Abstract
Background
The associations of egg consumption with cardiovascular disease (CVD) and diabetes are still unclear.Objective
We aimed to quantitatively summarize the literature on egg consumption and risk of CVD, cardiac mortality, and type 2 diabetes by conducting a meta-analysis of prospective cohort studies.Design
A systematic literature review was conducted for published studies in PubMed and EMBASE through March 2012. Additional information was retrieved through Google or a hand review of the reference from relevant articles. Studies were included if they had a prospective study design, were published in English-language journals, and provided HRs and 95% CIs for the associations of interest. Data were independently extracted by 2 investigators, and the weighted HRs and 95% CIs for the associations of interest were estimated by using a random-effects model.Results
A total of 22 independent cohorts from 16 studies were identified, including participants ranging in number from 1600 to 90,735 and in follow-up time from 5.8 to 20.0 y. Comparison of the highest category (≥1 egg/d) of egg consumption with the lowest (<1 egg/wk or never) resulted in a pooled HR (95% CI) of 0.96 (0.88, 1.05) for overall CVD, 0.97 (0.86, 1.09) for ischemic heart disease, 0.93 (0.81, 1.07) for stroke, 0.98 (0.77, 1.24) for ischemic heart disease mortality, 0.92 (0.56, 1.50) for stroke mortality, and 1.42 (1.09, 1.86) for type 2 diabetes. Of the studies conducted in diabetic patients, the pooled HR (95% CI) was 1.69 (1.09, 2.62) for overall CVD.Conclusions
This meta-analysis suggests that egg consumption is not associated with the risk of CVD and cardiac mortality in the general population. However, egg consumption may be associated with an increased incidence of type 2 diabetes among the general population and CVD comorbidity among diabetic patients.Free full text

Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis1,2,3
Abstract
Background: The associations of egg consumption with cardiovascular disease (CVD) and diabetes are still unclear.
Objective: We aimed to quantitatively summarize the literature on egg consumption and risk of CVD, cardiac mortality, and type 2 diabetes by conducting a meta-analysis of prospective cohort studies.
Design: A systematic literature review was conducted for published studies in PubMed and EMBASE through March 2012. Additional information was retrieved through Google or a hand review of the reference from relevant articles. Studies were included if they had a prospective study design, were published in English-language journals, and provided HRs and 95% CIs for the associations of interest. Data were independently extracted by 2 investigators, and the weighted HRs and 95% CIs for the associations of interest were estimated by using a random-effects model.
Results: A total of 22 independent cohorts from 16 studies were identified, including participants ranging in number from 1600 to 90,735 and in follow-up time from 5.8 to 20.0 y. Comparison of the highest category (≥1 egg/d) of egg consumption with the lowest (<1 egg/wk or never) resulted in a pooled HR (95% CI) of 0.96 (0.88, 1.05) for overall CVD, 0.97 (0.86, 1.09) for ischemic heart disease, 0.93 (0.81, 1.07) for stroke, 0.98 (0.77, 1.24) for ischemic heart disease mortality, 0.92 (0.56, 1.50) for stroke mortality, and 1.42 (1.09, 1.86) for type 2 diabetes. Of the studies conducted in diabetic patients, the pooled HR (95% CI) was 1.69 (1.09, 2.62) for overall CVD.
Conclusions: This meta-analysis suggests that egg consumption is not associated with the risk of CVD and cardiac mortality in the general population. However, egg consumption may be associated with an increased incidence of type 2 diabetes among the general population and CVD comorbidity among diabetic patients.
INTRODUCTION
Although elevated circulating cholesterol is an established risk factor for cardiovascular disease (CVD)4 (1–5), studies have shown inconsistent results on dietary cholesterol intake and risk of CVD (6–14). On the basis of 2 meta-analyses (15, 16), a 100-mg dietary cholesterol intake would increase plasma total cholesterol by 2.2–2.5 mg/dL, LDL cholesterol by 1.9 mg/dL, and HDL cholesterol by 0.4 mg/dL. In addition, some cholesterol feeding studies (17–19) observed that dietary cholesterol intake had little effect on the change in the ratio of LDL to HDL cholesterol. However, the association between dietary cholesterol intake and risk of CVD remains unclear (20).
Egg consumption is one of the main sources of dietary cholesterol. A medium egg contains ~225 mg cholesterol (21). A meta-analysis of clinical trials (22) found that the addition of 100 mg dietary cholesterol from eggs increased the ratio of total to HDL cholesterol by 0.02 units as well as plasma total cholesterol by 2.2 mg/dL and HDL cholesterol by 0.3 mg/dL. However, a few randomized controlled trials (RCTs) published later reported that egg consumption did not appreciably alter plasma total cholesterol, LDL cholesterol (23–25), HDL cholesterol (24, 25), or the ratio of total to HDL cholesterol (19, 25, 26). In addition, 2 RCTs (27, 28) found that consuming 2 eggs/d for 6 wk had no adverse effect on endothelial function. To date, no RCT data are available, whereas many observational studies relating egg consumption to the risk of CVD (29–36) and diabetes (37–41) have been published. However, the findings from these studies are inconsistent. To quantitatively investigate the overall longitudinal associations of egg consumption with incidence of CVD, type 2 diabetes, and cardiac mortality, we conducted a meta-analysis of prospective cohort studies.
SUBJECTS AND METHODS
Data sources and searches
We performed this meta-analysis according to the guidelines of the Meta-analysis Of Observational Studies in Epidemiology (42). A systematic literature search was conducted to identify published studies in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and EMBASE (http://www.embase.com/) through March 2012; the terms “egg intake or consumption,” “coronary heart disease,” “myocardial infarction,” “stroke,” “cardiovascular disease,” “mortality or death,” and “diabetes mellitus” were used. Additional information was retrieved through a Google search or a hand review of the references from relevant articles. To get additional data or de novo results for this meta-analysis, we contacted authors of the primary studies (43, 44).
Study selection
Studies were included in this meta-analysis if they had a prospective study design, were published in English-language journals, and provided HRs with 95% CIs for CVD, mortality, or type 2 diabetes in relation to egg consumption.
As shown in Figure 1, we retrieved 72 articles from PubMed and EMBASE or by hand-searching. Of these articles, 53 were excluded for one of the following reasons: 1) did not relate egg consumption to an outcome of interest; 2) was a review, editorial, abstract, or letter to editor; 3) was an animal study; 4) was not in English; or 5) was not a prospective cohort study. Of the 19 remaining articles (29–37, 41, 43–51), 2 (29, 45) were excluded because they did not report HRs with corresponding 95% CIs, and 1 study (49) was excluded because it used continuous variable (g/d) for egg consumption. Of the 16 identified eligible studies (30–37, 41, 43, 44, 46–48, 50, 51), 2 studies (30, 37) reported results from 4 independent cohorts, 3 studies (37, 43, 50) reported results stratified by sex, and 2 studies (33, 34) reported results for both CVD and mortality. In addition, 2 studies (31, 48) updated the results of early studies conducted in the same cohorts. In summary, 22 independent cohorts from 16 studies were included in the final data set for the meta-analysis: 8 eligible studies (30–36, 44) (8 cohorts) provided data on CVD, 6 studies (33, 34, 43, 46, 47, 50) (8 cohorts) on mortality, 3 studies (37, 41, 51) (5 cohorts) on type 2 diabetes in the general population, 4 studies (30, 33, 35, 48) (4 cohorts) on CVD, and 3 studies (33, 34, 50) (3 cohorts) on mortality in diabetic patients.
Data extraction and quality assessment
The following data were extracted from each included study: name of the first author, publication year, study population, country of origin, size of the sample, age range or mean age of the participants, proportion of men, duration of follow-up, methods for diet assessment, categories of egg intake, number of events, methods for case identification, adjusted covariates, and HRs or RRs and their 95% CIs in the corresponding exposure categories. All procedures, including the literature search, study selection, and data extraction, were independently performed by 2 authors (JYS and PX). Any discrepancies were resolved by group discussion.
To evaluate the quality of the studies included, we designed a scoring system with several a priori selected important characteristics that can affect study quality based on the literature. The scoring system was created to account for study eligibility (1 point if inclusion and exclusion criteria were clear), exposure (1 point if egg consumption was assessed by a validated tool, and 1 point if egg consumption was appropriately categorized), outcome (1 point if outcome of interest was not solely based on self-report), and statistical analysis (1 point if adjustment included a few major demographic and lifestyle variables such as age, BMI, smoking, and physical activity). The scoring system was designed with reference to the Meta-analysis Of Observational Studies in Epidemiology (42) and allowed a total score up to 5 points, with 5 reflecting the highest quality.
Statistical analysis
We estimated the pooled HRs (95% CIs), from a comparison of the highest category of egg consumption with the lowest, by using a random-effects model proposed by DerSimonian and Laird (52). All pooled HRs and 95% CIs were estimated by using data from the fully adjusted models. In this meta-analysis, the pooled HRs of overall CVD, type 2 diabetes, stroke, and ischemic heart disease (IHD) [including coronary artery disease, coronary heart disease (CHD), and myocardial infarction in relation to egg consumption were estimated. In adddition, the pooled HRs of mortality from overall CVD, IHD, and stroke were assessed. In addition, we computed the pooled HRs of overall CVD and mortality in patients with diabetes. Because the number of exposure categories was heterogeneous across primary studies or cohorts, we could not standardize egg consumption for all of the included studies. Thus, we calculated the pooled HRs (95% CI) by comparing the highest with the lowest category of egg consumption. If the results were generated from the same cohorts in different publications, we used data from the most recent publication. Because most of the studies reported results on total stroke, we combined ischemic and hemorrhagic stroke if the data on total stroke were not available, and the new results were used for the pooled estimation of stroke risk. In addition, the HRs with 95% CIs of overall CVD and mortality were pooled in studies among diabetic patients.
Cochran's test was used to evaluate the statistical heterogeneity and the I2 statistic was calculated to quantify the degree of inconsistency across individual studies. In addition, the Egger asymmetry test was used to examine publication bias (53). Sensitivity analyses were conducted to assess whether the results could have been affected markedly by any single study. All analyses were performed by using STATA statistical software (version 11.0; STATA Corp). All statistical tests were 2-sided, and a P value ≤0.05 was considered statistically significant.
RESULTS
Study characteristics
Characteristics of the 22 included cohorts from 16 eligible studies are shown in Table 1 (in the general population) and Table 2 (in diabetic individuals). Of 8 independent cohort studies of CVD in the general population, 7 were conducted in Western countries and 1 in Asia. The number of participants and duration of the follow-up ranged from 1600 to 90,735 and from 6 to 20 y, respectively. Of 8 independent cohort studies of mortality, 5 were conducted in Western countries and 3 in Asia. The number of individuals and duration of the follow-up ranged from 4077 to 37,130 and from 9 to 20 y, respectively. All 5 independent cohort studies of type 2 diabetes were conducted in Western countries. The number of participants and duration of follow-up ranged from 1669 to 36,295 and from 11 to 20 y, respectively (Table 1). In patients with diabetes, 4 studies (30, 33, 35, 48) reported HRs on overall CVD risk, and 3 studies (33, 34, 50) reported results on overall mortality. The number of patients and duration of follow-up ranged from 341 to 5672 and from 7 to 20 y, respectively. All studies in diabetic patients were conducted in Western countries (Table 2). Egg consumption was assessed by using a self-administered or interview-based food-frequency questionnaire and categorized into 3–6 groups. The quality scores for all included studies were ≥3 points based on our 5-point scoring system.
TABLE 1
Characteristics of the general population in the included studies1
| Source | Participants | Age | Men | Duration of follow-up | Exposure assessment | Exposure categories | No. of incidents | Case identification methods | Adjusted variables |
| n | y | % | y | ||||||
| Type 2 diabetes | |||||||||
 Vang et al, 2008 (51), AMS and AHS, USA Vang et al, 2008 (51), AMS and AHS, USA | 8401 | 45–88 | 61.1 | 17.0 | Semiquantitative questionnaire | 0, 1/wk, ≥1/wk | 543 diabetes | Self-administered questionnaires | Age and sex |
 Djousse et al, 2009 (37), PHS I, USA Djousse et al, 2009 (37), PHS I, USA | 20,703 | 40–86 | 100 | 20.0 | Self-administered questionnaire | 0, <1/wk, | 1921 diabetes | Physician's self-reported | Age, BMI, smoking, alcohol consumption, vigorous exercise, and history of hypercholesterolemia and hypertension |
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥7/wk | |||||||||
 Djousse et al, 2009 (37), WHS, USA Djousse et al, 2009 (37), WHS, USA | 36,295 | 39–90 | 0 | 11.7 | Self-administered questionnaire | 0, | 2112 diabetes | Self-reported diabetes was confirmed by telephone interviews, supplemental questionnaire, or review of medical records | Age, BMI, smoking, alcohol consumption, exercise, red meat intake, quintiles of energy intake, fruit and vegetables, SFAs, trans fatty acids, PUFAs, family history of diabetes, and history of hypercholesterolemia and hypertension |
| <1/wk, | |||||||||
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥7/wk | |||||||||
 Djousse et al, 2010 (41), CHS, USA Djousse et al, 2010 (41), CHS, USA | 1669 | 65–95 | 100 | 11.3 | Picture-sort questionnaire | Never, | 142 diabetes | Diagnosed by clinical examination according to ADA 2010 criteria | Age, race, BMI, smoking status, alcohol consumption, physical activity, cereal-fiber intake, and field center |
| <1/mo, | |||||||||
| 1–3/mo, | |||||||||
| 1–4/wk, | |||||||||
| almost daily | |||||||||
| Djousse et al, 2010 (41), CHS, USA | 2229 | 65–98 | 0 | 11.3 | Picture-sort questionnaire | Never, | 171 diabetes | Diagnosed by clinical examination according to ADA 2010 criteria | Age, race, BMI, smoking status, alcohol consumption, physical activity, cereal-fiber intake, and field center |
| <1/mo, 1–3/mo, 1–4/wk, almost daily | |||||||||
| Cardiovascular disease | |||||||||
 Hu et al, 1999 (30), HPFS, USA2 Hu et al, 1999 (30), HPFS, USA2 | 37,851 | 40–75 | 100 | 7.2 | Self-administered questionnaire | <1/wk, | 866 CHD, | Review medical record | Age, BMI, 2-y time periods, cigarette smoking, parental history of myocardial infarction, multivitamin supplement use, vitamin E supplement use, alcohol consumption, history of hypertension, physical activity, and total energy intake |
| 1/wk, | 258 stroke | ||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥1/d | |||||||||
 Hu et al, 1999 (30), NHS, USA Hu et al, 1999 (30), NHS, USA | 80,082 | 34–59 | 0 | 13.2 | Self-administered questionnaire | <1/wk, | 939 CHD, | Review medical record | Age, BMI, 2-y time periods, cigarette smoking, parental history of myocardial infarction, multivitamin supplement use, vitamin E supplement use, alcohol consumption, menopausal status and postmenopausal hormone use, history of hypertension, physical activity, and total energy intake |
| 1/wk, | 563 stroke | ||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥1/d | |||||||||
 He et al, 2003 (31), HPFS, USA2 He et al, 2003 (31), HPFS, USA2 | 43,732 | 40–75 | 100 | 14.0 | Semi-quantitative questionnaire | <1/wk, | 455 ischemic stroke, 125 hemorrhagic stroke | Medical record | Age, BMI, physical activity, history of hypertension, smoking status, aspirin use, multivitamin use, total servings of fruit and vegetables, total energy intake, hypercholesterolemia, and consumption of alcohol, potassium, fiber, and vitamin E |
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥1/d | |||||||||
 Nakamura et al, 2006 (32), JPHCPS, Japan Nakamura et al, 2006 (32), JPHCPS, Japan | Total: 90,735; cohort I: 41,234; cohort II: 49,471 | I: 40–59; II: 40–69 | I: 48.2;II: 47.4 | 10.2 | Self-administered questionnaire | <1 d/wk, | 462 CHD | Medical record | Age, sex, BMI, hypertension, diabetes, use of cholesterol-lowering drugs, smoking, alcohol, intention to avoid cholesterol-rich diets, cohort effects, and consumption frequencies of meat, fish, vegetables, and fruit |
| 1–2 d/wk, | |||||||||
| 3–4 d/wk, | |||||||||
| almost daily | |||||||||
 Qureshi et al, 2007 (33), NHANES I, USA Qureshi et al, 2007 (33), NHANES I, USA | 9734 | 25–74 | 38.6 | 16.6 | Self-administered questionnaire | <1/wk, | 655 total stroke, | Registry | Age, sex, race-ethnicity, systolic BP, DM, serum cholesterol, cigarette smoking, BMI, and education status |
| 1–6/wk, | 591 ischemic stroke | ||||||||
| ≥7/wk | (ICD-9-CM codes 430, 431–434.9, or 437.0–437.1); | ||||||||
| 1584 CAD (ICD-9-CM codes 410–414) | |||||||||
| Djousse and Gaziano, 2008 (34), PHS, USA | 21,327 | 40–86 | 100 | 20.0 | Self-administered questionnaire | <1/wk, | 1550 new MI, | Questionnaire | Age, BMI, smoking, history of hypertension, vitamin intake, alcohol consumption, vegetable consumption, breakfast cereal, physical activity, treatment arm a-fib, DM, hypercholesterolemia, and parental history of premature MI |
| 1/wk, | 1342 total stroke | ||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥7/wk | |||||||||
 Houston et al, 2011 (35), Health ABC Study, USA Houston et al, 2011 (35), Health ABC Study, USA | 1600 | 70–79 | 44.5 | 9.0 | Self-administered questionnaire | <1/wk, | 158 CVD | Annual clinical examination and medical record | Age, sex, race, education, field center, smoking, alcohol use, physical activity, BMI, total energy intake, protein intake, fiber intake, multivitamin use, supplemental vitamin E use, statin use, aspirin use, oral estrogen use (women), prevalent hypertension, and SFAs |
| 1–2/wk, | |||||||||
| ≥3/wk | |||||||||
 Zazpe et al, 2011 (36), SUN Project, Spain Zazpe et al, 2011 (36), SUN Project, Spain | 14,185 | 20–90 | 40.9 | 5.8 | Self-administered questionnaire | <1/wk, | 91 CVD | Self-reported questionnaire and medical record | Age, sex, total energy intake, adherence to the Mediterranean food pattern, alcohol intake, BMI, smoking status, physical activity, family history of CVD, diabetes, hypertension, and hypercholesterolemia |
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| >4/wk | |||||||||
 Yaemsiri et al, 2012 (44), WHI-OS, USA Yaemsiri et al, 2012 (44), WHI-OS, USA | 87,025 | 50–79 | 0 | 7.6 | Self-administered questionnaire | <1/wk, | 1049 ischemic stroke | Self-report and medical record | Age, race, education, family income, years as a regular smoker, hormone replacement therapy use, physical activity, alcohol intake, aspirin use, use of antihypertensive medication and cholesterol-lowering medication, BMI, systolic BP, total energy intake, dietary vitamin E, intake of fruit and vegetables and fiber, and history of CHD, a-fib, and diabetes |
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥1/d | |||||||||
| Mortality | |||||||||
 Mann et al, 1997 (46), UK Mann et al, 1997 (46), UK | 10,802 | 16–79 | 38.0 | 13.3 | Self-administered questionnaire | <1/wk, | 64 IHD death | Registry | Age, sex, smoking, and social class |
| 392 all-cause death | 1–5/wk, | ||||||||
| ≥6/wk | |||||||||
 Sauvaget et al, 2003 (47), LSS, Japan Sauvaget et al, 2003 (47), LSS, Japan | 37,130 | 34–103 | 38.3 | 16.0 | Self-administered questionnaire | Never, | 1462 stroke death | Registry | HR stratified by sex and birth cohort and adjusted for city, radiation dose, BMI, smoking status, alcohol habits, education level, history of diabetes, or hypertension |
| ≤1/wk, | |||||||||
| 2–4/wk, | |||||||||
| almost daily | |||||||||
 Nakamura et al, 2004 (43), NIPPON DATA80, Japan Nakamura et al, 2004 (43), NIPPON DATA80, Japan | 4077 | Mean | 100 | 13.0 | Self-administered questionnaire | <1/wk, | 39 IHD death, | Registry | Age, serum creatinine, total cholesterol, blood glucose, BMI, systolic and diastolic BP, use of BP-lowering drugs, cigarette smoking, and alcohol intake |
| 50.3 (>30) | 1–2/wk, 0.5/d, | 112 stroke death, | |||||||
| 1/d | 640 all-cause death | ||||||||
 Nakamura et al, 2004 (43), NIPPON DATA80, Japan Nakamura et al, 2004 (43), NIPPON DATA80, Japan | 5186 | Mean 50.7 (>30) | 0 | 13.3 | Self-administered questionnaire | <1/wk, | 41 IHD death, | Registry | Age, serum creatinine, total cholesterol, blood glucose, BMI, systolic and diastolic BP, use of BP-lowering drugs, cigarette smoking, and alcohol intake |
| 1–2/wk, | 107 stroke death, | ||||||||
| 0.5/d, | 562 all-cause death | ||||||||
| 1/d | |||||||||
 Qureshi et al, 2007 (33), NHANES-I, USA Qureshi et al, 2007 (33), NHANES-I, USA | 9734 | 25–74 | 38.6 | 16.6 | Self-administered questionnaire | <1/wk, | 3117 all-cause death | Registry | Age, sex, race-ethnicity, systolic BP, DM, serum cholesterol, cigarette smoking, BMI, and education status |
| 1–6/wk, | |||||||||
| ≥7/wk | |||||||||
 Djousse and Gaziano, 2008 (34), PHS, USA Djousse and Gaziano, 2008 (34), PHS, USA | 21,327 | 40–86 | 100 | 20.0 | Self-administered questionnaire | <1/wk, | 5169 all-cause death | Self or family-reported questionnaire | Age, BMI, smoking, history of hypertension, vitamin intake, alcohol consumption, vegetable consumption, breakfast cereal, physical activity, treatment arm a-fib, DM, hypercholesterolemia, and parental history of premature MI |
| 1/wk, | |||||||||
| 2–4/wk, | |||||||||
| 5–6/wk, | |||||||||
| ≥7/wk | |||||||||
 Scrafford et al, 2010 (50), NHANES III, USA Scrafford et al, 2010 (50), NHANES III, USA | 6833 | >17 | 100 | 8.8 | Self-administered questionnaire | <1/wk, | 198 CHD death | Registry | Age, energy, marital status, education status, race-ethnicity, BMI, diabetes, hypertension, and alcohol intake |
| 1–6/wk, | (ICD-10 codes I20–I25), 63 stroke death (ICD-10 codes I60–I69) | ||||||||
| ≥7/wk | |||||||||
 Scrafford et al, 2010 (50), NHANES III, USA Scrafford et al, 2010 (50), NHANES III, USA | 8113 | >17 | 0 | 8.9 | Self-administered questionnaire | <1/wk, | 168 CHD death, | Registry | Age, energy, marital status, education status, race-ethnicity, BMI, diabetes, hypertension, and alcohol intake |
| 1–6/wk, | 74 stroke death | ||||||||
| ≥7/wk |
TABLE 2
Characteristics of individuals with diabetes in the included studies1
| Source | Participants | Age | Men | Duration of follow-up | Exposure assessment | Exposure categories | Incidence | Case identification methods | Adjusted variables |
| n | y | % | y | ||||||
| Cardiovascular disease | |||||||||
 Hu et al, 1999 (30), HPFS, USA Hu et al, 1999 (30), HPFS, USA | 1187 | 40–75 | 100 | 7.2 | Self-administered questionnaire | <1/wk,1/wk,2–4/wk,5–6/wk,≥1/d | Not reported | Review medical record | Age, BMI, 2-y time periods, cigarette smoking, parental history of myocardial infarction, multivitamin supplement use, vitamin E supplement use, alcohol consumption, history of hypertension, physical activity, and total energy intake |
 Hu et al, 1999 (30), NHS, USA2 Hu et al, 1999 (30), NHS, USA2 | 4122 | 34–59 | 0 | 13.2 | Self-administered questionnaire | <1/wk,1/wk,2–4/wk,5–6/wk,≥1/d | Not reported | Review medical record | Age, BMI, 2-y time periods, cigarette smoking, parental history of myocardial infarction, multivitamin supplement use, vitamin E supplement use, alcohol consumption, menopausal status and postmenopausal hormone use, history of hypertension, physical activity, and total energy intake |
 Tanasescu et al, 2004 (48), NHS, USA2 Tanasescu et al, 2004 (48), NHS, USA2 | 5672 | Mean: 48.4 (≥30) | 0 | 10.1 | Semiquantitative questionnaire | <1/wk,1 to <3/wk,3/wk,>3–6/wk,>6/wk | 619 CVD | Review medical record | Age, smoking, postmenopausal hormone use, parental history of MI before 60 y of age, alcohol intake, physical activity, BMI, total caloric intake, protein intake, fiber intake, multivitamin use, vitamin E supplement use, and diabetes medication use |
 Qureshi et al, 2007 (33), NHANES I, USA Qureshi et al, 2007 (33), NHANES I, USA | 349 | 25–74 | 38.6 | 16.6 | Self-administered questionnaire | <1/wk,1–6/wk,≥7/wk | 57 total stroke, 132 CAD | Registry | Age, sex, race-ethnicity, systolic BP, DM, serum cholesterol, cigarette smoking, BMI, and education status |
 Houston et al, 2011 (35), Health ABC Study, USA Houston et al, 2011 (35), Health ABC Study, USA | 341 | 70–79 | 44.5 | 9.0 | Self-administered questionnaire | <1/wk,1–2/wk,≥3/wk | 45 CVD | Annual clinical examination and medical record | Age, sex, race, education, field center, smoking, alcohol use, physical activity, BMI, total energy intake, protein intake, fiber intake, multivitamin use, supplemental vitamin E use, statin use, aspirin use, oral estrogen use (women), prevalent hypertension, and SFAs |
| Mortality | |||||||||
 Qureshi et al, 2007 (33), NHANES I, USA Qureshi et al, 2007 (33), NHANES I, USA | 349 | 25–74 | 38.6 | 16.6 | Self-administered questionnaire | <1/wk,1–6/wk,≥7/wk | 253 all-cause death | Registry | Age, sex, race-ethnicity, systolic BP, DM, serum cholesterol, cigarette smoking, BMI, and education status |
 Djousse and Gaziano, 2008 (34), PHS, USA Djousse and Gaziano, 2008 (34), PHS, USA | 437 | 40–86 | 100 | 20.0 | Self-administered questionnaire | <1/wk,1/wk,2–4/wk,5–6/wk,≥7/wk | 263 all-cause death | Self or family-reported questionnaire | Age, BMI, smoking, history of hypertension, vitamin intake, alcohol consumption, vegetable consumption, breakfast cereal, physical activity, treatment arm a-fib, hypercholesterolemia, and parental history of premature MI |
 Scrafford et al, 2010 (50), NHANES III, USA Scrafford et al, 2010 (50), NHANES III, USA | 743 | >17 | 45.7 | 8.8 | Self-administered questionnaire | <1/wk,1–6/wk,≥7/wk | 66 CHD death,24 stroke death | Registry | Age, sex, and energy intake |
Egg consumption and risk of CVD
A total of 9839 incident cases of CVD from 348,420 participants in 8 cohorts were identified during an average of 11.3 y of follow-up, 5401 incident cases of IHD from 239,729 participants in 5 cohorts were documented with an average of 11.9 y follow-up, and 4189 incident cases of stroke from 241,900 participants in 5 cohorts were determined in 12.1 y follow-up. In comparison with those who never consumed egg or ate egg less than once per week, individuals who ate egg once per day or more did not have significantly higher risks of overall CVD, IHD, and stroke. The pooled HRs (95% CIs) were 0.96 ( 0.88, 1.05) for overall CVD, 0.97 (0.86, 1.09) for IHD, and 0.93 (0.81, 1.07) for stroke (Figure 2).

Pooled HRs and 95% CIs for incident IHD, stroke, and overall CVD. The pooled estimates were obtained by using a random-effects model. The dots indicate the adjusted HRs from a comparison of the highest category of egg consumption (≥1 egg/d) with the lowest (<1 egg/wk or never). The size of the shaded square is proportional to the weight of the individual study. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled HRs. CVD, cardiovascular disease; IHD, ischemic heart disease.
Egg consumption and risk of mortality
Of 103,202 participants in 8 cohorts, 11,845 overall deaths were documented during an average of 15.3 y follow-up. In particular, 510 deaths were from IHD and 1,818 deaths were from stroke. No significant associations were found between egg consumption and mortality. The pooled HRs were 1.13 (95% CI: 0.95, 1.33) for overall mortality, 0.98 (95% CI: 0.77, 1.24) for IHD mortality, and 0.92 (95% CI: 0.56, 1.50) for stroke mortality in a comparison of the highest (≥1 egg/d) with the lowest category (never or <1 egg/wk) of egg consumption (Table 3).
TABLE 3
Pooled HRs (95% CIs) of diabetes and mortality according to egg consumption1
| Egg consumption | Homogeneity | ||||||
| Source | No. ofcohorts | No. ofparticipants (events) | Duration offollow-up | Lowest | Highest | I2 | P |
| y | % | ||||||
| Mortality | |||||||
| Overall | 8 | 103,202 (11,845) | 15.3 | 1.0 | 1.13 (0.95, 1.33) | 59.2 | 0.01 |
| IHD2 | 5 | 35,011 (510) | 11.4 | 1.0 | 0.98 (0.77, 1.24) | 0.0 | 0.61 |
| Stroke | 5 | 61,339 (1818) | 13.8 | 1.0 | 0.92 (0.56, 1.50) | 40.5 | 0.15 |
| Overall in diabetic patients | 3 | 1529 (606) | 13.8 | 1.0 | 1.27 (0.78, 2.06) | 55.9 | 0.08 |
| Type 2 diabetes | 5 | 69,297 (4889) | 14.8 | 1.0 | 1.42 (1.09, 1.86) | 53.5 | 0.07 |
Egg consumption and risk of type 2 diabetes
A total of 4889 of 69,297 participants in 5 cohorts developed type 2 diabetes during an average of 14.8 y follow-up. Egg consumption was associated with an elevated risk of incident type 2 diabetes. The pooled HR was 1.42 (95% CI: 1.09, 1.86) in a comparison of the highest with the lowest category of egg consumption (Table 3).
Egg consumption and risk of CVD and mortality in patients with diabetes
The pooled database for CVD includes 7549 diabetic patients in 4 cohorts during an average of 9.5 y follow-up and 606 deaths were documented among 1529 diabetic participants in 3 cohorts with an average of 13.8 y follow-up. Patients with diabetes who ate eggs more than once per day were 1.69 times as likely to develop CVD comorbidity than were those who never ate eggs or ate eggs less than once per week (pooled HR: 1.69; 95% CI: 1.09, 2.62) (Figure 3). CVD, cardiovascular disease. However, egg consumption was not significantly associated with risk of overall mortality (Table 3).
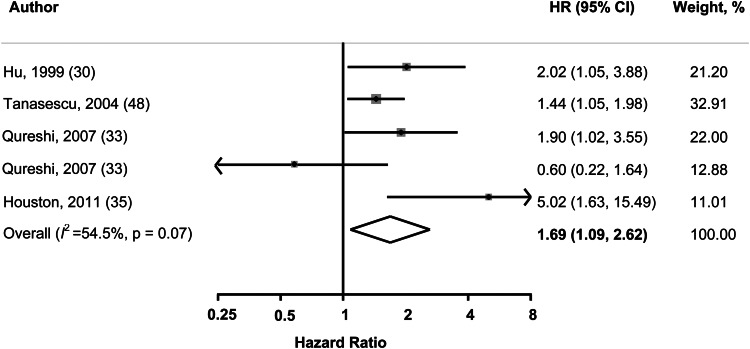
Pooled HRs and 95% CIs of incident overall CVD in diabetic patients. The pooled estimates were obtained by using a random-effects model. The dots indicate the adjusted HRs from a comparison of the highest category of egg consumption (≥1 egg/d) with the lowest (<1 egg/wk or never). The size of the shaded square is proportional to the weight of the individual study. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled HR.
Sensitivity analyses
We did not find significant heterogeneity (I2: 0.0–59.2%) among the included studies in the main analyses, except for pooled overall mortality (I2: 59.2%, P = 0.01), which combined death from all causes. In addition to comparing 2 extreme categories, we performed sensitivity analyses by replacing the highest category with the second highest category, and the results were generally consistent. In addition, we found that none of the studies markedly influenced the pooled estimate when we excluded one study at a time in the meta-analysis.
Publication bias
Publication bias was assessed by using the Egger's regression asymmetry test. No evidence of substantial publication bias was found: P = 0.67 (in the general population) and P = 0.66 (in diabetic patients) for overall CVD incidence, P = 0.999 (in the general population) and P = 0.29 (in diabetic patients) for overall mortality, and P = 0.47 for incidence of type 2 diabetes.
DISCUSSION
Accumulated epidemiologic evidence generated from this meta-analysis suggests that egg consumption is not associated with an increased risk of overall CVD, IHD, stroke, or mortality. However, compared with those who never consume eggs, those who eat 1 egg per day or more are 42% more likely to develop type 2 diabetes. Among diabetic patients, frequent egg consumers (ie, ≥ 1 egg/d) are 69% more likely to have CVD comorbidity.
A few strengths of this meta-analysis need to be highlighted. First, most of the studies or cohorts involved in this meta-analysis had a large sample size and long-term follow-up period, which potentiated the statistical power to evaluate the relation between egg consumption and risk of CVD, mortality and type 2 diabetes. For example, our meta-analysis for the risk of CVD comprises >345,000 male and female individuals with a wide age range (20–90 y) and an average of 11.3 y of follow-up. Also, our pooled estimation was based on prospective cohort studies, which largely reduced the likelihood of inherent selection bias and recall bias from the included primary studies. Because of practical and ethical considerations, it may not be feasible to conduct a long-term, double-blinded, and placebo-controlled randomized trial on egg consumption and chronic diseases. Thus, the value of this meta-analysis of prospective cohort studies is enhanced. In addition, this meta-analysis allowed us to examine the longitudinal associations of egg consumption with multiple chronic diseases. Moreover, sensitivity analyses showed that our findings were robust, and the pooled estimates were not substantially affected by any of the included primary studies.
Several limitations also need to be considered. First, our results may have been affected by the inherent limitations of primary studies. The possibility of residual confounding or bias, including diet measurement errors, cannot be excluded. In particular, our inability to standardize egg consumption in all of the included studies might have confounded our results, although the likelihood should be small because the reference group and the highest egg consumption group were similar in most primary studies. In addition, the heterogeneity of exposure categories across the individual studies limited our capability to examine a possible dose-response relation. In addition, although we found little evidence of publication bias by statistical tests, publication bias may have existed because of unpublished data or publications in non-English journals.
It has been thought that egg consumption may increase the risk of CVD because egg is a major dietary source of cholesterol. However, whether dietary cholesterol intake significantly affects the lipid profile and, consequently, is associated with the risk of CVD is still unclear. There are several possible explanations for the null association findings between egg consumption and the risk of CVD in this meta-analysis. First, the overall effect of egg consumption on circulating cholesterol concentrations may not be sufficient to appreciably increase the risk of CVD (54). A meta-analysis (22) found that the addition of 100 mg dietary cholesterol from eggs (approximately half of an egg) per day would increase plasma LDL cholesterol by 1.9 mg/dL, HDL cholesterol by 0.3 mg/dL, and the ratio of total to HDL cholesterol by 0.02 units. Another meta-analysis (15) reported that consuming 1 egg/d increased plasma LDL cholesterol by 4.1 mg/dL, increased HDL cholesterol by 0.9 mg/dL, and had little effect on the ratio of LDL to HDL cholesterol—a major predictor of CHD. Of note, the unfavorable effect of egg consumption on LDL and total cholesterol may be attenuated by its favorable effect on HDL cholesterol. Second, most of the included studies were conducted in Western countries that traditionally have a relatively high-cholesterol diet. Thus, it is likely that the background cholesterol concentration among these participants was already high, so that changes in their cholesterol concentrations might not be sensitive to egg consumption (55, 56). Third, egg is also a good source of nutrients, which may provide a cardioprotective effect. For example, egg is a good source of B vitamins, including choline, which may lower homocysteine concentrations (57, 58). Thus, the overall effect of egg consumption may reflect the interactions of cholesterol with other nutrients in egg. Notably, 2 RCTs showed that consumption of 2 eggs/d for 6 wk did not induce an adverse effect on endothelial function—an indicator of CHD risk (27, 28).
In addition, we found that egg consumption was associated with an increased risk of CVD in diabetic patients. The mechanisms are not fully understood. Presumably, diabetic patients are at high risk of developing CVD and may be more sensitive to egg consumption. A small change in circulating cholesterol may substantially increase the risk of CVD in patients with diabetes. Also, a study indicated that cholesterol synthesis and plasma lipid concentrations, including total cholesterol and triglyceride, were higher in type 2 diabetic patients with uncontrolled hyperglycemia (59). Hence, egg consumption may induce unfavorable effects on lipid profiles and lead to an increased risk of CVD in patients with diabetes. However, a diet intervention study (60) showed that an energy-restricted high-protein diet with high cholesterol (2 eggs/d) for 12 wk increased HDL cholesterol concentrations compared with an energy-restricted high-protein diet with low cholesterol (egg replaced by 100 g lean animal protein) in type 2 diabetic patients. Of note, both intervention and control diets significantly reduced body weight, total cholesterol, fasting plasma glucose and insulin, and blood pressure.
In the current meta-analysis, we observed a positive association between egg consumption and risk of type 2 diabetes. Although the findings were inconsistent, some animal studies suggested that high cholesterol feeding increased fasting plasma glucose concentrations or induced hyperinsulinemia and impaired glucose tolerance (61–63). Studies have also suggested that elevated serum cholesterol might be associated with an increased islet cholesterol content and directly induce β cell dysfunction by reducing glucose-stimulated insulin secretion (64, 65). In addition, studies have indicated that dietary cholesterol might be associated with chronic, low-grade inflammation (66, 67), which is a mechanism underlying the development of insulin resistance and type 2 diabetes. Moreover, our results on egg consumption and risk of diabetes are supported by some other human studies (38–40, 68) that do not meet our inclusion criteria. Furthermore, some prospective cohort studies found that dietary cholesterol intake was associated with risk of type 2 diabetes (69, 70) and gestational diabetes (71). However, RCTs in participants consuming a carbohydrate-restricted diet showed that consumption of 3 eggs/d for 12 wk did not significantly change plasma fasting glucose (72, 73) and insulin resistance (73) compared with placebo (equivalent amount of egg substitute without dietary cholesterol). Of note, the possibility cannot be completely excluded that the observed positive associations between egg consumption and risk of type 2 diabetes are explained by residual confounding from unmeasured factors, because diabetes was the secondary outcome in most included studies. Nevertheless, future studies are warranted.
In conclusion, this meta-analysis of prospective cohort studies does not support an increase in the risk of CVD and mortality in the general population with regular egg consumption. However, egg consumption may be associated with an increased risk of type 2 diabetes, and diabetic patients with frequent egg consumption may be more likely to develop CVD comorbidity. Clearly, more studies are needed to elucidate the potential mechanisms linking egg consumption to diabetes risk. Particularly, experimental studies are warranted to explore how dietary cholesterol is involved in glucose and insulin homeostasis. Although we have drawn attention to the association between high egg consumption and the risk of type 2 diabetes, and the potential increased risk of CVD with egg consumers in patients with type 2 diabetes, the results of this meta-analysis should be interpreted with caution and may not justify changes in current dietary advice on egg consumption until more scientific data become available.
Acknowledgments
The authors’ responsibilities were as follows—KH: conceived the study concept and design; JYS and PX: completed the literature search, study selection, and data extraction and prepared the tables and figures; PX: performed the data analysis; and JYS, PX, and KH: interpreted the results and drafted the manuscript. All authors: critically reviewed the manuscript for important intellectual content and approved the final version. None of the authors had any conflicts of interest to disclose.
Footnotes
4Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease; RCT, randomized controlled trial.
REFERENCES
Articles from The American Journal of Clinical Nutrition are provided here courtesy of American Society for Nutrition
Full text links
Read article at publisher's site: https://doi.org/10.3945/ajcn.112.051318
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ajcn/article-pdf/98/1/146/23828606/146.pdf
Citations & impact
Impact metrics
Article citations
Debunking the Myth: Eggs and Heart Disease.
Cureus, 16(5):e59952, 09 May 2024
Cited by: 0 articles | PMID: 38854339
Review
Eggs - a scoping review for Nordic Nutrition Recommendations 2023.
Food Nutr Res, 68, 06 Feb 2024
Cited by: 0 articles | PMID: 38370115 | PMCID: PMC10870976
Review Free full text in Europe PMC
Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022.
J Atheroscler Thromb, 31(6):641-853, 19 Dec 2023
Cited by: 22 articles | PMID: 38123343 | PMCID: PMC11150976
Precision Medicine in Type 2 Diabetes Mellitus: Utility and Limitations.
Diabetes Metab Syndr Obes, 16:3669-3689, 16 Nov 2023
Cited by: 4 articles | PMID: 38028995 | PMCID: PMC10658811
Review Free full text in Europe PMC
Dietary management of dyslipidemia.
Indian Heart J, 76 Suppl 1:S65-S72, 18 Dec 2023
Cited by: 0 articles | PMID: 38122980 | PMCID: PMC11019336
Review Free full text in Europe PMC
Go to all (143) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Egg consumption and cardiovascular disease according to diabetic status: The PREDIMED study.
Clin Nutr, 36(4):1015-1021, 29 Jun 2016
Cited by: 15 articles | PMID: 27448949
Association of Egg Consumption with Risk of All-Cause and Cardiovascular Disease Mortality: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies.
J Nutr, 152(10):2227-2237, 01 Oct 2022
Cited by: 2 articles | PMID: 35524693
Review
Associations of Dietary Cholesterol or Egg Consumption With Incident Cardiovascular Disease and Mortality.
JAMA, 321(11):1081-1095, 01 Mar 2019
Cited by: 135 articles | PMID: 30874756 | PMCID: PMC6439941
Is Butter Back? A Systematic Review and Meta-Analysis of Butter Consumption and Risk of Cardiovascular Disease, Diabetes, and Total Mortality.
PLoS One, 11(6):e0158118, 29 Jun 2016
Cited by: 73 articles | PMID: 27355649 | PMCID: PMC4927102
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDDK NIH HHS (1)
Grant ID: R21DK073812
NINDS NIH HHS (1)
Grant ID: R21NS056445