P) or “non-permissive” (NP) features that lastly determine the number of additional requirements to gain the self-renewal capacity, potency and tumorigenicity possessed by CSC cellular states.