Abstract
Aims
We compared neurodevelopmental outcomes of extremely low birth weight (ELBW) infants with and without bronchopulmonary dysplasia (BPD), using the physiologic definition.Study design
ELBW (birth weights<1000 g) infants admitted to the Neonatal Research Network centers and hospitalized at 36 weeks postmenstrual age (n=1189) were classified using the physiologic definition of BPD. Infants underwent Bayley III assessment at 18-22 months corrected age. Multivariable logistic regression was used to determine the association between physiologic BPD and cognitive impairment (score<70).Results
BPD by the physiologic definition was diagnosed in 603 (52%) infants, 537 of whom were mechanically ventilated or on FiO(2)>30% and 66 who failed the room air challenge. Infants on room air (n=505) and those who passed the room air challenge (n=51) were classified as "no BPD" (n=556). At follow up, infants with BPD had significantly lower mean weight and head circumference. Moderate to severe cerebral palsy (7 vs. 2.1%) and spastic diplegia (7.8 vs. 4.1%) and quadriplegia (3.9 vs. 0.9%) phenotypes as well as cognitive (12.8 vs. 4.6%) and language scores<70 (24.2 vs. 12.3%) were significantly more frequent in those with BPD compared to those without BPD. BPD was independently associated (adjusted OR 2.4; 95% CI 1.40-4.13) with cognitive impairment.Conclusions
Rates of adverse neurodevelopmental outcomes in early childhood were significantly higher in those with BPD. BPD by the physiologic definition was independently associated with cognitive impairment using Bayley Scales III. These findings have implications for targeted post-discharge surveillance and early intervention.Free full text

Outcomes of Extremely Low Birth Weight Infants with Bronchopulmonary Dysplasia: Impact of the Physiologic Definition
Abstract
Aims
We compared neurodevelopmental outcomes of extremely low birth weight (ELBW) infants with and without bronchopulmonary dysplasia (BPD), using the physiologic definition.
Study Design
ELBW (birth weights <1000 grams) infants admitted to the Neonatal Research Network centers and hospitalized at 36 weeks postmenstrual age (n=1,189) were classified using the physiologic definition of BPD. Infants underwent Bayley III assessment at 18-22 months corrected age. Multivariable logistic regression was used to determine the association between physiologic BPD and cognitive impairment (score < 70).
Results
BPD by the physiologic definition was diagnosed in 603 (52%) infants, 537 of whom were mechanically ventilated or on FiO2 > 30% and 66 who failed the room air challenge. Infants on room air (n=505) and those who passed the room air challenge (n=51) were classified as “no BPD” (n=556). At follow up, infants with BPD had significantly lower mean weight and head circumference. Moderate to severe cerebral palsy (7 vs. 2.1%) and spastic diplegia (7.8 vs. 4.1%) and quadriplegia (3.9 vs. 0.9%) phenotypes as well as cognitive (12.8 vs. 4.6%) and language scores < 70 (24.2 vs. 12.3%) were significantly more frequent in those with BPD compared to those without BPD. BPD was independently associated (adjusted OR 2.4; 95% CI 1.40-4.13) with cognitive impairment.
Conclusions
Rates of adverse neurodevelopmental outcomes in early childhood were significantly higher in those with BPD. BPD by the physiologic definition was independently associated with cognitive impairment using Bayley Scales III. These findings have implications for targeted post-discharge surveillance and early intervention.
Introduction
Bronchopulmonary dysplasia (BPD) is a serious morbidity among preterm infants, with a reported incidence of 42% among infants 501-750 grams birth weight and 25% among those with 751-1000 grams birth weight (1). Traditional BPD, defined as supplemental oxygen (O2) administration at 36 weeks postmenstrual age was diagnosed in 42% of a recent cohort of preterm infants 22 to 28 weeks gestation from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) who survived to 36 weeks postmenstrual age (2). BPD is associated with an increased risk of neurodevelopmental impairment with mental, psychomotor and language delays in early childhood (3, 4). At school age, children with BPD have greater risk of growth retardation and academic difficulties (5, 6).
The diagnosis of BPD has evolved in recent years from that based solely on duration of O2 administration to one incorporating a standardized assessment of O2 need. The traditional definition of BPD is based on O2 administration at a postnatal age of 28 days or postmenstrual age of 36 weeks. The National Institutes of Health (NIH) consensus definition of BPD includes a classification of severity (mild, moderate and severe) based on O2 use at 28 days and at 36 weeks postmenstrual age or discharge home for preterm infants < 32 weeks gestation (7). The predictive validity of the NIH consensus definition for 18 month outcomes has been demonstrated by Ehrenkranz and colleagues in the NICHD NRN in a large cohort of extremely low birth weight (ELBW) infants born between 1995 and 1999 (8). The incidence of any neurodevelopmental impairment, cerebral palsy, mental and psychomotor developmental indices < 70 on the Bayley Scales of Infant Development II and blindness and hearing impairment significantly increased as the severity of BPD increased. In another study, compared with children with mild or moderate BPD, those with severe BPD perform poorly on IQ tests at 3 years and had lower performance IQs and poorer perceptual organization at 8 years of age (9). In both these studies, moderate BPD was based on FiO2 < 30% at 36 weeks postmenstrual age, an imperfect definition due to disparate individual and center practices related to target O2 saturations and thresholds for O2 therapy.
The physiologic definition of BPD, developed by Walsh et al, is based on a standardized assessment of O2 saturation during a timed stepwise reduction of administered O2 to room air, with clearly defined criteria for ‘passing’ or ‘failing’ the challenge (10). It has been demonstrated to be feasible, safe and reliable and to reduce the reported rate of BPD in individual centers of the NICHD NRN by a mean of 10% (11). The relationship between BPD by the physiologic definition and early childhood growth and neurodevelopmental outcomes has not been previously investigated. In this prospective observational cohort study from the NICHD NRN, we compared the growth and neurodevelopmental outcomes at 18-22 months corrected age of a recent cohort of ELBW (birth weights 401-1000 grams) infants with and without physiologic BPD. We further examined the association between BPD by the physiologic definition and cognitive impairment on the Bayley Scales III. Our hypothesis was that preterm ELBW infants with BPD by the physiologic definition would have a higher rate of adverse growth and neurodevelopmental outcomes at 18-22 months corrected age, compared to those without BPD by the physiologic definition and that BPD would be an independent predictor of cognitive impairment.
Material and Methods
This was a secondary analysis of data collected prospectively as part of the NICHD NRN very low birth weight registry (Generic Database) and Follow-up Study. Institutional Review Boards at each participating center approved both the database and follow-up studies.
Study Population
Inclusion criteria consisted of the following: preterm infants with birth weights of 401-1000 grams, born between January 1, 2006 and June 30, 2007, eligible for follow-up (< 27 weeks gestation and inborn, or in an approved study with follow-up), and still hospitalized at 36 weeks postmenstrual age. Infants were classified as having “physiologic BPD” if they fulfilled either of two conditions: a) any form of assisted ventilation or continuous positive airway pressure (CPAP) or supplemental O2 with an effective FiO2 > 30% at 36 weeks postmenstrual age or b) O2 via nasal cannula or hood with effective FiO2 < 30% and failed the stepwise O2 reduction challenge in the 36th postmenstrual week, using previously published criteria (O2 saturation 80% to 89% for 5 consecutive minutes or <80% for 15 seconds) (11). The “No physiologic BPD” group comprised infants on room air at 36 weeks postmenstrual age or those who passed the room air challenge, irrespective of clinical O2 administration.
Data collected included demographic characteristics such as gestational age at birth (by best obstetric estimate), ethnicity, gender and small for gestational age status and clinical data such as 5-minute Apgar score, surfactant therapy, presence of respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC) requiring surgery (modified Bell’s stage III), retinopathy of prematurity (ROP), bloodstream infections, severe intracranial hemorrhage (ICH) and cystic periventricular leukomalacia (PVL). Maternal data on antenatal steroids were also obtained. Severe intracranial hemorrhage was defined as blood/echodensity in the cerebral parenchyma or if ventricular enlargement occurred in association with blood/echodensity in the ventricular system (Grade III or IV intracranial hemorrhage). Follow-up information included growth parameters, results of a structured neurologic examination by trained examiners and language and cognitive scores on Bayley Scales of Infant Development III at 18-22 months corrected age. Blindness was defined as no useful function in either eye or possibly blind with some functional vision and deafness as requiring hearing aids in both ears. Moderate to severe cerebral palsy were defined as Gross Motor Functional Classification system level 2 or higher. Maternal education was defined as either less than high school diploma or high school diploma or higher level. Medicaid insurance, defined as public insurance or uninsured was used as surrogate for low socioeconomic status.
Statistical Analysis
Descriptive data were expressed as mean (SD) and number (percent) as appropriate. Data were compared between groups of infants with and without physiologic BPD. Wilcoxon rank-sum tests or t-tests were used for between-group comparisons of continuous data. Categorical data were analyzed using Fisher’s exact test or Chi-square test, as appropriate. Multivariable logistic regression analysis was used to determine the association between BPD using the physiologic definition and cognitive impairment (cognitive score < 70), after adjusting for confounding variables that have been previously demonstrated to impact developmental outcomes. The basic model included gestational age, male gender, maternal education, and BPD by the physiologic definition. Center as a random effect was not significant. With center in the model, a fitting problem occurred, caused by the scarcity or absence of cases among a number of the centers. In light of its insignificance as a covariate, the decision was made to exclude center from the model. The other factors examined were small for gestational age status, surgical NEC, severe IVH or cystic PVL, bloodstream infection, and antenatal steroids. Because bloodstream infection and antenatal steroids were insignificant and contributed nothing to the model fit, they were dropped from the model. Associations with p value < 0.05 were considered statistically significant.
Results
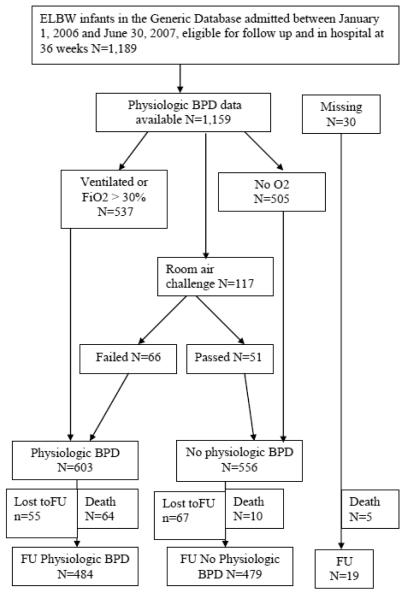
A total of 2,716 extremely low birth weight (ELBW) infants were admitted to the centers of the NICHD NRN between January 1, 2006 and June 30, 2007, 2065 of whom were eligible for the follow-up study. Of these, 1,189 infants were in the hospital at 36 weeks (725 had died by 36 weeks, 89 had transferred, and 62 had been discharged). Figure 1 is a flowchart describing the study cohort.
Short-term outcomes
The vast majority of infants had sufficient data to diagnose physiologic BPD (1,159; 97.5%). BPD by the physiologic definition was diagnosed in 603 (52%) infants. A total of 117 (10%) of our cohort underwent the room air challenge, 51 (44%) of whom passed. Table 1 compares the demographic characteristics of ELBW preterm infants with and without BPD by the physiologic definition. Infants with physiologic BPD were significantly more premature and had lower birth weights, were more likely to be male, to have a 5 minute Apgar score < 5 and to have less exposure to antenatal steroids compared to infants without BPD. Table 2 describes NICU outcomes of infants with and without BPD, using the physiologic definition. Infants with BPD had significantly lower survival, more prolonged duration of ventilation, greater postnatal steroid and surfactant therapies, and higher incidence of severe ICH, PVL, bloodstream infections, ROP stage III or higher and NEC requiring surgery.
Table 1
Demographic characteristics of ELBW preterm infants with and without BPD, as determined by the physiologic definition
| Characteristic | BPD, N=603 | No BPD, N=556 | p-value1 |
|---|---|---|---|
| Birth weight, mean (SD) | 726 (139) | 801 (128) | <.0001 |
| Gestational age, mean (SD) | 25.2 (1.5) | 26.2 (1.8) | <.0001 |
| Male Gender, % | 55.6 | 41.6 | <.0001 |
| Ethnicity, % | |||
| Caucasian | 56.2 | 49.7 | |
| Black | 38.8 | 46.6 | 0.08 |
| Am. Indian/Alaskan native | 0.3 | 0.4 | |
| Asian/Pacific Islander | 4.0 | 2.9 | |
| More than one race | 0.7 | 0.4 | |
| Apgar score at 5 min < 5, % | 17.4 | 7.6 | <.0001 |
| Small for gestation, % | 11.8 | 14.4 | 0.22 |
| Maternal Age, mean (SD) | 26.9 (6.4) | 27.1 (6.6) | 0.47 |
| Prenatal care, % | 93.7 | 92.8 | 0.62 |
| Outborn, % | 6.3 | 5.0 | 0.42 |
| Cesarean delivery, % | 66.5 | 71.4 | 0.08 |
|
| |||
| Any Antenatal steroids, % | 81.4 | 86.2 | 0.03 |
|
| |||
| Singleton, % | 76.6 | 74.1 | 0.35 |
Prenatal care was defined as at least 1 prenatal care visit prior to delivery
Table 2
NICU outcomes of infants with and without BPD, as determined by the physiologic definition
| Outcome | BPD, N=603 | No BPD, N=556 | p- value1 |
|---|---|---|---|
| Surfactant, % | 95.5 | 76.1 | <.0001 |
| ICH grades 3-4, % | 18.5 | 10.3 | 0.0001 |
| PVL, % | 7.6 | 4.5 | 0.04 |
| Postnatal steroids, % | 25.4 | 4.7 | <.0001 |
| ROP stage III or higher, % | 32.7 | 14.1 | <.0001 |
| Days on ventilator, mean | 52.6 (29.4) | 16.4 (18.6) | |
| (SD) | <.0001 | ||
| Bloodstream infection, % | 54.0 | 33.5 | <.0001 |
| Necrotizing enterocolitis, | 6.3 | 2.9 | 0.005 |
| surgical, % | |||
|
| |||
| Survival, n (%) | 539 (89.4) | 546 (98.2) | <.0001 |
Follow up outcomes
Seventy four (6.4%) infants died between 36 weeks postmenstrual age and 18-22 months corrected age. The vast majority of deaths (86.5%) occurred in those with BPD. Data at 18-22 months corrected age were available for 963 (88.7%) survivors, 92% and 88% in the groups with and without BPD. Growth and neurodevelopmental outcomes were compared between groups of surviving infants with and without physiologic BPD (Table 3). The mean weight and head circumference at follow up was significantly lower in those with BPD. A significantly higher proportion of infants with BPD had weight, length and head circumference < 10th centiles at the follow-up visit. Moderate to severe cerebral palsy (7.0 vs. 2.1%) and spastic diplegia (7.8 vs.4.1%) and spastic quadriplegia (3.9 vs. 0.9%) phenotypes were significantly more frequent in those with BPD as were cognitive score< 70 (12.8 vs. 4.6%) and language score < 70 (24.2 vs. 12.3%), compared to those without BPD. A comparison of neurodevelopmental outcomes between the small numbers of infants who passed (n=49) and failed (n=60) the room air challenge revealed no significant differences (table 4).
Table 3
Follow-up outcomes of infants at 18-22 months corrected age.
| Follow-up Outcome | BPD, N=484 | No BPD, N=479 | p-value |
|---|---|---|---|
| Maternal education, | 0.14 | ||
| highest level <12 y, % | 23.1 | 23.8 | |
| HS diploma or greater, % | 72.5 | 74.1 | |
| Medicaid or uninsured, % | 62.8 | 67.2 | 0.17 |
| Weight, kg, mean (SD) | 10.5 (1.5) | 10.7 (1.5) | 0.01 |
| Length, cm, mean (SD) | 80.9 (4.2) | 81.3 (5.4) | 0.15 |
| Head CF, cm, mean (SD) | 46.7 (2.0) | 47.0 (2.0) | 0.02 |
| Weight < 10th percentile, % | 49.8 | 39.4 | 0.002 |
| Length < 10th percentile, % | 37.7 | 25.4 | <.0001 |
| Head CF < 10th centile., % | 30.4 | 21.3 | 0.002 |
| Blind1, % | 1.9 | 0.6 | 0.15 |
| Deaf2, % | 2.3 | 0.6 | 0.06 |
| Cerebral palsy, moderate.-severe., % | 7.0 | 2.1 | 0.0004 |
| Spastic diplegia, % | 7.8 | 4.1 | 0.03 |
| Spastic hemiplegia, % | 2.2 | 1.3 | 0.45 |
| Spastic quadriplegia, % | 3.9 | 0.9 | 0.005 |
| Cognitive score3 | |||
 <70, % <70, % | 12.8 | 4.6 | <.0001 |
 70-85, % 70-85, % | 36.9 | 29.4 | |
 >85, % >85, % | 50.3 | 66.0 | |
| Language score 4 | |||
 <70, %
<70, %
| 24.2 | 12.3 | <.0001 |
 70-85, %
70-85, %
| 33.8 | 27.8 | |
 >85, %
>85, %
| 42.1 | 59.9 |
Table 4
Follow-up outcomes of infants who underwent the room air challenge at 18-22 months corrected age.
| Outcome | Room air challenge, N=109 | ||
|---|---|---|---|
| Failed N=60 | Passed N=49 | p-value | |
| Maternal education, highest level | |||
| <12 y, % | 16.7 | 20.4 | 0.11 |
| HS diploma or greater, % | 75.0 | 79.6 | |
| Medicaid or uninsured, % | 63.3 | 52.1 | 0.33 |
| Weight, kg, mean (SD) | 10.6 (1.26) | 10.8 (1.64) | 0.81 |
| Length, cm, mean (SD)* | 80.6 (4.17) | 81.1 (4.28) | 0.60 |
| Head CF, cm, mean (SD) | 47.0 (1.53) | 47.2 (1.85) | 0.88 |
| Weight < 10th percentile, % | 41.7 | 40.8 | 1.00 |
| Length < 10th percentile, % | 38.3 | 22.5 | 0.12 |
| Head CF < 10th centile., % | 26.7 | 14.3 | 0.18 |
| Blind1, % | 0.0 | 0.0 | NA |
| Deaf2, % | 0.0 | 0.0 | NA |
| Cerebral palsy, mod.-sev., % | 3.3 | 0.0 | 0.50 |
| Spastic diplegia, % | 6.9 | 2.2 | 0.38 |
|
| |||
| Spastic hemiplegia, % | 1.7 | 0.0 | 1.00 |
| Spastic quadriplegia, % | 1.7 | 0.0 | 1.00 |
| Cognitive score3 | |||
 <70, % <70, % | 1.7 | 4.1 | |
 70-85, % 70-85, % | 43.3 | 26.5 | 0.14 |
 >85, % >85, % | 55.0 | 69.4 | |
|
| |||
| Language score4 | |||
 <70, % <70, % | 16.7 | 10.4 | |
 70-85, % 70-85, % | 33.3 | 29.2 | 0.49 |
 >85, % >85, % | 50.0 | 60.4 | |
We further explored the association of BPD with cognitive impairment in a multivariate logistic regression model. Table 5 presents the adjusted odds ratios (with 95% CI) of the risk of cognitive impairment from logistic regression models. Physiologic BPD (adjusted OR 2.41; 95% CI 1.40-4.13) was independently associated with cognitive impairment. Other factors that were independently associated with adverse outcomes at 18 -22 months age included severe ICH or PVL, surgical NEC and small for gestation status.
Table 5
Adjusted odds ratios of the risk of Cognitive Score<701 from multivariate logistic regression, modeled with predictors and physiologic BPD.
| Predictor variable | Odds Ratio | 95% CI |
|---|---|---|
| Gestational age2 | 0.91 | (0.76, 1.08) |
| Male gender | 1.39 | (0.86, 2.24) |
| Small for gestational age | 2.60 | (1.23, 5.50) |
| Maternal education | ||
 Unknown vs HS Unknown vs HS | 1.79 | (0.62, 5.15) |
 diploma+ diploma+ | 1.41 | (0.81, 2.43) |
 < HS vs HS diploma+ < HS vs HS diploma+ | ||
| Surgical NEC | 3.35 | (1.42, 7.91) |
| IVH or PVL | 3.97 | (2.40, 6.55) |
| Physiologic BPD | 2.41 | (1.40, 4.13) |
Discussion
This is the first investigation of the relationship between BPD determined by the physiologic definition and growth and neurodevelopmental outcomes using Bayley Scales III. Our cohort comprised a select subset of ELBW (< 1000 grams) infants, the majority of whom were < 27 weeks gestation and still hospitalized at 36 weeks postmenstrual age. We chose these restrictive inclusion criteria in order to obtain physiologic BPD data, using the standardized O2 reduction challenge for infants with effective FiO2 < 0.3. The relatively high rates of BPD in our cohort of extremely low gestation infants reflect our inclusion criteria. About 10% of our cohort underwent the room air challenge, similar to 14% in the original study on the impact of the physiologic definition of BPD; an identical 44% passed the challenge (11). The proportion of infants who died (6.4%) between 36 weeks and 18 months is consistent with previous reported rates between 2.2 and 11.2% (12, 13). In our data, death beyond 36 weeks postmenstrual age was overwhelmingly more frequent in those with BPD.
BPD is a recognized antecedent of cerebral palsy and adverse motor outcomes. Skidmore et al showed that cerebral palsy occurs more frequently (15% vs. 3-4%) in very low birth weight infants with BPD (defined as oxygen dependence at 28 days or beyond) (14). BPD by the traditional definition (supplemental oxygen at 36 weeks or discharge home) was one of the identified significant risk factors for cerebral palsy, neurodevelopmental impairment and psychomotor developmental index (PDI) < 70 at 18-22 months corrected age among 839 infants < 25 weeks gestation in the NRN (15). Singer and colleagues demonstrated that BPD was a significant, independent predictor of poorer motor outcome at 3 years of age after controlling for confounding variables and was associated with a 10-12 point decrement in PDI scores (16). In the more recent Extremely Low Gestational Age Newborn (ELGAN) study cohort, BPD accompanied by mechanical ventilation at 36 weeks postmenstrual age was associated with 6-fold increased risk of quadriparesis and 4-fold increased risk of diparesis (21). Our findings of a significantly higher rate of moderate or severe cerebral palsy, spastic diplegia and quadriplegia in those with BPD by the physiologic definition are consistent with these data.
Data on the association between BPD and cognitive impairment are conflicting. In one previous study, significantly higher rates of specific receptive language impairment were identified in VLBW infants with BPD compared to those without BPD (17). In the EPICure study, preterm infants ≤ 25 weeks gestation who were receiving supplemental oxygen therapy at 36 weeks postmenstrual age, when compared to those breathing room air had lower mean MDI score at 30 months corrected age after adjustment for the main predictor variables (18). In a secondary analysis of the trial of indomethacin prophylaxis in preterm infants, Schmidt and colleagues found that, among ELBW infants who survived to a postmenstrual age of 36 weeks, traditional BPD was independently associated (OR 2.4; 95% CI 1.8-3.2) with a poor 18-month outcome, defined as death or any of: cerebral palsy, cognitive delay, hearing loss requiring amplification, or bilateral blindness (13). A significant negative linear relationship between the “severity of BPD” and cognitive outcomes in infancy has been previously demonstrated (8, 19). In a recent analysis from the ELGAN study, Laughon and colleagues examined the association between chronic lung disease (defined as O2 administration at 36 weeks postmenstrual age) and developmental delay at 2 years of age (20). The lowest risk of MDI or PDI < 55 was in those without chronic lung disease but chronic lung disease, without or with mechanical ventilation, was not associated with the risk of a low MDI. There are several possible reasons for this discordance between our results and the ELGAN study. In the ELGAN analysis, infants who were not able to walk independently and had moderate or severe gross motor impairments were excluded. Since cerebral palsy may be associated with cognitive impairment, it is possible that the association between cognitive impairment and BPD was underestimated. In addition, cognitive impairment was defined as MDI < 55 using Bayley II scales. The physiologic definition was used only in our cohort to define BPD. The mechanisms proposed for adverse neurodevelopment of infants with BPD include postnatal corticosteroid use impacting brain growth and recurrent episodes of hypoxia which may affect neuronal organization, myelination and cellular apoptosis (18). In addition, it is possible that antecedents of BPD are risk factors for delayed development (20).
Although a substantial body of work has examined the association between BPD and later outcomes, this is the first investigation in which BPD was rigorously defined based on performance of the room air challenge. The physiologic definition has been previously shown to obviate variability in oxygen use and provide a more uniform outcome for clinical comparisons between centers (22). BPD by the physiological definition is now being used in preference to the traditional definition in major randomized controlled trials (23). The present analysis supports the predictive validity of the physiologic definition of BPD for adverse neurodevelopmental outcomes at 18-22 months corrected age. Our data were prospectively collected from a large recent cohort when non-invasive modes of ventilation and surfactant were common and postnatal steroids were used selectively. The other major strength of the data is that neurodevelopmental assessment included structured neurologic examinations by certified examiners and the Bayley Scales of Infant Development III for the first time. The Bayley Scales III separately measure cognitive and language scores and have been recently shown to have significantly improved positive predictive value for low IQ at 4 years, compared to Bayley Scales II (0.87 vs. 0.29), with comparable predictive value for normal IQ (0.93 vs. 0.95) among extremely preterm infants (Bode MM, Mettelman BB, Maslak SE, Gross SJ. Predictive validity of the Bayley Scales of Infant Development 3rd edition at age 24 months to predict preschool cognitive function in children born at ≤ 30 weeks gestation. EPAS 20112300.2).
The rates of cerebral palsy in the current data set were strikingly low (7% in those with BPD and 2.1% in those without BPD). Among infants with moderate and severe BPD born between 1995 and 1999 in any of the NRN sites, the reported rates of cerebral palsy were 17 and 26.8% respectively and in another large multicenter trial in the late 90s, rates in the groups with and without BPD were 17 and 9% (8, 13). The ELGAN study from a 2002-2004 cohort reported a cerebral palsy rate of 11.5% overall (21). Whether our data reflect improved outcomes over the past decade remains unclear. The proportion of infants with Bayley Scales II MDI < 70 in previous studies have ranged between 35.1 and 49.8% in infants with moderate and severe BPD and 19% in those without BPD (8, 13). The inherent differences between the Bayley Scales II and III, however, preclude direct comparisons with previous data from similar populations. The technical report of the Bayley-III states that mean scores on the Bayley–III are approximately 7 points higher than the Bayley–II MDI and PDI scores (24).
Our study has certain limitations. Our cohort comprised a select group of infants in hospital at 36 weeks postmenstrual age. Despite use of the room air challenge and the ‘physiologic’ diagnosis of BPD, practice variations in home oxygen use may have confounded subsequent outcomes. We did not collect data on the precise indication for supplemental oxygen use at 36 weeks or the timing of discontinuation of O2. The numbers of infants who underwent the room air challenge were relatively small, and therefore, statistical differences in 18 month outcomes between those who passed and failed were not detected. Our focus was on neurodevelopmental outcomes; post-discharge pulmonary morbidities, potential modulators of developmental outcomes, were not examined. Finally, our results are confined to 18-22 months corrected age, a relatively early developmental stage.
We conclude that rates of adverse neurodevelopmental outcomes in early childhood were significantly higher in those with BPD. BPD by the physiologic definition was independently associated with cognitive impairment using the Bayley Scales III. These data on the prognostic importance of the physiologic BPD suggest that performing the room air challenge in infants on low supplemental oxygen at 36 weeks postmenstrual age may have implications for parental counseling and may allow for targeted post-discharge surveillance and early intervention.
Abbreviations
| BPD | Bronchopulmonary dysplasia |
| NICHD | National Institute of Child Health and Development |
| NRN | Neonatal Research Network |
| RDS | Respiratory distress syndrome |
| ROP | Retinopathy of prematurity |
| NEC | Necrotizing enterocolitis |
| PVL | Periventricular leukomalacia |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure:
Conflict of Interest: None
Contributor’s Statement Page: All authors have made substantive intellectual contributions to the study conception and design, acquisition of data, or analysis and interpretation of data. They have all contributed to the drafting of the article or revising it critically for important intellectual content and have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.earlhumdev.2011.12.013
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3686277?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.earlhumdev.2011.12.013
Article citations
The association of the room air challenge with long-term outcomes in extremely preterm infants.
J Perinatol, 44(9):1300-1306, 03 Aug 2024
Cited by: 0 articles | PMID: 39095524
The Diaphragmatic Initiated Ventilatory Assist (DIVA) trial: study protocol for a randomized controlled trial comparing rates of extubation failure in extremely premature infants undergoing extubation to non-invasive neurally adjusted ventilatory assist versus non-synchronized nasal intermittent positive pressure ventilation.
Trials, 25(1):201, 20 Mar 2024
Cited by: 1 article | PMID: 38509583 | PMCID: PMC10953115
Brain age predicted using graph convolutional neural network explains neurodevelopmental trajectory in preterm neonates.
Eur Radiol, 34(6):3601-3611, 14 Nov 2023
Cited by: 2 articles | PMID: 37957363
Postnatal corticosteroids and developmental outcomes in extremely preterm or extremely low birth weight infants: The Victorian Infant Collaborative Study 2016-17 cohort.
Acta Paediatr, 112(6):1226-1232, 15 Feb 2023
Cited by: 6 articles | PMID: 36719082 | PMCID: PMC10953334
Safety and efficacy of a novel double-lumen tracheal tube in neonates with RDS: A prospective cohort study.
Front Pediatr, 10:1032044, 05 Dec 2022
Cited by: 0 articles | PMID: 36545664 | PMCID: PMC9760922
Go to all (113) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Impact of a physiologic definition on bronchopulmonary dysplasia rates.
Pediatrics, 114(5):1305-1311, 01 Nov 2004
Cited by: 330 articles | PMID: 15520112
Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia.
Pediatrics, 116(6):1353-1360, 01 Dec 2005
Cited by: 618 articles | PMID: 16322158
Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia.
J Perinatol, 23(6):451-456, 01 Sep 2003
Cited by: 219 articles | PMID: 13679930
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.
Neonatology, 107(4):358-359, 05 Jun 2015
Cited by: 15 articles | PMID: 26044104
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000454
NCRR NIH HHS (15)
Grant ID: M01 RR30
Grant ID: M01 RR44
Grant ID: M01 RR8084
Grant ID: M01 RR7122
Grant ID: M01 RR39
Grant ID: M01 RR997
Grant ID: M01 RR59
Grant ID: M01 RR70
Grant ID: M01 RR80
Grant ID: M01 RR32
Grant ID: M01 RR750
Grant ID: M01 RR125
Grant ID: M01 RR54
Grant ID: UL1 RR24139
Grant ID: M01 RR633
NICHD NIH HHS (23)
Grant ID: U10 HD021385-16
Grant ID: U10 HD027904
Grant ID: U10 HD027904-12
Grant ID: U10 HD21364
Grant ID: U10 HD27871
Grant ID: U10 HD21373
Grant ID: U10 HD53089
Grant ID: U10 HD021385
Grant ID: U10 HD21385
Grant ID: U10 HD27856
Grant ID: U10 HD27904
Grant ID: U10 HD34216
Grant ID: U10 HD40492
Grant ID: U10 HD27851
Grant ID: U10 HD27880
Grant ID: U10 HD40689
Grant ID: U10HD53109
Grant ID: U10 HD36790
Grant ID: U10 HD53119
Grant ID: U10 HD27853
Grant ID: U10 HD40461
Grant ID: U10 HD40498
Grant ID: U10 HD40521