Abstract
Background
Dengue is the most prevalent mosquito-borne viral disease in humans and a major urban public health problem worldwide.Methods
A prospective cohort study of approximately 3800 children initially aged 2-9 years was established in Managua, Nicaragua, in 2004 to study the natural history of dengue transmission in an urban pediatric population. Blood samples from healthy subjects were collected annually prior to the dengue season, and identification of dengue cases occurred via enhanced passive surveillance at the study health center.Results
Over the first four years of the study, seroprevalence of anti-dengue virus (DENV) antibodies increased from 22%-40% in the 2-year-old cohort and 90%-95% in the 9-year-old cohort. The incidence of symptomatic dengue cases and the ratio of inapparent to symptomatic DENV infection varied substantially from year to year. The switch in dominant transmission from DENV-1 to DENV-2 was accompanied by an increase in disease severity but, paradoxically, a decrease in transmission. Phylogeographic analysis of full-length DENV-2 sequences revealed strong geographic clustering of dengue cases.Conclusions
This large-scale cohort study of dengue in the Americas demonstrates year-to-year variation of dengue within a pediatric population, revealing expected patterns in transmission while highlighting the impact of interventions, climate, and viral evolution.Free full text

Trends in patterns of dengue transmission over four years of a pediatric cohort study in Nicaragua
Abstract
Background
Dengue is the most prevalent mosquito-borne viral disease in humans and a major urban public health problem worldwide.
Methods
A prospective cohort study of ~3,800 children initially aged 2-9 years old was established in Managua, Nicaragua, in 2004 to study the natural history of dengue transmission in an urban pediatric population. Blood samples from healthy subjects were collected annually prior to the dengue season, and identification of dengue cases occurred via enhanced passive surveillance at the study health center.
Results
Over the first four years of the study, seroprevalence of anti-dengue virus (DENV) antibodies increased from 22-40% in the 2-year-old cohort and 90-95% in the 9-year-old cohort. The incidence of symptomatic dengue cases and the ratio of inapparent to symptomatic DENV infection varied substantially year-to-year. The switch in dominant transmission from DENV-1 to DENV-2 was accompanied by an increase in disease severity but, paradoxically, a decrease in transmission. Phylogeographic analysis of full-length DENV-2 sequences revealed strong geographic clustering of dengue cases.
Conclusions
This large-scale cohort study of dengue in the Americas demonstrates year-to-year variation of dengue within a pediatric population, revealing expected patterns in transmission while highlighting the impact of interventions, climate, and viral evolution.
INTRODUCTION
Dengue is a major public health problem worldwide, caused by four dengue virus serotypes (DENV-1-4) of the Flavivirus genus. DENV is transmitted principally by Aedes aegypti and Ae. albopictus mosquitoes and causes dengue fever (DF) and the more severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) throughout tropical and subtropical regions. Forty million dengue cases are estimated to occur annually worldwide, with 250,000-500,000 hospitalizations and 25,000 fatalities [1]. In the Americas, the incidence, distribution and severity of dengue have increased dramatically over the past three decades, and dengue is now endemic throughout the region [2]. In Nicaragua, the first documented dengue epidemic occurred in 1985 [3]; since 1990, dengue has been endemic, with circulation of all four serotypes and tens of thousands of cases each year [4, 5].
A number of multi-year cohort studies have documented incidence and transmission patterns of DENV in Southeast Asia [6-12]. However, in the Americas, few long-term prospective cohort studies have been performed [13, 14]; most have been short-term, spanning 1-2 years [15-17]. Given the variations in DENV transmission and disease severity between Southeast Asia and the Americas, a large-scale, cohort study in the Americas is needed to more fully understand the epidemiology of dengue and prepare for possible vaccine and drug trials.
Since 2004, a seroepidemiological cohort study of ~3,800 children aged 2-12 years has been ongoing in Managua, Nicaragua [14] to document the natural history of pediatric dengue transmission, measure symptomatic and inapparent DENV infections, identify circulating DENV serotypes, and maintain a biobank of clinical specimens and viruses. Additionally, this study serves to establish infrastructure for potential dengue vaccine and drug trials. Here, we present the epidemiological observations for 2004-8, years that encompassed substantial variation in overall DENV infection, the ratio of symptomatic to inapparent infection, disease severity, circulating serotypes, and geographical clustering.
MATERIALS AND METHODS
Study Design
This is a community-based prospective seroepidemiological cohort study, with passive surveillance for illnesses among participants at the study health center enhanced by periodic home visits [14]. Participants were followed closely for all illnesses, and children presenting with fever were screened for signs and symptoms of dengue. Those presenting WHO criteria for dengue syndromes and those with undifferentiated fever were evaluated for acute DENV infection using virological, serological, and molecular biological assays. A blood sample was collected annually from all subjects in July-August, prior to the dengue season. The study was initiated in August 2004 and continues to date; this report includes data through July 2008.
Study site and population
The study is being conducted in the catchment area of the Health Center “Sócrates Flores Vivas” (HCSFV), the principal primary care facility serving neighborhoods along Lake Xolotlán in Managua. Children 2-9 years old were recruited in August-September of 2004, and new 2-year olds were recruited each year in July [14]. Parents or legal guardians of participants signed informed consent forms and children >5 years old provided verbal assent. The cohort population varied between 3,693 and 3,947 (table 1), with the increase in size attributable to the aging of participants up to 12 years old. This study was approved by the Institutional Review Boards of the Nicaraguan Ministry of Health, the study hospital “Hospital Infantil Manuel de Jesús Rivera” (HIMJR), the University of California, Berkeley, and the International Vaccine Institute.
Table 1
Cohort Characteristics, Infestation and Precipitation in District II, Managua, Nicaragua, 2004- 2008
| 2004-5 | 2005-6 | 2006-7 | 2007-8 | |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
| Total cohort size | 3,713 | 3,689 | 3,563 | 3,676 |
| Gender | ||||
 Female Female | 1,821 (49.0) | 1,816 (49.2) | 1,756 (49.3) | 1,823 (49.6) |
 Male Male | 1,892 (51.0) | 1,873 (50.8) | 1,807 (50.7) | 1,853 (50.4) |
| Age (years) | ||||
 2-5 2-5 | 1998 (53.8) | 1725 (46.8) | 1392 (39.1) | 1385 (37.7) |
 6-9 6-9 | 1715 (46.2) | 1674 (45.4) | 1569 (44.0) | 1578 (42.9) |
 10-12 10-12 | 0 | 290 (7.9) | 602 (16.9) | 713 (19.4) |
| Category of initial visit | ||||
 A (Suspected dengue) A (Suspected dengue) | 210 (2.5) | 335 (3.7) | 195 (1.9) | 233 (2.3) |
 B (Undifferentiated febrile illness) B (Undifferentiated febrile illness) | 159 (1.9) | 267 (2.9) | 246 (2.3) | 201 (2.0) |
 C (Febrile with defined focus) C (Febrile with defined focus) | 3,916 (48.4) | 3,500 (38.3) | 4,565 (43.4) | 4,074 (40.2) |
 D (Afebrile) D (Afebrile) | 4,177 (49.4) | 5,047 (55.2) | 5,507 (52.4) | 5,619 (55.5) |
 Total initial visits Total initial visits | 8,462 | 9,149 | 10,513 | 10,127 |
| Ae. aegypti infestation | ||||
 House Index (%) House Index (%) | 13.5 | 8.8 | 4.1 | 7.9 |
 Block Index (%) Block Index (%) | 9.5 | 9.0 | 5.3 | 10 |
 Container Index (%) Container Index (%) | 2.3 | 3.1 | 1.3 | 2.6 |
 Breteau Index Breteau Index | 24.2 | 15.8 | 6 | 13 |
| Precipitation (mm) | 819 | 1395 | 683 | 1412 |
Symptomatic dengue cases and annual sample collection
To facilitate identification of symptomatic dengue, participants were urged to attend the HCSFV at the onset of fever or illness, where study physicians classified them into one of four clinical categories (A-D) [14]. Febrile illnesses were classified as those which met the WHO’s dengue case definition (category A) [18], those with undifferentiated fever (category B) or those with an apparent focus other than dengue (category C). Non-febrile complaints were classified as category D. Category A and B cases (“possible dengue cases”) were followed by study physicians and nurses daily while fever and symptoms persisted, either at the HCSFV or by home visits. A complete blood count (CBC) was taken daily, and acute- and convalescent-phase blood samples sent for virological testing at the National Virology Laboratory of the Nicaraguan Ministry of Health (NVL/CNDR). Possible dengue cases presenting signs of alarm were transferred to the HIMJR, where hospital-based study physicians and nurses provided case management and continued data collection. Each July, blood samples were collected from all children to determine DENV infection incidence and seroprevalence through serological testing of paired samples from the current and previous year.
Sample management and laboratory assays
Blood samples from category A and B patients were sent to the NVL/CNDR for processing and storage [14]. After separation from red blood cells, acute-phase serum samples were tested for DENV RNA using a nested RT-PCR assay that targets the capsid gene and allows both DENV detection and serotyping [19]. Positive samples were confirmed by a second RT-PCR amplification and then attempted viral isolation by inoculation onto Aedes albopictus C6/36 cells [20]. Serotyping after viral isolation was performed by indirect immunofluorescence or, from 2007, by RT-PCR. Acute- and convalescent-phase samples were paired and tested simultaneously for anti-DENV IgM antibodies using an in-house IgM capture ELISA (MAC-ELISA) [21] and for total anti-DENV antibodies by an Inhibition ELISA containing antigens from DENV1-4 [5, 15, 22]. Annual serum samples were processed by Inhibition ELISA.
A possible dengue case was considered positive when DENV RNA was demonstrated by RT-PCR, DENV was isolated, or seroconversion of DENV-specific IgM was observed by MAC-ELISA or a >4-fold increase in anti-DENV antibody titer was measured using Inhibition ELISA in paired samples. Children with paired annual serum samples demonstrating seroconversion or >4-fold increase in DENV-specific antibody titer, but who had not presented to the HCSFV with acute DENV illness were considered to have experienced inapparent DENV infections [14]. Symptomatic dengue cases (henceforth “cases”) were classified as primary infections when acute antibody titer was <10 or convalescent antibody titer was <2,560 and as secondary infections when the titer was >10 (acute) or >2560 (convalescent), as determined by Inhibition ELISA. In paired annual samples, seroconversion from a titer of <1:10 to >1:10 by Inhibition ELISA was considered primary DENV infection, whereas a >4-fold increase in antibody titer was considered secondary infection.
Phylogeography of DENV-2
Phygeographic analysis was performed on 58 DENV-2 sequences. To determine the strength and pattern of spatial structure, we first inferred Bayesian Markov chain Monte Carlo (MCMC) phylogenetic trees utilizing the GTR+I+Γ4 model of nucleotide substitution, a relaxed molecular clock, a Bayesian skyline coalescent prior, and a total run of 50 million generations, all conducted using the BEAST package [23]. To estimate the strength of geographical clustering, we employed another Bayesian MCMC approach (BaTS; [24]), using 1000 replications of the distribution of Bayesian trees inferred above (thereby accounting for phylogenetic uncertainty), with 10% of trees removed as burn-in. This allowed us to compute the association index (AI) and parsimony score (PS) statistics of clustering strength [24]. We also used a parsimony approach [25] to explore patterns of viral movement between neighborhoods based on the Maximum Clade Credibility (MCC) tree, which summarizes all the phylogenies produced from BEAST analysis. All DENV-2 sequences were assigned a character state (A to J) reflecting the neighborhood of origin. The minimum number of changes in character state needed to produce the observed distribution of character states on the MCC tree was then estimated using the parsimony method in PAUP* [26] (excluding ambiguous changes). To determine the expected number of changes under the null hypothesis of random mixing among neighborhoods, the character states of all isolates were randomized 1000 times. The analysis was then repeated as above.
Annual precipitation and entomological indices
Annul precipitation was measured at Managua’s International Airport, approximately 13km from the HCSFV, and retrieved from the Meteorologic Service of the Nicaraguan Institute of Territorial Studies. Infestation rates of Ae. aegypti larvae in District II were collected each year by the Municipal Health Service (SILAIS) of Managua.
Data management and statistical analysis
All demographic, clinical and laboratory data was collected using standardized forms and entered into Access databases. Double data entry, frequent quality control queries, and numerous customized informatics tools ensured minimal errors [14, 27, 28]. Statistical analysis was performed in Epi Info 2003 (Centers for Disease Control and Prevention, Atlanta, GA) and Access 2003 (Microsoft, Seattle, WA).
RESULTS
Characteristics of study population
Throughout the four years, the cohort remained stable with respect to gender and age (table 1). All 17 neighborhoods served by the HCSFV were represented in the cohort. Compliance with bringing febrile children to the HCSFV was very high, with 97% of possible dengue cases presenting within the first 72 hours of symptom onset and 94% providing a convalescent sample. During the study period, 92% of participants attended the HCSFV at least once for a medical consult. There was a 4-7% loss to follow-up per year (the majority of these had moved and could not be located), and only 2% of participants reported seeking healthcare providers other than HCSFV. The number of initial consults averaged ~9-10,000 per year, of which 4.2-6.6% were categories A and B (table 1). Confirmed DENV infection among possible cases ranged from 4-22% among category A patients and from 1.3-7% among category B patients. On average, 26% of laboratory-confirmed dengue cases were category B (table 2).
Table 2
Confirmed Symptomatic and Inapparent Dengue Infections in PDVI Cohort, Managua, Nicaragua, 2004-2008
| 2004-5 | 2005-6 | 2006-7 | 2007-8 | Total | |
|---|---|---|---|---|---|
|
| |||||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Symptomatic infections 1 | |||||
 Total symptomatic cases (n) Total symptomatic cases (n) | 17 | 65 | 13 | 64 | 159 |
 Category A Category A | 15 (88.2) | 44 (67.7) | 8 (61.5) | 51 (79.7) | 118 (74) |
 Category B Category B | 2 (11.8) | 21 (32.3) | 5 (38.5) | 13 (20.3) | 41 (26) |
 DHF & DSS2 DHF & DSS2 | 0 | 0 | 1 (7.7) | 8 (12.5) | 9 (6) |
 Hospitalized cases3 Hospitalized cases3 | 2 (11.8) | 2 (3.1) | 1 (7.7) | 15 (23.4) | 20 (13) |
|
Incidence proportion of DENV
infection in the cohort (%) 4 | |||||
 Symptomatic infections Symptomatic infections | 0.4 | 1.9 | 0.4 | 1.8 | 1.1 |
 Total infections Total infections | 8.6 | 11.1 | 5.8 | 7.3 | 8.3 |
| Immune response | |||||
 Symptomatic DENV infections Symptomatic DENV infections | |||||
  Primary Primary | 10 (70.0) | 24 (38.0) | 7 (60.0) | 19 (32.0) | 60 (40.0) |
  Secondary Secondary | 5 (30.0) | 40 (62.0) | 4 (40.0) | 41 (68.0) | 90 (60.0) |
 Inapparent DENV infections Inapparent DENV infections | |||||
  Primary Primary | 89 (30.0) | 130 (41.0) | 79 (40.0) | 100 (54.9) | 398 (41.8) |
  Secondary Secondary | 187 (70.0) | 188 (59.0) | 97 (60.0) | 82 (45.1) | 554 (59.2) |
 Ratio of inapparent:symptomatic Ratio of inapparent:symptomatic | 18.4:1 | 5.0:1 | 16.0:1 | 3.0:1 | 6.4:1 |
  DENV infections DENV infections | |||||
 Ratio of 1° inapparent: Ratio of 1° inapparent:  symptomatic DENV infections symptomatic DENV infections | 8.9:1 | 5.4:1 | 11.3:1 | 5.3:1 | 6.6:1 |
 Ratio of 2° inapparent: Ratio of 2° inapparent:  symptomatic infections symptomatic infections | 37.4:1 | 4.7:1 | 24.3:1 | 2.0:1 | 6.2:1 |
 Ratio of % 2° inapparent: Ratio of % 2° inapparent:  symptomatic infections symptomatic infections | 0.49 | 1.06 | 0.66 | 1.52 | 1.03 |
Incidence Proportion of symptomatic and inapparent DENV infections
Cases were observed primarily during Nicaragua’s dengue season, July-December (figure 1A). Many more dengue cases were documented in 2005-6 and 2007-8, with 1.9 and 1.8% of subjects experiencing symptomatic infection, versus 0.4% in both 2004-5 and 2006-7 (figure 1A,B; table 2). In the years with higher numbers of cases, older children were more affected (≥9 years old) (figure 1B). Despite greater numbers of symptomatic dengue cases in 2005-6 and 2007-8, only in 2005-6 was there a noticeable increase in the incidence proportion of total DENV infections (11.1%), as compared to 8.6, 5.8 and 7.3% for 2004-5, 2006-7, and 2007-8, respectively (figure 1C; table 2).
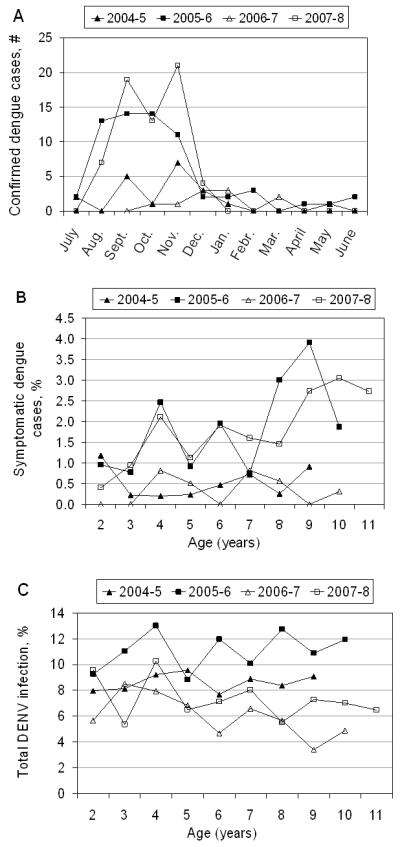
A, Number of symptomatic dengue cases by month and year. B, Age-stratified incidence proportion of symptomatic dengue cases among subjects by age and year. C, Age-stratified incidence proportion of total DENV infection (symptomatic and inapparent) by year.
The ratio of inapparent to symptomatic DENV infection varied greatly, from 18.4:1 and 16.0:1 in 2004-5 and 2006-7 to 5.0:1 and 3.0:1 in 2005-6 and 2007-8, respectively (figure 2A). In 2005-6, there were high numbers of both total DENV infections and symptomatic cases; however, in 2007-8, the number of DENV infections was similar to 2004-5 and 2006-7, yet there was a larger proportion of cases. Focusing on 2007-8, we observe an age-dependent shift in the ratio of inapparent to symptomatic infection, from 4.2:1 in children aged 3-8 to 1.4:1 for 9-11-year olds (figure 2B), reflecting the greater number of cases among older children that year. Severity -- both hospitalizations and DHF/DSS cases -- also increased markedly in later years. No DHF/DSS cases were documented in the first two years, whereas in 2006-7 and 2007-8 (figure 2C) 8% and 13%, respectively, of dengue cases were classified as DHF/DSS (table 2). An increase in hospitalized cases was also observed in 2007-8, with a total of 15 (23%) hospitalizations (table 2).
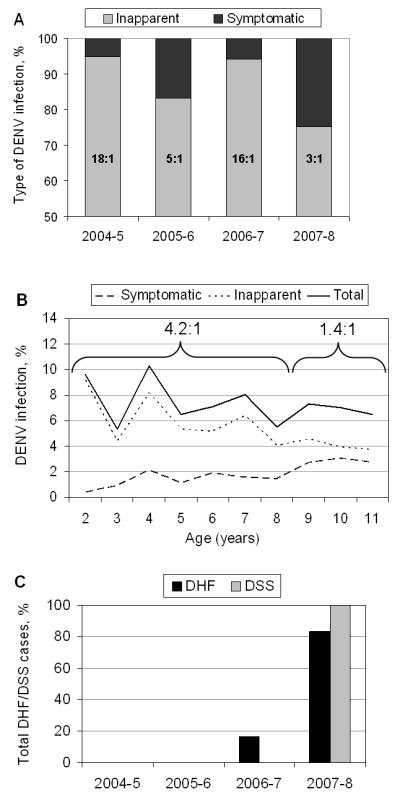
A, Proportion of symptomatic and inapparent DENV infections by year. B, Incidence proportion of symptomatic, inapparent and total DENV infections in 2007-8. Older children had a greater proportion of symptomatic infections compared to younger children. C, Percent of all DHF/DSS cases by year. 2006-7, DHF (n=1); 2007-8, DHF (n=5), DSS (n=3). The average age of DHF/DSS was 9.5 years-old. The average age of DHF/DSS cases was 9.5 years-old.
Primary and secondary DENV infections
Analysis of immune status yielded expected results: primary cases and infections were more frequent in younger children, while secondary cases and infections predominated in older children (figure 3A,B). The ratio of inapparent to symptomatic infections is lower for primary infections in 2004-5 and 2006-7, and for secondary infections in 2005-6 and 2007-8 (table 2). We noticed the ratio of percent of secondary cases to secondary infections increased, especially comparing 2004-5 to 2007-8 (table 2), which could be due to the shift from DENV-1 to DENV-2 (see below). When immune status was examined in relation to serotype, DENV-2 was associated with a significantly higher percentage of secondary than primary cases as compared to DENV-1 (p<0.001) (figure 3C).
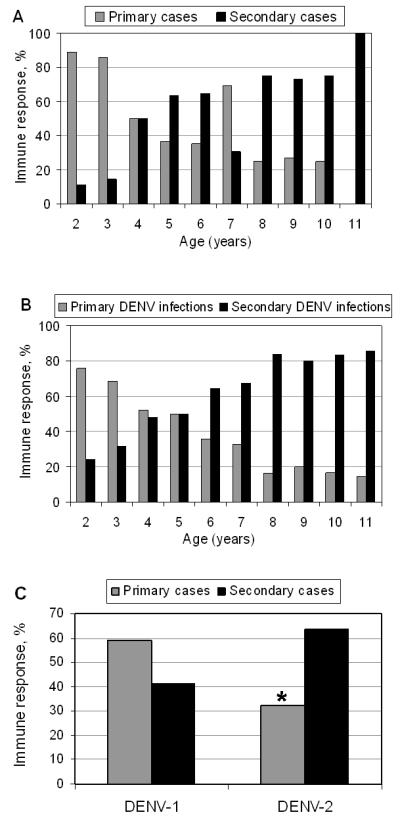
A, Percent primary and secondary immune response by age among symptomatic dengue cases. B. Percent primary and secondary immune response by age among all symptomatic and inapparent DENV infections. C, Percent primary and secondary cases by DENV serotype. DENV-2 was associated with a significantly higher percentage of secondary than primary cases as compared to DENV1; *, p<0.001.
Seroprevalence and circulating DENV serotypes
Increasing seroprevalence was observed in the cohort, with an 12.8% overall rise during the study period (figure 4A). However, the rate of DENV transmission decreased; for instance, 79% seroprevalence was observed in 6-year-old children in 2004-5, compared to 47% seroprevalence among 6-year-olds (the 3-year old cohort) in 2007-8. Concomitant with the decrease in seroprevalence, the circulating serotype shifted between 2004 and 2008 (figure 4B). Among the 89.3% of cases with serotype information, DENV-1 prevailed in 2004-5 (53%), but was almost exclusively replaced by DENV-2 in 2006-7 (70%) and 2007-8 (95%). The continued predominance of DENV-2 in later years was accompanied by increasing numbers of symptomatic dengue cases among older children (figure 1B).
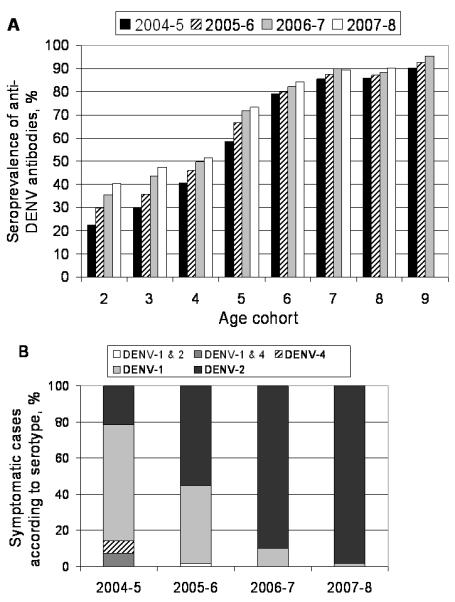
A, Seroprevalence of anti-DENV antibodies by age cohort beginning with year of enrollment, showing the expected increase in anti-DENV antibodies over time. The 9-year old cohort has seroprevalence data through 2006, as subjects turning 12-years old were not re-enrolled in 2007 and therefore did not complete the 2007 annual sample. B, , Circulating DENV serotypes identified in symptomatic infections by year. There was 100% serotype concordance between RT-PCR and virus isolation for samples with positive results for both tests.
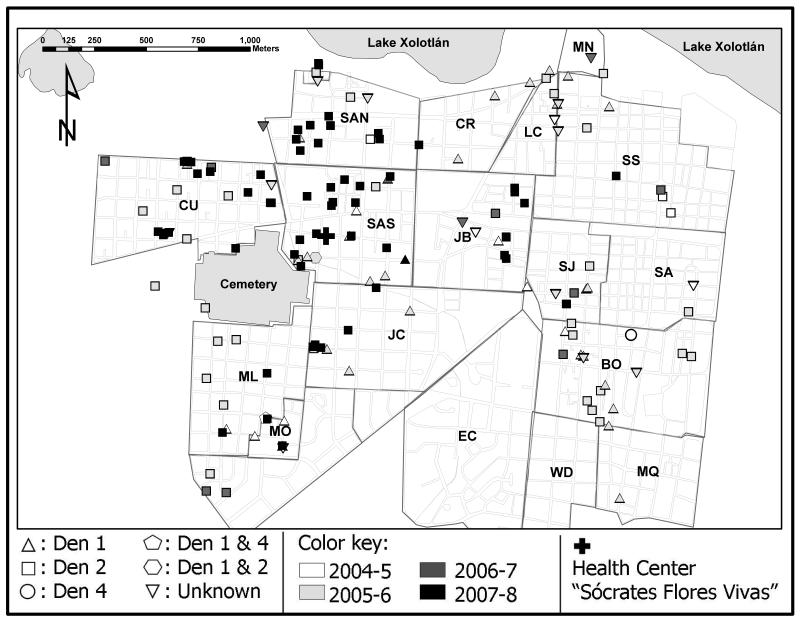
Spatial shifts occurred over the four years of the study, as cases clustered geographically according to year and serotype (figure 5). Phylogeographic analysis revealed strong clustering by neighborhood, using both Bayesian (AI, p<0.001; PS, p<0.001) and parsimony (p<0.001) approaches. In addition, parsimony analysis revealed relatively little viral movement between individual neighborhoods compared to that expected by chance alone. The majority of statistically significant instances of viral movement involved either Santa Ana Norte or Bóer neighborhoods, indicating that viruses move into, or out of, these locations more often than the other neighborhoods studied. In addition, there was phylogenetic evidence for movement between Santa Ana Norte and Santa Ana Sur, Bóer and Monseñor Lezcano, and Bóer and Cuba.
Climate and entomological indices
Precipitation was substantially higher in 2005-6 and 2007-8 than 2004-5 and 2006-7, the latter experiencing the lowest rainfall in the 50 years from which precipitation data is available. Ae. aegypti indices in the catchment area of the HCSFV were consistently quite high, except for 2006-7, when all indices were substantially lower (table 1).
DISCUSSION
Here we present results from the first four years of a large-scale prospective study of dengue in Latin America, documenting the natural history of dengue transmission in an urban pediatric population and demonstrating that variations in climate and interventions are associated with annual trends. Understanding the entire spectrum of DENV infection is essential to combating disease, whether via vector control, improved case identification and management, or vaccine and drug development.
This study was characterized by a high degree of participant compliance and comprehensive quality control of data and biological samples [14]. The community-based cohort design was chosen over a school-based design to maximize case capture, as a school-based study conducted previously in Managua was not optimal for dengue surveillance due to numerous healthy absences and frequent school vacations [15]. Others, in Iquitos, Perú, also found that community-based surveillance was more effective than a school-based approach [13], although studies in schoolchildren have been useful in other settings [6, 12, 17, 29]. This study included children from one district of Managua, but as a low-to middle-income area affected by dengue and other infectious diseases, we believe it to be representative of Managua and other Latin American cities.
Indeed, the incidence proportions of DENV infections in our cohort (5.8%-11.1%) fall within the range reported in both Southeast Asian and Latin American pediatric populations. In a similar Nicaraguan pediatric population in 2001-2003, we reported an annual DENV infection incidence of 6-12% [15], while in 2003-4 during the re-introduction of DENV-1, total infection incidence of 25% was documented in the HCSFV catchment area (A.Balmaseda and E.Harris, unpublished). In comparison, a prospective study in Maracay, Venezuela, reported 25.8% and 16.9% total infection rates in 2001-2 and 2002-3, respectively [17]. Incidence rates of pediatric DENV infection in Southeast Asia fall within the same range: 5.6% in Bangkok, Thailand in 1980-81 [29]; an overall annual incidence of 5.8% (2.2-7.9%) in 1998-2000 in Kamphaeng Phet, Thailand [6]; and 29.2% in Yogyakarta, Indonesia in 1995-6 [8].
In terms of clinical manifestations of DENV infection, 74% of symptomatic DENV infection throughout the study period met the WHO case definition for dengue, while the remaining 26% of cases were diagnosed as undifferentiated fever. Undifferentiated fever accounted for the greatest proportion of cases (38.5%) in 2006-7, which had fewest symptomatic cases. Similar findings have been reported in Vietnam and Pakistan [30, 31]. This implies that dengue surveillance programs are likely underestimating the number of cases, excluding those that present with fever but without characteristic signs and symptoms.
We document substantial year-to-year variation in dengue cases, transmission and serotype circulation. The low numbers of dengue cases in 2004-5 may be because DENV-1, the dominant serotype that year, had caused many infections the previous year (2003) leaving a smaller susceptible population [4]. In 2005-6, the dominant serotype switched to DENV-2, which had not circulated widely since 2002, allowing for higher infection rates. With the continued circulation of DENV-2 in 2006-7 and 2007-8, we documented an expected decrease in transmission rates. However, while the decrease in incidence proportion of DENV infection from 2005-6 too 2007-8 (11.1% to 7.3%) is expected, the large drop in 2006-7 (5.8%) may be explained by unusually low precipitation and a particularly vigorous vector control campaign that year (F.Acevedo, personal communication), resulting in exceptionally low Ae. aegypti indices (table 1). Variations were also witnessed in disease by age. Older children presented greater numbers of symptomatic dengue after the introduction of DENV-2, which produces symptomatic disease mainly in secondary infections [32, 33].
DENV-2 caused the majority of symptomatic cases from 2005 to 2008, yet despite decreasing transmission and fewer infections, it produced more severe cases in 2006-7 and 2007-8. Sequencing of the complete DENV-2 genome identified two different clades in the cohort; Clade 1 circulated in 2004-5 and 2005-6, while Clade 2 -- which is significantly associated with DHF/DSS -- circulated only during 2006-7 and 2007-8 (A. Balmaseda, M. Henn, and E. Harris, unpublished). The appearance of Clade 2 could explain the increase in severity in later years, as DENV-2 from all of the cohort’s sequenced DHF/DSS cases (7/7) were Clade 2, and may account for the higher proportion of symptomatic DENV infections in 2007-8.
Seroprevalence in this cohort, whose oldest subjects were born in 1995, reflects the recent history of DENV circulation in Nicaragua. DENV-3 caused a large epidemic in 1998, then DENV-2 circulated from the end of 1998 until 2002. Older cohort participants were exposed to DENV-3 and DENV-2 in this phase when both serotypes circulated. In 2003, there was an epidemic caused by DENV-1, which had not circulated for a decade; thus, both younger and older children were susceptible to DENV-1 infection in 2003-4 and the first year of this study (2004-5). However, since DENV-1 and then to a greater extent DENV-2 circulated throughout the study, the rate of DENV transmission started to decline, leading to an accumulation of DENV-susceptible population – exactly what we are currently observing in the fifth year of the study (2008-9), with the introduction of DENV-3 for the first time since 1998 and a correspondingly high proportion of DENV-3 cases (A. Balmaseda, unpublished).
A detailed phylogeographic analysis of DENV-2 utilizing full-length genome sequences revealed a marked spatial clustering according to neighborhood. A similarly strong phylogeographic spatial structure was recently observed for DENV-1 and DENV-3 sequences in Singapore [34] and previously using classical epidemiological methods in Puerto Rico [35, 36] and Thailand [37, 38]. Hence, despite the population fluidity characterizing many urban environments, DENV spreads within highly localized epidemic foci. Nonetheless, some inter-neighborhood viral traffic was observed, particularly involving Santa Ana Norte and Bóer. In some cases, such viral traffic is clearly explained by the spatial proximity of neighborhoods, such as Santa Ana Norte and Santa Ana Sur. In others, it may be explained by mobile populations, for example, Bóer is situated near numerous Central American regional bus terminals and a seasonal settlement of banana workers from northwestern Nicaragua.
One limitation of our study design is that case capture is dependent on presentation of children to the HCSFV when febrile, thus non-attendance may lead to misclassification of symptomatic infections as inapparent. However, only 2% of participants reported visiting a healthcare provider outside the HCSFV, so misclassification is unlikely to significantly affect results. Additionally, secondary infections may be underestimated if a primary infection occurs just after the first annual sample and a second infection occurs just prior to the second sample. Scheduling annual sample collection in July when few infections occur limits this type of misclassification. In addition, data is restricted to children 2-12 years of age, but nonetheless represents a broad pediatric age range. Voluntary drop-out and withdrawal hampers the collection of a subset of paired annual samples, but as the annual rate of loss was quite low (4.3-7.1%), the overall results were not grossly affected. It is possible that there was incomplete capture of inapparent infections due to undetectable antibody titers in the post-infection annual sample, but more frequent sample collection was not possible in this population. Finally, detailed information about the Ministry of Health intervention in 2006 was unfortunately not available.
The results of this community-based cohort study underscore the year-to-year variation of dengue within a pediatric population, revealing expected patters in transmission and infection while highlighting the impact of climate, interventions, and viral evolution. The continuation of this cohort will allow additional insight into these trends as circulating DENV serotypes and subtypes and environmental factors vary. A carefully monitored pediatric cohort not only affords critical data and biological samples to study DENV infection and disease, but also enables detailed surveillance of epidemiologic and immunologic trends in the study population necessary for potential vaccine and drug trials.
ACKNOWLEDGMENTS
We thank the phenomenal study team based at the Centro de Salud Sócrates Flores Vivas, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Hospital Infantil Manuel de Jesús Rivera for their dedication and high-quality work, particularly Magaly Amador, Sonia Arguello, José Ramon Cisneros, Carolina Flores, Nicole Fitzpatrick, Oscar Ortega, Juan Carlos Matute, Berman Moraga, Juan Carlos Mercado, Mirtha Monterrey, Zoila Orozco, Sergio Ojeda, Leonel Perez, Miguel Reyes, Crisanta Rocha, Carlos Romero, Saira Saborio, Leyla Saenz, Nery Sanchez, Sheyla Silva, Yolanda Tellez, and Maria José Vargas. We are especially grateful to Dr. Alcides Gonzalez and Dr. Juan José Amador for their continued support over the years. We thank Patrick Charlebois for his technical support on the comparative bioinformatic analysis of the complete genome data. We are extremely grateful to the participants and their families.
This study was funded by grant VE-1 from the Pediatric Dengue Vaccine Initiative. Additional support was received from the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200400001C to the Broad Institute.
2)This study was funded by the Pediatric Dengue Vaccine Initiative (grant # VE-1), which played no role in performing the study or preparing the manuscript.
Footnotes
1)None of the authors have any relationships or support that might be perceived as constituting a conflict of interest.
3)This manuscript has not been, and will not be, submitted elsewhere for publication as long as it is under consideration of The Journal of Infectious Diseases. Part of the information in this manuscript was presented at the 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene in Philadelphia, PA, November 4-8, 2007, and at the Fifth Network Meeting of the Pediatric Dengue Vaccine Initiative, in Montreal, Canada, June 5-8, 2008.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1086/648592
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jid/article-pdf/201/1/5/18059923/201-1-5.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Pooled Cohort Profile: ReCoDID Consortium's Harmonized Acute Febrile Illness Arbovirus Meta-Cohort.
JMIR Public Health Surveill, 10:e54281, 23 Jul 2024
Cited by: 1 article | PMID: 39042429 | PMCID: PMC11288473
Cohort study: Neurological and cognitive-behavioral sequelae of acquired Zika virus infection among Nicaraguan children.
Pediatr Res, 02 Jul 2024
Cited by: 0 articles | PMID: 38956218
Primary Dengue and Long-Term Health Status in Madeira Island, Portugal: A Retrospective Questionnaire-Based Study.
Am J Trop Med Hyg, 111(2):403-411, 02 Jul 2024
Cited by: 0 articles | PMID: 38955194 | PMCID: PMC11310610
Introduction of New Dengue Virus Lineages of Multiple Serotypes after COVID-19 Pandemic, Nicaragua, 2022.
Emerg Infect Dis, 30(6):1203-1213, 01 Jun 2024
Cited by: 3 articles | PMID: 38782023 | PMCID: PMC11138998
Flying under the radar - impact and factors influencing asymptomatic DENV infections.
Front Cell Infect Microbiol, 13:1284651, 24 Nov 2023
Cited by: 0 articles | PMID: 38076464 | PMCID: PMC10704250
Review Free full text in Europe PMC
Go to all (121) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year.
PLoS Negl Trop Dis, 7(8):e2357, 08 Aug 2013
Cited by: 146 articles | PMID: 23951377 | PMCID: PMC3738476
High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua.
Trop Med Int Health, 11(6):935-942, 01 Jun 2006
Cited by: 100 articles | PMID: 16772016
Dynamics and determinants of the force of infection of dengue virus from 1994 to 2015 in Managua, Nicaragua.
Proc Natl Acad Sci U S A, 115(42):10762-10767, 28 Sep 2018
Cited by: 18 articles | PMID: 30266790 | PMCID: PMC6196493
Spatial dimensions of dengue virus transmission across interepidemic and epidemic periods in Iquitos, Peru (1999-2003).
PLoS Negl Trop Dis, 6(2):e1472, 21 Feb 2012
Cited by: 60 articles | PMID: 22363822 | PMCID: PMC3283551
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: HHSN272200900018C
Grant ID: HHSN266200400001C