Abstract
Free full text

Exenatide as a Weight-Loss Therapy in Extreme Pediatric Obesity A Randomized, Controlled Pilot Study
Abstract
The objective of this pilot study was to evaluate the effects of exenatide on body mass index (BMI) (primary endpoint) and cardiometabolic risk factors in non-diabetic youth with extreme obesity. Twelve children and adolescents (age 9–16 years old) with extreme obesity (BMI ≥1.2 times the 95th percentile or BMI ≥35 kg/m2) were enrolled in a 6-month, randomized, open-label, crossover, clinical trial consisting of two, 3-month phases: 1) a control phase of lifestyle modification and 2) a drug phase of lifestyle modification plus exenatide. Participants were equally randomized to phase-order (i.e., starting with control or drug therapy) then crossed-over to the other treatment. BMI, body fat percentage, blood pressure, lipids, oral glucose tolerance (OGTT), adipokines, plasma biomarkers of endothelial activation, and endothelial function were assessed at baseline, 3-, and 6-months. The mean change over each 3-month phase was compared between treatments. Compared to control, exenatide significantly reduced BMI (−1.7 kg/m2, 95% CI (−3.0, −0.4), P = 0.01), body weight (−3.9 kg, 95% CI (−7.11, −0.69), P = 0.02), and fasting insulin (−7.5 mU/L, 95% CI (−13.71, −1.37), P = 0.02). Significant improvements were observed for OGTT-derived insulin sensitivity (P = 0.02) and beta cell function (P = 0.03). Compliance with the injection regimen was excellent (≥94%) and exenatide was generally well-tolerated (the most common adverse event was mild nausea in 36%). These preliminary data suggest that exenatide should be evaluated in larger, well-controlled trials for its ability to reduce BMI and improve cardiometabolic risk factors in youth with extreme obesity.
Introduction
Extreme pediatric obesity, defined as an age- and gender-specific body mass index (BMI) ≥1.2 times the 95th percentile or BMI ≥35 kg/m2, is the fastest growing obesity category in youth and afflicts approximately 3–7% of the U.S. pediatric population (1–3). While obesity in childhood is associated with an increased risk of type 2 diabetes mellitus (T2DM) (4–6), cardiovascular disease (CVD) (7;8), and early mortality (9;10), extreme obesity is associated with even greater risk. Approximately 84% of youth with extreme obesity already have at least one CVD risk factor (11) and up to 5% have impaired glucose tolerance (12), a harbinger of T2DM. Obesity in childhood tracks strongly into adulthood, particularly in the extremely obese (11;13). Targeting adiposity early in life offers an opportunity to reduce the risk of T2DM and CVD since interventions instituted in childhood may be more effective than when initiated in adulthood (14).
Lifestyle modification is the preferred strategy for treating pediatric obesity. However, many youth with extreme obesity are unable to reduce their weight and improve their risk factor profile to an acceptable level with lifestyle modification alone (15). While bariatric surgery is becoming more common in extreme pediatric obesity, it is invasive, life-altering, and not without risk. Intermediate interventions, such as pharmacotherapy, may be beneficial for some patients and should be considered as a treatment option. Unfortunately, drug treatments are limited. Sibutramine, orlistat, and metformin are the only medications that have been evaluated for the treatment of pediatric obesity. Sibutramine was recently removed from the U.S. market due to cardiovascular safety concerns (16) and orlistat (17) and metformin (18;19) only modestly reduce BMI. Therefore, a significant need exists to evaluate novel drug therapies aimed at reducing adiposity and improving risk factors for T2DM and CVD in youth with extreme obesity.
Exenatide is a glucagon like peptide-1 (GLP-1) receptor agonist used in adults with T2DM to improve glycemic control. Of primary relevance to the treatment of obesity, data in adults have demonstrated that exenatide significantly reduces BMI, body weight, and body fat (20;21) through its ability to heighten feelings of satiety and suppress appetite. To our knowledge, exenatide has not been evaluated as a weight-loss agent in children or adolescents. Therefore, we performed a 6-month randomized, open-label, crossover, pilot clinical trial evaluating the effects of exenatide on BMI (pre-specified primary endpoint) and T2DM/CVD risk factors (pre-specified secondary endpoints) in children and adolescents with extreme obesity. We hypothesized that, compared to control, exenatide would significantly reduce BMI.
Methods and Procedures
Study Design and Eligibility Criteria
This was a 6-month, randomized, open-label, crossover, clinical trial in 12 children and adolescents. An open-label design was chosen due to concern about using a placebo injection within the context of a pediatric pilot/feasibility study. Inclusion criteria consisted of age 8–19 years old and extreme obesity (BMI ≥1.2 times the 95th percentile or BMI ≥35 kg/m2). Exclusion criteria included: diabetes mellitus (type 1 or 2), use of weight-loss medication within 3-months of screening, initiation of new drug therapy within 30 days of screening, BMI ≥ 55 kg/m2, history of bariatric surgery, and obesity from an established genetic cause or known syndrome (e.g., Prader-Willi). Participants were recruited from three pediatric obesity/endocrinology centers in the Minneapolis-St. Paul metropolitan area (University of Minnesota, Children's Hospitals and Clinics of Minnesota, and the International Diabetes Center at Park Nicollet) and were enrolled and followed at the University of Minnesota.
The study consisted of two, 3-month phases: 1) a control phase (C) consisting of clinically-offered lifestyle modification and 2) a drug phase (E) consisting of clinically-offered lifestyle modification plus exenatide therapy. The clinical-offered lifestyle modification varied by center but generally consisted of counseling by a physician (and registered dietician at some centers) with particular focus on making healthier food choices and increasing levels of physical activity. Participants were assigned at random to one of 12 phase-order sequences: 6 C-E and 6 E-C (i.e., starting with control or drug therapy then crossed-over to the other treatment regimen, no washout period). The protocol was approved by the University of Minnesota Institutional Review Board and consent/assent was obtained from parents/participants. An investigational new drug exemption was obtained from the United States Food and Drug Administration prior to study initiation and the study was registered on the clinicaltrials.gov website (NCT00886626).
Exenatide Dosing, Administration, and Tracking
For the drug treatment phase, participants initiated exenatide at a dose of 5 mcg, twice per day (BID), delivered by subcutaneous injection. After 1-month, exenatide was uptitrated to 10 mcg, BID for the remaining 2-months of the drug treatment phase. If the 10 mcg dose was not tolerated, the exenatide dose was reduced to 5 mcg. Compliance was assessed by requiring participants to complete a medication administration log and bring exenatide pens to each study visit for visual inspection (estimation of the percentage of drug utilized). The pre-determined threshold for compliance with the drug regimen was designated as missing no more than 20% of the required doses. Although hypoglycemia was not expected, participants were given a glucometer and instructed to check blood glucose level if any symptoms of hypoglycemia were experienced.
Measurement of Clinical Variables, Glucose Tolerance, and Blood Biomarkers
Height and weight were obtained with participants in light clothes and without shoes using the same standardized stadiometer and electronic scale, respectively. Body composition was determined using dual-energy x-ray absorptiometry (DXA) (Prodigy, 3M, Madison, WI, USA). Seated blood pressure was obtained after five minutes of quiet rest, on the right arm using an automatic sphygmomanometer and appropriately-fitted cuff. Tanner stage (pubertal development) determinations were performed by trained pediatricians. Fasting (at least 12 hours) blood samples were collected and assays for lipids, glucose, and insulin were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention–certified laboratory. Standard 2-hour oral glucose tolerance tests (OGTT) were performed using a 75-gram oral glucose load. Serial glucose and insulin levels were performed at baseline and every 30-minutes for 2-hours. For the post-drug phase visit, exenatide (10 mcg) was administered 30-minutes before the start of the OGTT (fasting blood samples were obtained prior to exenatide administration). Area-under-the-curve (AUC) for glucose and insulin, the whole body insulin sensitivity index (WBISI/Matsuda index), and beta cell function were calculated from the OGTT as previously described (22). Blood plasma was stored frozen at −70 C for a batched analysis of the following (unless otherwise noted, all kits from R&D Systems, Minneapolis, MN): high-sensitivity C-reactive protein (CRP) (ALPCO, Salem, NH), high-sensitivity interleukin-6 (IL-6), high-sensitivity tumor necrosis factor-alpha (TNF-α), adiponectin, leptin, oxidized LDL (oxLDL) (Mercodia, Winston-Salem, NC), vascular cell adhesion molecule-1 (VCAM-1), and von Willebrand factor (vWF) (Aushon BioSystems, Billerica, MA) in the University of Minnesota Cytokine Reference Laboratory (CLIA licensed).
Measurement of Endothelial Function
Endothelial function was measured by digital reactive hyperemia (EndoPAT 2000, Itamar Medical, Caesarea, Israel). Following 10 minutes of quiet rest in the supine position, finger probes were placed on the index fingers to measure baseline and reactive hyperemic pulse amplitude. After collection of baseline data, a blood pressure cuff on the upper forearm was inflated to a suprasystolic level for five minutes. Following cuff release, the change in pulse amplitude during reactive hyperemia was measured for five minutes. The ratio of the hyperemic and the baseline pulse amplitude (corrected for the same ratio on the control finger) was calculated and expressed as the reactive hyperemic index (RHI). Reproducibility of this technique in children and adolescents has shown a mean difference in RHI, measured one week apart, of 0.12 and within subject variation of 0.16 (23).
Statistical Analyses
Baseline characteristics were tabulated with respect to randomized order of cross-over treatment regimens. Outcomes were evaluated at baseline and at the end of each of the two, 3-month treatment phases. Estimated treatment effects were based on an individual's change over each treatment phase. Generalized estimating equations (GEE) were used with exchangeable correlation structure to take into account possible correlation between the changes observed over the first treatment phase with the changes observed over the second treatment phase within an individual. Due to incomplete follow-up on all participants who entered the study, the number of measurements for each treatment regimen was unbalanced. The primary analysis followed a pre-specified per-protocol analysis where participants were included if they completed at least one treatment phase and missed no more than 20% of the required exenatide doses. All statistical analyses were performed using R v2.12.0 (R Foundation for Statistical Computing, Vienna, Austria) and with `gee' library v4.13–16. Glucose and insulin AUC during OGTT was calculated with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
Enrollment occurred from October 2009 – August 2010. A total of 29 subjects who qualified for the study according to information in the medical chart were approached about the study and 12 agreed to participate. Three subjects failed to complete the study - two voluntarily withdrew due to lack of interest and one was withdrawn from the study by the investigators due to right upper quadrant pain that was later determined to be not-related to the study medication. Of these three dropouts, two had complete data (pre and post) from at least one phase (these data were included in the analyses) and one did not have data beyond randomization (lack of interest) so was not included in any of the analyses. One participant initiated an ADHD stimulant medication (associated with weight-loss and increases in blood pressure and heart rate) after the 3-month assessment; therefore, data for this individual was only included for the initial phase in the primary analysis.
All participants who completed the exenatide phase tolerated the 10 mcg dose. However, two participants required reduction to 5 mcg for one week due to GI symptoms but were then able to resume the 10 mcg without further problems for the remainder of the treatment phase. All participants who had complete data (pre and post) from the exenatide phase were compliant with the required injection regimen. Study medication compliance ranged from 94–100% of the required doses (mean of 98%).
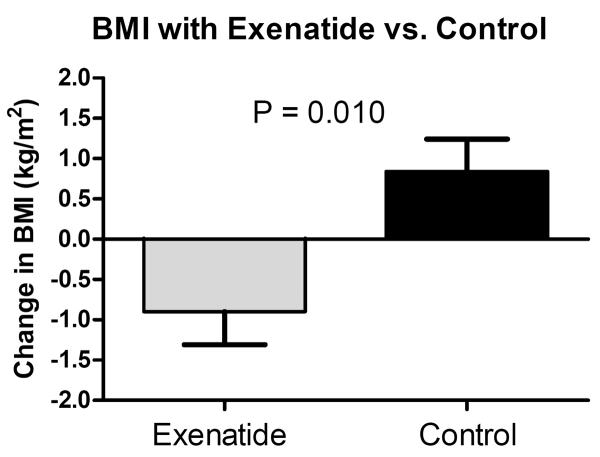
Descriptive baseline characteristics of the participants overall and by randomized group are shown in Table 1. Nine participants were white and three were biracial. The age range of those enrolled was 9–16 years old and all participants had started puberty and Tanner stage at baseline ranged from 2–5 (9/12 were in Tanner stage 4 or 5). The estimated treatment effects within and between treatments are presented in Table 2. For the primary outcome, the change in BMI for the exenatide group compared to control was significantly lower (–1.7 kg/m2, 95% CI (−3.0, −0.4), P = 0.01) (Figure 1). The change in several secondary endpoints also showed significant differences compared to control; lower percent BMI (−4.92%, 95% CI (−8.61, −1.23), P = 0.009), body weight (−3.9 kg, 95% CI (−7.11, −0.69), P = 0.02), percent body weight (−4.58%, 95% CI (−8.12, −1.04), P = 0.011), fasting insulin (−7.5 mU/L, 95% CI (−13.71, −1.37), P = 0.02), and increased surrogate markers of insulin sensitivity (6.1, 95% CI (1.01, 11.25), P = 0.02) and beta cell function (18.0, 95% CI (1.40, 34.54), P = 0.03). Percent body fat was not statistically significantly different between exenatide and control. Although not a pre-specified endpoint, exenatide treatment was associated with a mild increase in the liver enzyme aspartate transaminase (AST). There were trends toward improved glucose and insulin response (AUC) during the OGTT (Figure 2 shows the glucose and insulin responses pre- and post-exenatide phase). When the month six BMI value from the participant who started the ADHD medication was included in the analysis, the BMI estimate was attenuated, but remained statistically significant (−1.5 kg/m2, 95% CI (−2.94, −0.06), P = 0.04).
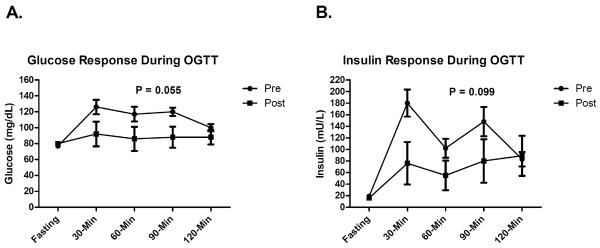
Glucose (panel A) and insulin (panel B) response during the 2-hour OGTT in participants with data pre- and post-exenatide treatment. P-value is for the estimated treatment effect between study arms for AUC over the 2-hour OGTT period. Error bars represent SEM.
Table 1
Participant baseline characteristics by randomized treatment-order group.
| Covariate | Overall (N=11) | Group E-C (N=5) | Group C-E (N=6) |
|---|---|---|---|
| Age (years) | 12.7 (2.1) | 13.0 (1.87) | 12.5 (2.43) |
| Male | 2 (18.2%) | 0 (0.0%) | 2 (33.3%) |
| BMI (kg/m2) | 36.7 (4.8) | 37.4 (4.18) | 36.2 (5.59) |
| Weight (kg) | 93.8 (20.6) | 94.4 (17.2) | 93.3 (24.7) |
| Body Fat (%) | 47.9 (5.27) | 48.7 (4.39) | 47.3 (6.25) |
| Heart Rate (bpm) | 78.9 (15.6) | 79.8 (22.8) | 78.2 (8.5) |
| SBP (mmHg) | 119 (6.9) | 119 (9.04) | 118 (5.47) |
| DBP (mmHg) | 58.9 (10.2) | 64.0 (11.4) | 54.7 (7.47) |
| Cholesterol (mg/dL) | 145 (20.8) | 143 (26.8) | 146 (17.1) |
| LDL (mg/dL) | 85.6 (23.6) | 88.4 (25.1) | 83.3 (24.4) |
| HDL (mg/dL) | 36.5 (5.89) | 35.4 (4.72) | 37.3 (7.03) |
| Triglycerides (mg/dL) | 90.7 (31.7) | 97.2 (30.1) | 84.2 (35.3) |
| ALT (U/L) | 21.82 (9.23) | 17.60 (10.36) | 25.33 (7.20) |
| AST (U/L) | 31.18 (11.93) | 26.40 (6.47) | 35.17 (14.47) |
| Glucose (mg/dL) | 75.4 (4.11) | 73.8 (3.03) | 76.7 (4.68) |
| Glucose AUC (per 100) | 51.1 (24.4) | 45.4 (21.1) | 55.9 (27.8) |
| Insulin (mU/L) | 14.6 (6.15) | 17.0 (5.29) | 13.0 (6.6) |
| Insulin AUC (per 100) | 132 (68.6) | 100 (32.3) | 153 (80.6) |
| WBISI/Matuda | 2.88 (1.31) | 2.88 (0.52) | 2.88 (1.79) |
| β-Cell Function | 8.56 (3.94) | 7.47 (2.05) | 9.43 (5.07) |
| CRP (mg/L) | 1.79 (1.55) | 1.26 (1.09) | 2.32 (1.86) |
| IL-6 (pg/mL) | 3.21 (1.76) | 3.85 (2.05) | 2.67 (1.44) |
| TNF-a (pg/mL) | 1.36 (0.76) | 1.47 (0.92) | 1.26 (0.67) |
| Adiponectin (μg/mL) | 16.1 (5.0) | 15.9 (2.08) | 16.2 (6.81) |
| Leptin (ng/mL) | 135 (54.0) | 143 (47.4) | 128 (62.6) |
| OxLDL (U/L) | 42.9 (7.81) | 42.2 (8.68) | 43.5 (7.8) |
| VCAM-1 (ng/mL) | 580 (139.8) | 564 (156.7) | 594 (137.7) |
| vWF (U/mL) | 23.0 (9.3) | 24.8 (5.99) | 21.5 (11.8) |
| RHI | 1.83 (0.48) | 1.84 (0.44) | 1.82 (0.55) |
Values presented are mean (SD) or N (%) where indicated. E = exenatide; C = control. Two participants in group C-E only completed the control phase (the first 3-months).
Table 2
Estimated treatment effects within and between study arms.
| Outcome | Δ Control | Δ Exenatide | Treatment Effect (95% CI) | P-value |
|---|---|---|---|---|
| BMI (kg/m2) | 0.84 (1.28) | −0.90 (1.22) | −1.71 (−3.01, −0.42) | 0.010 |
| Percent BMI | 1.72 (4.19) | −2.57 (3.46) | −4.92 (−8.61, −1.23) | 0.009 |
| Weight (kg) | 2.97 (2.88) | −0.99 (2.90) | −3.90 (−7.11, −0.69) | 0.017 |
| Percent Weight | 2.68 (3.71) | −1.20 (3.19) | −4.58 (−8.12, −1.04) | 0.011 |
| Body Fat (%) | 0.92 (2.75) | −0.36 (3.07) | −1.28 (−4.66, 2.09) | 0.457 |
| Heart Rate (bpm) | −4.80 (7.45) | 2.22 (11.26) | 7.60 (−3.01, 18.21) | 0.160 |
| SBP (mmHg) | 3.10 (8.72) | −3.11 (8.51) | −5.31 (−14.89, 4.27) | 0.277 |
| DBP (mmHg) | −0.30 (13.91) | −1.78 (11.96) | −3.00 (−18.33, 12.33) | 0.701 |
| Cholesterol (mg/dL) | −8.60 (18.31) | 4.33 (19.54) | 10.70 (−11.92, 33.32) | 0.354 |
| LDL (mg/dL) | −4.50 (16.75) | 2.89 (16.70) | 6.93 (−11.77, 25.62) | 0.468 |
| HDL (mg/dL) | −0.20 (6.84) | 3.44 (3.40) | 3.25 (−2.47, 8.97) | 0.266 |
| Triglycerides (mg/dL) | −4.22 (37.45) | −9.89 (41.24) | −12.57 (−59.41, 34.28) | 0.599 |
| ALT (U/L) | 0.89 (10.49) | 0.71 (7.63) | 1.48 (−10.65, 13.60) | 0.811 |
| AST (U/L) | −3.67 (3.61) | 3.29 (3.64) | 6.04 (2.54, 9.54) | <0.001 |
| Glucose (mg/dL) | 1.60 (6.40) | 3.22 (5.85) | 1.68 (−3.65, 7.01) | 0.537 |
| Glucose AUC (per 100) | 11.69 (29.50) | −17.67 (22.98) | −27.96 (−56.51, 0.59) | 0.055 |
| Insulin (mU/L) | 6.00 (5.58) | −1.62 (8.42) | −7.54 (−13.71, −1.37) | 0.017 |
| Insulin AUC (per 100) | 30.29 (98.76) | −38.95 (73.74) | −76.01 (−166.30, 14.28) | 0.099 |
| WBISI/Matuda | −2.00 (3.52) | 4.12 (7.10) | 6.13 (1.01, 11.25) | 0.019 |
| β-Cell Function | −1.16 (6.33) | 14.50 (26.09) | 17.97 (1.40, 34.54) | 0.034 |
| CRP (mg/L) | −0.50 (0.76) | −0.36 (1.66) | 0.12 (−1.11, 1.34) | 0.853 |
| IL-6 (pg/mL) | −0.54 (0.78) | −0.83 (1.62) | −0.35 (−1.21, 0.50) | 0.419 |
| TNF-a (pg/mL) | −0.16 (0.49) | −0.33 (0.67) | −0.17 (−0.82, 0.48) | 0.610 |
| Adiponectin (μg/mL) | −1.35 (5.47) | −0.20 (3.93) | 0.98 (−4.22, 6.17) | 0.712 |
| Leptin (ng/mL) | −7.95 (41.31) | −12.71 (22.02) | −4.44 (−40.29, 31.41) | 0.808 |
| OxLDL (U/L) | −0.73 (7.49) | −0.40 (5.02) | 0.34 (−5.44, 6.13) | 0.907 |
| VCAM-1 (ng/mL) | 73.31 (154.41) | −7.37 (142.68) | −79.33 (−252.28, 93.63) | 0.369 |
| vWF (U/mL) | −3.81 (11.24) | −2.17 (10.87) | 2.08 (−10.97, 15.12) | 0.755 |
| RHI | 0.23 (0.65) | −0.03 (0.69) | −0.26 (−0.88, 0.36) | 0.413 |
Values presented are mean (SD).
Adverse event data were available from all but one participant (two of the three dropouts had 1-month data while in the exenatide phase). The most commonly reported adverse event was nausea, which was experienced in 4/11 (all classified as mild). Vomiting (3/11) and headache (3/11) were the second most commonly reported adverse events – all were categorized as mild. Three subjects experienced abdominal pain (two classified as mild and one as moderate). One subject experienced injection site bruising, which was classified as mild. No subjects experienced hypoglycemia or pancreatitis.
Discussion
Few weight-loss drugs have been evaluated in youth and we believe ours is the first study to examine the effects of a GLP-1 receptor agonist on BMI and/or weight-loss in obese children and adolescents. While lifestyle modification is considered the cornerstone of obesity treatment, the results of this approach tend to be dismal in youth with extreme obesity, who are more likely to continue to gain weight (15;17;18). Our control findings are consistent with this observation - participants in our study experienced an increase in BMI of 0.84 kg/m2 over just three months during the lifestyle modification-only phase of the study. When the BMI change during the control period was examined separately for those starting on exenatide vs. control, there was some suggestion of a possible order effect (i.e., greater increase in BMI during the post-exenatide control period), although not statistically significant. Nevertheless, these results are in line with the previous studies of orlistat and metformin, which reported weight-gain in the placebo groups (17;18). In contrast, when exenatide therapy was provided, participants not only stopped their BMI gain, but reduced their BMI by 0.90 kg/m2. Although trending in a positive direction, percent body fat was not statistically significantly different between exenatide and control. Ideally, any weight-loss intervention would reduce body fat along with BMI and body weight. Regardless, data from this clinical trial offer preliminary evidence that short-term treatment with exenatide can change the trajectory of weight-gain and significantly reduce BMI and body weight in youth with extreme obesity.
Fasting insulin, insulin sensitivity, and beta cell function were significantly improved with exenatide. The observed increase in the WBISI/Matsuda index, a surrogate measure of insulin sensitivity derived from the OGTT, suggests that exenatide may improve insulin action, at least in the postprandial setting. This, along with the finding of improved beta cell function, is encouraging considering the pivotal dual roles of insulin resistance and pancreatic beta cell dysfunction/failure in the pathophysiology of T2DM. Since exenatide was administered prior to the OGTT, it is unclear whether long-term, durable improvements would persist following withdrawal of therapy. Further studies using more direct methods of quantifying insulin sensitivity (e.g., the euglycemic, hyperinsulinemic clamp) and beta cell function (e.g., the hyperglycemic clamp or the intravenous glucose tolerance test), will be required to confirm these findings. There was a non-statistically significant trend toward reduced glucose and insulin responses during OGTT. The reduction in the glucose AUC was expected based upon the well-documented mechanism of action of exenatide (increased first-phase insulin response and inhibition of glucagon secretion). However, the reduced insulin AUC was somewhat unexpected but is likely explained by the ability of exenatide to slow gastric emptying, resulting in a slower rate of appearance of glucose in the blood. Together, these preliminary findings suggest that exenatide may offer multiple beneficial metabolic effects, which may have implications for the prevention of T2DM. This is of particular importance in the context of extreme pediatric obesity since these youth are at greatly elevated risk of developing T2DM (24).
Cardiovascular risk reduction is an important consideration in obese youth. There were no statistically significant improvements in any of the cardiovascular risk factors, adipokines, or markers of endothelial activation measured in this study. It is possible that a larger amount of weight-loss than was achieved in this study is required in order to improve many of these variables since the effects of GLP-1 receptor agonists on cardiovascular risk factors may be predominantly weight-loss dependent. However, it should be noted that compared to control, exenatide was associated with a clinically-meaningful reduction in SBP (−5 mmHg), which did not reach statistical significance with the sample size here. Reduction in SBP has been a consistent finding in exenatide studies in adults and evidence suggests that the blood pressure lowering effect of exenatide may be independent of weight-loss (25). Larger trials of exenatide in obese youth will be able to more conclusively characterize the effect of this medication on SBP and other cardiovascular risk factors.
Exenatide was generally well-tolerated. The reports of nausea, vomiting, headache, and abdominal pain were transient and mild in severity and were consistent with reports from the adult literature. There were no reports of hypoglycemia. Mild elevations in the liver enzyme AST appeared to be associated with exenatide treatment. An explanation for this finding is not clear, since to our knowledge, no such association has been reported in the adult literature. Despite the inconvenience of twice daily injections, adherence was excellent. A long-acting, once-weekly formulation of exenatide should be available in the relatively near future, which may reduce the main disincentive of this therapy – frequency of injections.
It is important to compare exenatide to the two drugs currently being used to treat obesity in youth. Although the current data are preliminary and should be viewed with caution due to the lack of blinding and the potential over-estimation of the treatment effect due to possible post-exenatide BMI rebound, the control-subtracted change in BMI of −1.71 kg/m2 with exenatide was twice that which has been reported for orlistat (approximately −0.86 kg/m2) (17) and 50% greater than that reported for metformin (approximately −1.1 kg/m2) (18). Reductions in percent BMI and body weight with exenatide approached 5% over 3-months of treatment. Because of the relatively short duration of treatment used in the current study compared to the previous studies of orlistat and metformin (most studies ranged from 6–12 months), direct comparisons cannot be made; however, data in adults demonstrate further weight-loss beyond 3-months with continued use of exenatide (20;21), suggesting a potential persistent effect over time may also hold for this population. Nevertheless, conclusions regarding durability cannot be made based upon our data and further studies evaluating exenatide treatment over longer periods of time will be needed to address this issue. Orlistat has GI side effects which are often intolerable to youth including oily fecal spotting, oily evacuation, fecal urgency, and abdominal pain (17;26) and neither orlistat nor metformin offer much in the way of metabolic or cardiovascular risk factor modification (17–19). Thus, exenatide may be an attractive alternative to these drugs but further investigation is required.
Strengths of the study included the randomized, controlled design and the comprehensive assessments that were performed (body fat, OGTT, endothelial function, plasma biomarkers of inflammation, oxidative stress, and endothelial activation). Since the study was un-blinded, participants' knowledge of the treatment and control phases may have affected the responses. We chose to utilize this design because of concerns about using placebo injections within the context of a first-run pediatric pilot/feasibility study. This study did not include a washout phase so the results may have been influenced by a potential carry-over effect. It is possible that pubertal development may have affected some of the variables measured in this study; however, most (9/12) were in Tanner stage 4 or 5 at baseline. Finally, the sample size was relatively small. Follow-up studies, with larger sample sizes, will be needed to more comprehensively evaluate the safety and efficacy of exenatide in this patient population.
In conclusion, we provide preliminary evidence that exenatide reduces BMI and body weight and improves important risk factors for T2DM in children and adolescents with extreme obesity. The twice per day dosing, method of administration, and side-effect profile appear to be acceptable to obese youth. These findings provide justification for the further evaluation of exenatide as a weight-loss therapy in the context of extreme pediatric obesity.
Acknowledgments
Funding for this study was provided the Minnesota Obesity Center (NIH Grant P30DK050456 NORC) and GCRC (M01-RR00400, General Clinical Research Center Program, NCRR/NIH). Glucose meters were generously donated by Bayer HealthCare. We would like to thank the participants and their families for donating their time to make this study possible. We are also grateful to Julia Steinberger, M.D., M.S. (University of Minnesota) for consulting on the study.
Clinical Trials Registration: This study is registered on the www.clinicaltrials.gov website (ClinicalTrials.gov identifier: NCT00886626)
Footnotes
Disclosures
Dr. Kelly receives research funding from Amylin/Eli Lilly and served on a pediatric obesity advisory board (clinical trial design) for Novo Nordisk. Dr. Abuzzahab receives research funding from Amylin/Eli Lilly. None of the other authors have relevant disclosures.
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1038/oby.2011.337
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1038/oby.2011.337
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/oby.2011.337
Article citations
The Antiobesity Effect and Safety of GLP-1 Receptor Agonist in Overweight/Obese Adolescents Without Diabetes Mellitus: A Systematic Review and Meta-Analysis.
Cureus, 16(8):e66280, 06 Aug 2024
Cited by: 0 articles | PMID: 39238716 | PMCID: PMC11376316
Review Free full text in Europe PMC
Financial Incentives and Treatment Outcomes in Adolescents With Severe Obesity: A Randomized Clinical Trial.
JAMA Pediatr, 178(8):753-762, 01 Aug 2024
Cited by: 1 article | PMID: 38884967
Effective and appropriate use of weight loss medication in pediatric obesity: a narrative review.
J Yeungnam Med Sci, 41(3):158-165, 02 Jul 2024
Cited by: 1 article | PMID: 38952016 | PMCID: PMC11294794
Review Free full text in Europe PMC
Comparative Efficacy and Safety of Glucagon-like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity or Overweight: A Systematic Review and Network Meta-Analysis.
Pharmaceuticals (Basel), 17(7):828, 24 Jun 2024
Cited by: 1 article | PMID: 39065679 | PMCID: PMC11279917
Review Free full text in Europe PMC
Special considerations for the child with obesity: An Obesity Medicine Association (OMA) clinical practice statement (CPS) 2024.
Obes Pillars, 11:100113, 23 May 2024
Cited by: 0 articles | PMID: 38953014 | PMCID: PMC11216014
Go to all (73) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT00886626
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years.
Curr Med Res Opin, 24(1):275-286, 01 Jan 2008
Cited by: 441 articles | PMID: 18053320
The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial.
JAMA Pediatr, 167(4):355-360, 01 Apr 2013
Cited by: 80 articles | PMID: 23380890 | PMCID: PMC4010226
Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: Sustained reductions in body weight, glycaemia and blood pressure over 1 year.
Diabetes Obes Metab, 19(9):1276-1288, 31 May 2017
Cited by: 35 articles | PMID: 28345814 | PMCID: PMC5575470
Exenatide in obese or overweight patients without diabetes: A systematic review and meta-analyses of randomized controlled trials.
Int J Cardiol, 219:293-300, 15 Jun 2016
Cited by: 8 articles | PMID: 27343423
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000114
NCRR NIH HHS (2)
Grant ID: M01-RR00400
Grant ID: M01 RR000400
NIDDK NIH HHS (2)
Grant ID: P30 DK050456
Grant ID: P30DK050456