Abstract
Free full text

Identification and quantitation of platelet-associated fibronectin antigen.
Abstract
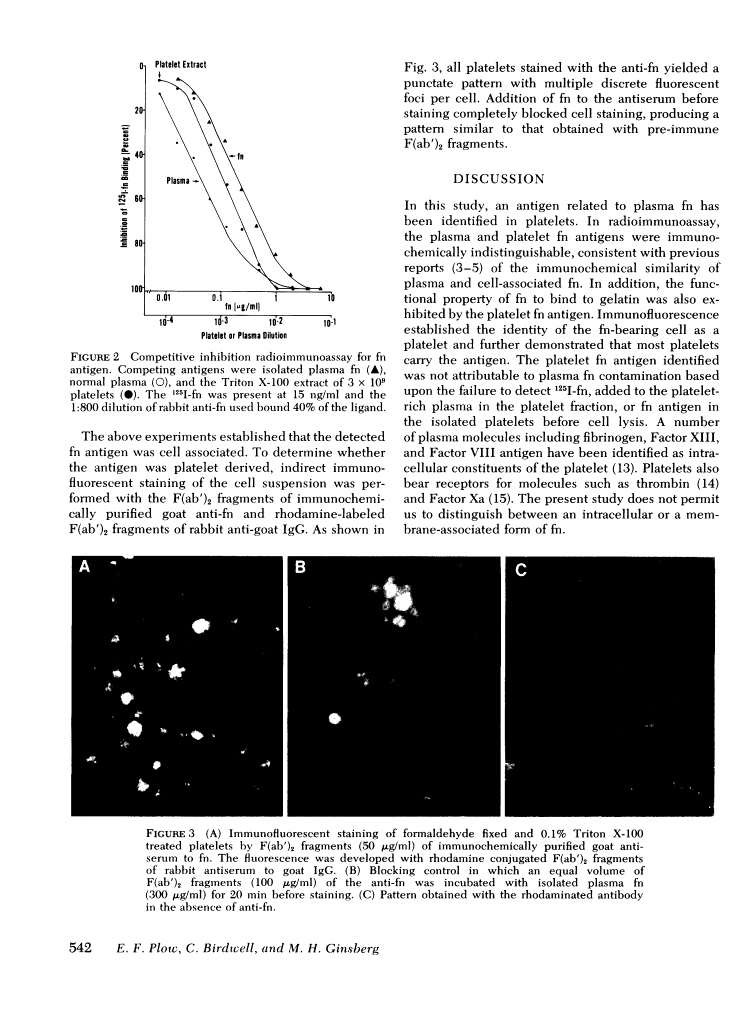
Platelet-associated fibronectin antigen has been identified by radioimmunoassay and immunofluorescent techniques. In radioimmunoassay, platelet fibronectin was immunochemically indistinguishable from plasma fibronectin. Platelet and plasma fibronectin were bound and eluted from gelatin-sepharose under similar conditions. The level of platelet fibronectin in detergent extracts of washed platelets from 12 healthy adults was 2.85 +/- 1.24 microgram/10(9) platelets. Immunofluorescence with F(ab')2 fragments of immunochemically purified antifibronectin showed that all platelets stained with a discrete punctate pattern. The identification of platelet fibronectin antigen raises the possibility that this protein may participate in platelet-platelet or platelet-surface interactions.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (859K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hynes RO, Bye JM. Density and cell cycle dependence of cell surface proteins in hamster fibroblasts. Cell. 1974 Oct;3(2):113–120. [Abstract] [Google Scholar]
- Yamada KM, Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. [Abstract] [Google Scholar]
- Vaheri A, Mosher DF. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. [Abstract] [Google Scholar]
- Plow EF, Hougie C, Edgington TS. Neoantigenic expressions engendered by plasmin cleavage of fibrinogen. J Immunol. 1971 Nov;107(5):1496–1500. [Abstract] [Google Scholar]
- Ginsberg MH, Kozin F, O'Malley M, McCarty DJ. Release of platelet constituents by monosodium urate crystals. J Clin Invest. 1977 Nov;60(5):999–1007. [Europe PMC free article] [Abstract] [Google Scholar]
- Tangen O, Berman HJ, Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [Abstract] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [Abstract] [Google Scholar]
- Mosesson MW, Chen AB, Huseby RM. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. [Abstract] [Google Scholar]
- Mosher DF. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [Abstract] [Google Scholar]
- Niewiarowski S. Proteins secreted by the platelet. Thromb Haemost. 1977 Dec 15;38(4):924–938. [Abstract] [Google Scholar]
- Tollefsen DM, Feagler JR, Majerus PW. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [Abstract] [Google Scholar]
- Miletich JP, Jackson CM, Majerus PW. Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4033–4036. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci109334
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/109334/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Delivery technologies for therapeutic targeting of fibronectin in autoimmunity and fibrosis applications.
Adv Drug Deliv Rev, 209:115303, 06 Apr 2024
Cited by: 1 article | PMID: 38588958
Review
Plasma and cellular fibronectin: distinct and independent functions during tissue repair.
Fibrogenesis Tissue Repair, 4:21, 16 Sep 2011
Cited by: 285 articles | PMID: 21923916 | PMCID: PMC3182887
Review Free full text in Europe PMC
Role of fibronectin assembly in platelet thrombus formation.
J Thromb Haemost, 4(7):1461-1469, 01 Jul 2006
Cited by: 59 articles | PMID: 16839338
Review
Differential expression of thrombospondin and cellular fibronectin during remodeling in proliferative glomerulonephritis.
J Histochem Cytochem, 47(4):533-544, 01 Apr 1999
Cited by: 13 articles | PMID: 10082755
Low-density lipoprotein enhances platelet secretion via integrin-alphaIIbbeta3-mediated signaling.
Arterioscler Thromb Vasc Biol, 19(2):239-247, 01 Feb 1999
Cited by: 23 articles | PMID: 9974403
Go to all (53) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The detection, immunofluorescent localization, and thrombin induced release of human platelet-associated fibronectin antigen.
J Supramol Struct, 11(2):167-174, 01 Jan 1979
Cited by: 29 articles | PMID: 398428
Fibronectin gelatin-binding activity: effects of platelet thrombospondin, serum, and plasma.
J Lab Clin Med, 110(5):548-557, 01 Nov 1987
Cited by: 3 articles | PMID: 3312442
Biochemical characterization of PECAM-1 (CD31 antigen) on human platelets.
Thromb Haemost, 66(6):700-707, 01 Dec 1991
Cited by: 30 articles | PMID: 1796415
Fibronectin expression on the platelet surface occurs in concert with secretion.
J Supramol Struct Cell Biochem, 17(1):91-98, 01 Jan 1981
Cited by: 22 articles | PMID: 7198692