Abstract
Free full text

Preparing for the First Breath: Genetic and Cellular Mechanisms in Lung Development
Abstract
The mammalian respiratory system—the trachea and the lungs—arises from the anterior foregut through a sequence of morphogenetic events involving reciprocal endodermal-mesodermal interactions. The lung itself consists of two highly branched, tree-like systems—the airways and the vasculature—that develop in a coordinated way from the primary bud stage to the generation of millions of alveolar gas exchange units. We are beginning to understand some of the molecular and cellular mechanisms that underlie critical processes such as branching morphogenesis, vascular development, and the differentiation of multipotent progenitor populations. Nevertheless, many gaps remain in our knowledge, the filling of which is essential for understanding respiratory disorders, congenital defects in human neonates, and how the disruption of morphogenetic programs early in lung development can lead to deficiencies that persist throughout life.
Our lungs fulfill multiple functions related to terrestrial living and breathing air. The most critical, of course, is to facilitate gas exchange. To achieve this goal the lung consists of two intertwined and highly branched tree-like tubular systems—one conducting air; the other, blood. While this arrangement is highly efficient, breathing air is not without risks, such as exposure to airborne pathogens and dehydration. The lung has evolved various strategies to overcome these problems. They include mechanisms of innate immunity, the secretion and clearing of mucus, maintenance of proper surface tension, and fluid and electrolyte transport, all functions carried out in large part by specialized epithelial cell types lining the airways. A major challenge is to understand how the two sophisticated tubular systems of the lung develop in a coordinated way from small primordial buds, each consisting of a few hundred epithelial progenitors surrounded by a layer of mesenchyme. Basic research into the mechanisms controlling this transformation is important for a number of reasons. First, premature birth, intrauterine infection, or genetic mutation can disrupt developmental cues, resulting in defective lungs in the neonate. While the normal adult lung can undergo repair, it appears that some prenatal deficiencies cannot be rescued by postnatal regeneration, and result in lifelong respiratory problems and further clinical burdens (Greenough, 2009). A deeper understanding of development may tell us why this is so and how to improve outcomes. Second, many of the intercellular signaling networks controlling the morphogenesis of the respiratory system are conserved and used in the development of other branched tubular organs such as the kidney, mammary gland, and prostate. Thus, advances in our understanding of one organ system can inform another. Here, we highlight recent advances in our knowledge of lung development, building on earlier reviews (Cardoso and Lü, 2006; Maeda et al., 2007). We also point to some of the many questions that are still underexplored.
Early Foregut Morphogenesis in Relation to the Respiratory System
The earliest known step in the development of the respiratory system (the trachea and lungs) is the establishment of a localized domain of expression of Nkx2-1 (also known as Titf1) in the ventral wall of the anterior foregut (Figure 1A). Within this domain, at E9.5 in the mouse (~22 somite stage) and ~28 days in the human, the two primary lung buds appear. Around the same time, the single foregut tube, just anterior of the lung buds, begins to separate into two–a dorsal esophagus that leads into the stomach and a ventral trachea that connects to the lung buds.

For references other than those cited, see Cardoso and Lü (2006).
(A) The anterior foregut begins as a single epithelial tube surrounded by mesoderm. Early specification of the future trachea and lungs (here marked by Nkx2-1, blue) and other endodermal organs is regulated by the dose and timing of Wnt and FGF signals from the mesoderm (Goss et al., 2009; Serls et al., 2005). Shh in the endoderm regulates Bmp4 in the mesoderm in part through the transcription factors Foxf1 and Gli1 and Gli3. The localized expression of Fgf10, which drives lung bud outgrowth, requires retinoic acid (RA) signaling and involves inhibition of Tgfβ (Chen et al., 2007). In the chick, Fgf10 is downstream of Tbx4. Embryos lacking Hoxa5 have defects in tracheal cartilage development, but roles for other Hox genes have not been determined.
(B) Schematic section through undivided foregut at the level of the dotted box in (A) showing the highest Sox2 levels dorsally, and Nkx2-1, ventrally. The action of Bmp4 (and earlier, Bmp7) is antagonized by noggin (Li et al., 2008b; Que et al., 2006). Asterisks mark the site of separation of foregut into two tubes. Deficiencies in Shh, Gli1/Gli3, RARs, Fgfr2, Fgf10, Foxf1, Sox2, Nkx2-1, Bmp4, noggin, and Wnts lead to defects in foregut development including tracheoesophageal fistula and esophageal atresia (EA/TEF).
(C) Around E9.5, the primary lung buds appear with the right bud larger than the left. This asymmetry is downstream of the genetically regulated L/R patterning of the whole-body axis. Separation of the foregut tube into trachea and esophagus begins just anterior of the lung buds (asterisk). The domain of separation extends over time. There is also extensive elongation of the two tubes (green arrows) as well as outgrowth of primary lung buds.
(D) Whole-mount in situ hybridization for Shh showing expression in the endoderm with highest levels in distal tips. Domain branching gives rise to the primordial buds of the right cranial (Cr), medial (Md), caudal (Cd), and accessory (Ac) lobes.
Some of the molecular players known to be involved in these early morphogenetic events are summarized in Figure 1. In particular, recent studies have provided new information about the patterning of the endoderm along the D/V axis (Figure 1B). For example, greatest Sox2 expression is observed dorsally in the future esophagus while Nkx2-1 expression marking the future trachea is greatest ventrally. This patterning depends on signals from the surrounding mesenchyme including Bmps and their antagonist, noggin, as well as Fgfs and Wnts. Deficiencies in some of these genes result in a failure in foregut separation and abnormal differentiation of the epithelium and mesenchyme. Wnt signaling plays a particularly important role at this stage. Combined loss of Wnt2 and Wnt2b, which are expressed in the mesoderm surrounding the anterior foregut, or of beta-catenin (Ctnnb1) in the endoderm, leads to loss of Nkx2-1 expression, expansion of Sox2 expression, and failure in foregut separation (Chen et al., 2007; Goss et al., 2009; Harris-Johnson et al., 2009; Li et al., 2008b; Que et al., 2006, 2009).
While some progress has been made, a great deal more remains to be learned about the cellular mechanisms involved in the separation of the foregut into trachea and esophagus. Separation is a very dynamic process and likely involves coordinated changes in the shape and alignment of cells in the foregut epithelium, as well as the inward movement of lateral mesodermal ridges, that proceed in a posterior to anterior direction. There is also rapid elongation of the tracheal and esophageal tubes once they have separated, though again the mechanisms involved are not clear (Figure 1C). Associations have already been made between mutations in genes encoding some of the known players in foregut morphogenesis and a spectrum of quite common human birth defects, including esophageal atresia, tracheoesophageal fistula, and tracheal or lung agenesis (Brunner and van Bokhoven, 2005; Que et al., 2006; Stankiewicz et al., 2009). Identifying new players in the process would likely expand the number of candidate genes for both familial and sporadic cases of these congenital defects.
Another aspect of tracheal development that deserves further investigation is the differentiation of the mesoderm into a specific number of C-shaped cartilage rings ventrolaterally and strips of smooth muscle (SM) dorsally. Reciprocal interactions between the epithelium and mesenchyme involving a number of signaling pathways have been implicated (recently reviewed in Rock et al., 2008). In some cases the defects are sufficient to cause congenital collapse of the trachea, a condition known as tracheomalacia in humans.
The study of foregut morphogenesis may provide new clues not only about human birth defects but also about the evolution of the vertebrate respiratory system. One theory is that the lung arose from a swim-bladder-like organ of an aquatic ancestor. Recent studies show that, as in the lung, Shh expression in the endoderm plays a critical role in zebrafish swim bladder development (Winata et al., 2009). In addition, surfactant proteins, lipids, and lamellar bodies (the organelles that traffic surfactant to the cell surface) are found in epithelial cells lining the swim bladder (Prem et al., 2000). Another recent finding is that signals from the pulmonary and pharyngeal endoderm regulate the development of the inflow and outflow tracts of the heart (Hoffmann et al., 2009). Thus, the lungs and the cardiovascular system may have coevolved during the emergence of the first terrestrial animals.
Overview of Lung Development
Once the primary lung buds have formed, they extend into the surrounding mesenchyme and begin the process of branching morphogenesis. The development of the buds is entirely dependent on the localized expression of Fgf10 in the mesoderm overlying the buds and Fgfr2 in the endoderm. No bud extension occurs in mutants lacking Fgf10, although the trachea does separate (Min et al., 1998; Sekine et al., 1999). Between E9.5 and E16.5 in the mouse (the “pseudoglandular stage”), the primary buds generate a complex tree-like structure ending in thousands of terminal tubules. Remarkably, during this stage the branching pattern is highly stereotyped and essentially identical between inbred mouse strains (Metzger et al., 2008). Morphometric analysis of lungs fixed at different times was elegantly used to show that the branching pattern can be broken down into three distinct local “modes” or subroutines that are repeatedly in different combinations (Metzger et al., 2008). The first mode is called “domain branching.” It involves the orderly sprouting of new buds at specific distances from the tip of a stalk and at positions around the circumference that are either dorsal/ventral or medial/lateral relative to the axis of the parent stalk. The other two subroutines relate to the bifurcation of the tip; they are called “planar” and “orthogonal bifurcation,” depending on the axis along which the two new buds are formed.
After about E16.5 in the mouse, the process of branching morphogenesis changes. During the “canalicular” stage (E16.5–17.5) the terminal buds become narrower, and then during the “saccular” stage (E18.5 to postnatal day 5 [P5]), they develop numerous small sacs that are the precursors of the alveoli (Figure 4). At the same time the blood vessels, which from the beginning have developed in parallel with the airways, become closely associated with the epithelium. This behavior of the blood vessels (to be discussed later) is critical for the development of the alveoli and for subsequent gas exchange.

(A) Alveolar development begins in late gestation as the endothelial plexus becomes tightly associated with the distal epithelial saccules. Bottom panel shows terminal bronchioles with saccules attached while top panel is a higher magnification after the saccules have been removed to reveal the lumen.
(B) The epithelium of the developing saccules/primary septae differentiates into several important cell types found in the mature alveolus. These include AEC1 cells, which are very flat and thin walled and when mature characteristically express aquaporin 5 and T1alpha (podoplanin), and the much larger, cuboidal AEC2 cells. These cell types are closely juxtaposed to each other and AEC1 cells form intimate interactions with the underlying vascular endothelium.
(C) Mature AEC2 cells secrete abundant surfactant proteins and lipids that are trafficked to the cell surface in organelles called lamellar bodies marked by the ACBC3 transporter protein. Mature AEC2 cells express high levels of the gene encoding surfactant protein C (Sftpc). Maturation of the alveolar compartment is accompanied by generation of secondary septae, which involves growth of alveolar crests. Crest formation requires elastin deposition and Pdgfa for myofibroblast development (Boström et al., 1996).
(D) Hand Estained histological sections demonstrating the change in distal lung morphology from the canalicular/saccular stages at approximately E17.5 through the adult where mature alveoli are found with secondary septae (arrows).
While branching morphogenesis does not continue postnatally, the lungs increase in size for a significant time after birth as a result of increases in the length and diameter of the airways and the subdivision of the alveoli into smaller units (Bourbon et al., 2009; Kauffman, 1980).
The Establishment of the Basic Lobulation Pattern of the Lung
The two primary lung buds give rise to the future main stem bronchi. Subsequently, domain branching establishes the basic “skeleton” of the respiratory tree; in the mouse this consists of four lobes on the right (cranial, medial, caudal, and accessory) and one on the left (Figure 1D). The main driver of this domain branching is the localized expression of Fgf10 in the distal mesoderm acting through Fgfr2IIIb in the endoderm. Remarkably, this mechanism is very similar to that regulating the early branching of the Drosophila larval respiratory system. Here, the epithelial cells of the tracheal placode express the Fgf receptor breathless. The pattern of primary branching is controlled by the localized expression of the Fgf ligand branchless (Bnl), in small clusters of ectodermal and mesodermal cells localized around the placode (Affolter and Caussinus, 2008). The position of these clusters is determined by D/V and A/P patterning genes acting within each segment. This raises the possibility that the position of Fgf10 domains in the mesoderm of the early mouse lung is also genetically “hard-wired” through local patterning genes. This idea predicts that mutations will be found that affect primarily the number, position, or spacing of specific lobes. In support of this idea, loss of either Fog2 or Gata4, genes encoding interacting transcription factors with localized expression in the mesoderm, results in the deletion of the founding accessory bud (Ackerman et al., 2005, 2007). Other candidates might include Hox family members that, at least in the chick, show nested expression in the mesoderm along the D/V and A/P axes of the primary lung buds (Sakiyama et al., 2000). However, the Hox genes likely work in combination because mutants in single genes do not have striking defects in lung patterning. Rigorous testing of the idea that the early pattern of Fgf10 expression in the lung is hard-wired will require, among other things, the identification of region-specific enhancers in the Fgf10 gene and their evolutionarily conserved transcription factor binding sites.
Epithelial-Mesodermal Interactions Maintain a Distal Tip Signaling Center
Once established, the localized source of Fgf10 in the tip of the mesoderm becomes a critical component of a distal organizing and signaling center. This involves a core group of evolutionarily conserved signaling pathways—those driven by Bmps/Tgfβ, Wnts, Shh, and retinoic acid (RA) (for references see Cardoso and Lü, 2006 and the legend to Figure 2). Recent evidence has also implicated Notch signaling downstream of FGF10 at this stage (Tsao et al., 2008). Together, these pathways coordinate reciprocal interactions between the epithelium and mesoderm. They also regulate the size of progenitor pools by balancing their expansion through proliferation with reduction through differentiation. Consequently, the distal signaling center plays a major role in determining the ultimate size and morphology of the lung.

(A) Fgf10 in distal mesoderm acts on the epithelium through the Fgfr2IIIb isoform to promote cell proliferation and bud morphogenesis, possibly through different downstream pathways. Signaling through Tgfβr2 in the mesoderm inhibits Fgf10 expression (Li et al., 2008a). RA expressed in a gradient in the mesoderm, with highest levels proximally, also inhibits Fgf10. Fgf9 made in the mesothelium and epithelium promotes the proliferation of Fgf10-express-ing mesenchymal cells that express Fgfr2IIIc, and inhibits the expression of Bmp4 and noggin in response to Shh.
(B) Epithelial Shh promotes proliferation and expression of Bmp4 in the mesenchyme, as well as inhibits Fgf10 expression and upregulates hedgehog-interacting inhibitory protein (Hhip). The phenotype of Shh null lungs is partially rescued by deleting Gli3, suggesting that the repressor form of Gli3 (Gli3R) functions in the mesenchyme to inhibit proliferation and to regulate Foxf1.
(C) Absence of Wnt7b results in lungs that are small but almost normally proportioned. Wnt7b regulates the expression of Bmp4 and Idb2 in the epithelium through independent pathways and promotes proliferation of the mesoderm through Axin2 and Lef1 (Rajagopal et al., 2008; Shu et al., 2002). Loss of Wnt2 results in significant lung hypoplasia due to decreased proliferation in both epithelial and mesenchymal compartments (Goss et al., 2009). Wnt5a is expressed in both endoderm and mesoderm.
(D) Bmp4 is transcribed in distal epithelium and in the mesoderm with highest levels around bud stalks. Bmp5 is expressed in mesoderm. Bmpr1a is expressed in both the epithelium and mesenchyme, while Bmpr1b is restricted to the proximal epithelium. The level of Bmp4lacZ in the epithelium increases as the bud elongates, suggesting a role in limiting this process (Weaver et al., 2000). Moreover, addition of Bmp4 to buds in culture inhibits their directed outgrowth toward a source of Fgf10 (Weaver et al., 2000). Conversely, deletion of Bmpr1a in the epithelium reduces proliferation in vivo and inhibits the ability of epithelium to bud in response to Fgf in vitro. The precise response of cells to Bmps likely depends on the level of ligand, the activity of other signaling pathways, and cell type. Bmps likely play additional roles in later lung development and postnatally (Alejandre-Alcázar et al., 2007).
An essential feature of the distal signaling network is that it involves multiple antagonists: for example, Sproutys (Sprys), noggin, and hedgehog-interacting protein (Hhip). In other developing systems such antagonists are components of complex feedback loops that limit or refine the range, magnitude, and duration of mitogenic and morphogenetic signals (Affolter and Basler, 2007; Alon, 2007; Dikic and Giordano, 2003). There is evidence that they also fulfill this role in the developing lung. For example, Spry2 mutants have subtle increases in the number and degrees of altered position of lateral branches in some regions, which is consistent with Spry2 being a negative inhibitor of receptor tyrosine kinase signaling in the epithelium (Metzger et al., 2008). In Hhip mutants the primary lung buds form, but the early invariant domain branching of the right bud does not occur. However, lateral budding from the primary branches is seen later (Chuang et al., 2003). This phenotype suggests a model for how Hhip normally regulates branching. The high levels of Shh in the tips of the buds (Figure 1D) induce Hhip in the adjacent mesoderm. Shh also has the potential to inhibit Fgf10 expression in the mesoderm but this activity is attenuated locally by the Hhip. By contrast, Hhip is not induced around the stalks of the buds, where Shh expression is lower. This allows the Shh to inhibit the expression of Fgf10 laterally and so reduces the probability of lateral budding close to the tip (Figure 2).
Another feature of the distal organizing center is that different signaling pathways cross-regulate one other. Examples of this crosstalk are that Shh stimulates Wnt2 and Bmp4 in the mesenchyme (Pepicelli et al., 1998), Wnt7b/beta-catenin signaling promotes the expression of Bmp4 and Fgfr2 in the epithelium (Rajagopal et al., 2008; Shu et al., 2005), and Fgf9 (secreted by both the early epithelium and the mesothelium) promotes the expression of Fgf10 in the distal mesoderm (del Moral et al., 2006).
A distal tip organizing center with feedback loops and cross-regulation between pathways is not unique to the developing lung. Outgrowth and branching of the ureter bud of the embryonic kidney involves expression of glial cell-line-derived neurotrophic factor (GDNF) in the mesenchyme. This functions through the transmembrane tyrosine kinase receptor, cRet, expressed in the epithelium. Recent work suggests that GDNF works cooperatively with other signaling factors in the mesenchyme, including Fgf10 and Fgf7. Together they overcome the inhibitory effect of Spry proteins in the epithelium (Basson et al., 2005; Costantini, 2006).
Regulation of Epithelial Cell Proliferation during Branching Morphogenesis
It is clear from the above discussion that no one signaling component—Shh, FGF9/10, Wnt2/7b, or Bmp4—is a master regulator of lung development. Rather, they work together as a team to control downstream effectors such as Nmyc, Etv4/5, Erk1/2 (Mapk3 and Mapk1) and p38 (Mapk14) (Goss et al., 2009; Liu et al., 2008; Shu et al., 2005). Additional factors in this regulatory network are still being identified, including micro-RNAs (miRNAs). Deletion of Dicer from the epithelium inhibits lung development (Harris et al., 2006) and the miR17–92 cluster promotes early distal progenitor proliferation, possibly by controlling factors that regulate cell division (Lu et al., 2007; Ventura et al., 2008). Identification of more regulatory miRNAs will certainly expand our understanding of the regulatory networks in the lung.
In the E12.5 lung the proportion of cells that incorporate BrdU during a short pulse is higher in the distal compared with proximal epithelium (Okubo et al., 2005). Epithelial cells in mitosis are localized throughout the growing buds. Surprisingly, their precise location in relation to distance from the bud tip, and their plane of division relative to the axes of the branch and sites of budding, has not yet been quantified in vivo. One question that needs to be resolved is whether initial bud formation requires a localized increase in cell proliferation. Such an association has not been seen in other budding systems. For example, in the Drosophila tracheal system, initial placode invagination occurs without cell division (Affolter and Caussinus, 2008). The budding of lung endoderm in response to exogenous FGF also occurs without localized cell division in vitro (.Nogawa et al., 1998). Therefore it seems likely that bud initiation involves primarily changes in cell shape and cell-cell organization in vivo (Liu et al., 2004).
Changes in Cell Behavior Associated with Bud Elongation and Bifurcation
During branching morphogenesis the lung buds undergo repetitive cycles of four processes: bud elongation, cessation of outgrowth, expansion of the tip, and bifurcation (either planar or orthogonal) (Metzger et al., 2008). These processes are associated with dynamic changes in the localization of FGF10 transcripts in the mesoderm, in the expression level of genes such as Bmp4 in the endoderm, and in the deposition of extracellular matrix molecules (Figure 3). Surprisingly, we still know relatively little about the cellular mechanisms involved in lung bud morphogenesis. Clues about potential mechanisms used to drive the process of elongation come from studies on kidney morphogenesis, in which both convergence extension (the alignment of cells along one axis) and changes in the plane of cell division are used to modulate the length and diameter of epithelial tubes at different stages (Karner et al., 2009; Yu et al., 2009). Defects in these processes as a result of mutations in Wnt genes lead to abnormal tube morphologies and epithelial “cysts” similar to those frequently described in mutant lungs. When driven by Wnt ligands, alterations in cell polarity within a planar sheet (planar cell polarity or PCP) involve the noncanonical pathway, including Rho-GTPase and Jun kinases (JNK) (Klein and Mlodzik, 2005; Segalen and Bellaïche, 2009). Several Wnts are expressed in the developing lung (Figure 2) and attenuation in their levels leads to abnormal morphogenesis. This is particularly striking in the case of Wnt5a (Li et al., 2002). Changes in gene expression have been described in these mutant lungs that may be mediated through the canonical Wnt pathway (Li et al., 2002, 2005). However, the mutant phenotypes have not yet been examined in relation to potential changes in epithelial cell alignment, polarity, or planes of division, which could be mediated by noncanonical signaling. The roles in lung development of genes encoding other proteins that work through the PCP pathway (for example Celsr1,2,3; Vangl1,2; and Scrb) have also not yet been determined. This will be important not only for understanding branching morphogenesis but for determining how multiciliated cells become polarized so that the cilia all beat in the same direction, clearing mucus out of the lungs.

(A) Movement of isolated lung endoderm in Matrigel from an E11.5 lung toward a bead soaked in recombinant Fgf10. Images are from a continuous series over 36 hr. To date there have been no detailed studies on dynamic cell rearrangements in these buds as with ureter bud (Costantini, 2006) and mammary gland epithelium (Ewald et al., 2008). (B) Schematic representation of the spatial expression in the distal lung of some semaphorins, plexins, and neuropilins first described as “axonal guidance” factors. Based on in vitro studies, netrin 4 may help to restrict expansion of the stalk and lateral budding while sema3a may help to restrict outgrowth of the bud (Hinck, 2004). (B–D) Schematic representation of (B) bud elongation, (C) cessation of outgrowth, and (D) bifurcation. The basal lamina (dashed line) surrounds the epithelial cells in the stalk and extends into the tip but is less dense in this region, meaning that the basal surface of the tip epithelium can be in contact with mesenchyme (Bluemink et al., 1976). There is also evidence for differential sulfation of the matrix associated with budding (Izvolsky et al., 2003). As the bud elongates (arrow in A) toward Fgf10 in the distal mesenchyme (red), expression of the Bmp4lacZ knockin reporter (blue) increases in the tip (blue) (Weaver et al., 2000). PhosphERK1/2 expression is confined to cells at the tip (Liu et al., 2004). There is deposition of new matrix adjacent to the epithelium in the cleft formed by the bifurcation process; this may be promoted by Tgfβ signaling (Goss et al., 2009).
There is even less information about mechanisms that may control cessation of lung bud outgrowth as well as bud expansion and bifurcation. Changes in Bmp4lacZ expression during budding and the response of epithelium to Bmp4 in vitro suggest that the protein functions in an autocrine mechanism to limit bud outgrowth (Figure 3). However, precisely which cell behaviors are regulated by Bmp4 is unknown. Various roles have also been proposed for extracellular matrix molecules and Tgfβ signaling in modulating bud outgrowth and bifurcation during the pseudoglandular stage (Cardoso and Lü, 2006 for references). In addition, there is evidence for the involvement of molecules first identified for their role in axonal guidance (Figure 3). For example, netrins are made by the epithelium of the stalk and localize in the surrounding matrix. Here, they may act through receptors in the epithelium as a “sleeve” restricting bud outgrowth and helping to refine tubule diameter and elongation (Liu et al., 2004). Likewise, it has been proposed that branching is regulated by slit, robo, semaphorins, and plexins that are all dynamically expressed in the developing lung (reviewed in Hinck, 2004). However, rigorous testing of these ideas has not been straightforward. This is because the proteins are often members of families with overlapping expression, necessitating the deletion of multiple genes to overcome functional redundancy. In addition, some factors are expressed in both the developing airways and vascular system, so that the ablation will have to be tissue-specific to distinguish primary from secondary effects.
From this discussion it is clear that a great deal more needs to be learned about cell behavior during the process of lung branching morphogenesis. In the future it will be important to describe mutant phenotypes that involve abnormal branching in very specific terms, not only in relation to the three modes of branching used in lung development but also in relation to changes in cell shape, polarity, and alignment relative to different axes. To facilitate this deeper level of analysis, we need to improve methods for visualizing and quantifying dynamic change in cell behaviors in real time, both in lungs undergoing morphogenesis in organ culture and in isolated endoderm responding to exogenous ligands (Figure 3). In addition, we need to know more about the role of components such as integrins and metalloproteinases that are dynamically expressed during development (Greenlee et al., 2007). Another exciting aspect of cell biology that has hardly been touched upon in relation to lung development is the role of the primary cilium in epithelial and mesenchymal cells of the developing lung. There are many human congenital abnormalities, such as the diverse group of Bardet-Biedl syndromes, associated with defects in primary cilia (Zaghloul and Katsanis, 2009). Given the wide range of tissues affected in these patients, it would be surprising if no specific lung phenotypes were found in these and other examples of ciliopathies.
Finally, we need to understand more about the role of fluid pressure within the lumen in lung development. The liquid in the developing lung comes from two sources: amniotic fluid and secretions of the epithelial cells themselves, associated with the active transport of chloride ions. Reduction in fluid pressure, due for example to reduction in the level of amniotic fluid, is associated with lung hypoplasia, whereas experimentally increasing the pressure can enhance the rate of branching. However, the mechanisms by which pressure is sensed, and the ion channels involved in chloride transport in the embryonic lung, have not yet been determined (Wilson et al., 2007). The idea that fluid pressure has a morphogenetic role is supported by observations that in the Drosophila tracheal system, regulated secretion/absorption is important in controlling tube diameter (Affolter and Caussinus, 2008).
Forming the Alveolar Gas Exchange Unit: The Culmination of Lung Development
At around E16.5 in the mouse, lung development switches from branching morphogenesis to the canalicular and saccular stages. These, in turn, lead to the final process of alveologenesis (also known as alveolization) that generates the functional units for gas exchange (Figure 4). The timing of alveolar development varies between species. In mice it occurs postnatally (~P5–30), but in humans some alveoli have formed before birth and the process continues for many months afterwards. Significantly, the lungs of very low birth-weight (VLBW) premature babies may have only progressed to the canalicular stage at delivery, highlighting the clinical challenges of protecting the organ from damage. Almost nothing is known about what controls the ending of the pseudoglandular stage, and the morphogenetic mechanisms underlying subsequent development remain poorly understood (Bourbon et al., 2005, 2009; Prodhan and Kinane, 2002).
During the canalicular and saccular stages, the terminal or acinar tubes narrow and give rise to small saccules. The walls of these sacs, the primary septae, are tightly associated with the vascular plexus, with an extracellular matrix rich in elastin, and with as yet poorly defined mesenchymal cell types, including precursors of the myofibroblasts. The endoderm begins to differentiate into the two main specialized cell types of the future alveolus–the alveolar epithelial type 1 and type 2 cells (AEC1 and AEC2) (Figure 4). During alveolization the sacs are subdivided by the ingrowth of ridges or crests known as secondary septae. Both myofibroblast progenitors and endothelial cells migrate into these crests, and a scaffold of matrix proteins is deposited, enriched in elastin at the tip. The capillary units are initially double, but as the alveoli mature, the capillaries remodel to form one unit and the endothelial cells become tightly apposed to the AEC1 cells, allowing efficient gas exchange (Figure 4). Several signaling factors are important for the formation of secondary septae. The differentiation of the myofibroblasts and their production of elastin is regulated by Pdgfa (Bostro¨m et al., 1996; Lindahl et al., 1997). The matrix also includes critical proteins such as fibrillin-1. These may function in part by specifically binding and acting as a “sink” for growth factors (such as Tgfβ) required for coordinating development (Neptune et al., 2003). Recent studies have demonstrated an important role for ephrin B2 on the endothelial cells at this time. In ephrin-B2-signaling-defective mutants, the secondary septae do not develop normally and the deposition of several matrix proteins is disrupted (Wilkinson et al., 2008).
From this brief description and from recent reviews (Bourbon et al., 2005, 2009), it can be seen that alveolar formation, one of the most critical steps in lung development, involves the precise temporal and spatial coordination of multiple cell lineages. This makes the process particularly susceptible to disruption by cellular stress, intrauterine infection, and even nutritional restriction (Das, 1984). This, in turn, may lead to conditions such as emphysema (enlarged air spaces) and alveolar capillary dysplasia that are not easily reversed. Many signaling pathways and intercellular interactions are obviously involved in the generation and differentiation of the vascular and mesenchymal components needed to make alveoli, and these will be discussed later. In addition to Pdgfα and ephrin B2, Fgf and RA signaling have been shown to be especially important for alveolization. Multiple Fgfs are expressed in the late-stage lung, including Fgf2 and Fgf18. A critical role for the Fgf pathway in alveolar development was demonstrated by the phenotype of lungs of Fgfr3/Fgfr4 double null mice that fail to undergo secondary septation. Moreover, they do not show the downregulation of elastin production that normally occurs at the end of the process (Weinstein et al., 1998). RA receptor-beta (RARb) deficient mice have defects in distal airspace development and a progressive loss of respiratory function (Snyder et al., 2005). In fact, both increased and decreased RA signaling impair alveolar development. Increased RA signaling by transgenic expression of a dominant active RARα receptor in the epithelium results in lung immaturity, with a block in distal epithelial maturation and no discernable development of AEC1 cells (Wongtrakool et al., 2003). In contrast, vitamin A deficiency impairs lung epithelial function in rodents and causes bronchopulmonary dysplasia in humans (Biesalski and Nohr, 2003; Verma et al., 1996). Precisely how RA signaling functions to regulate alveolization remains unclear. There is evidence for crosstalk with Pdgf and Fgf signaling leading to myofibroblast growth (Liebeskind et al., 2000; Snyder et al., 2005). Moreover, RA regulation of lung myofibroblast proliferation was recently shown to be dependent upon intact Fgf signaling (Perl and Gale, 2009).
In conclusion, a great deal needs to be learned about the whole process of alveolar development, a need driven by the huge clinical challenge posed by VLBW neonates. Moreover, glucocorticoids, widely administered to accelerate the maturation of AEC2 cells and surfactant production in premature babies, appear to inhibit secondary septation and vascular development (Bourbon et al., 2005, 2009). New experimental models to study lung alveolarization are badly needed. Paradigm-changing concepts may emerge if we could follow in real time the migration and rearrangement of cells during alveolar development in ectopic grafts (Vu et al., 2003). The generation of new genetic tools for conditionally manipulating genes in developing alveolar cells and following their lineage relationships is also a high priority.
The Development of the Mesodermal Components of the Lung
The mesoderm around the primary lung buds is an important source of signals for driving epithelial growth and differentiation and branching morphogenesis (Figure 2). It is also a source of specialized cell types, including pericytes, parabronchial SM, myofibroblasts, and other cells of the alveoli such as lipid storage cells underlying the AEC2 cells. In the human lung the mesoderm also gives rise to cartilage around the distal airways. The proliferation and differentiation of the mesoderm has to be coordinated with that of the endoderm, and this is achieved through a complex signaling network involving the endoderm, mesoderm, and mesothelium. Known players include Shh, Bmps, Wnts, Vegf, Pdgf, Fgfs, Tgfβ, and RA.
One current model of mesoderm development during the pseudoglandular stage is depicted in Figure 5. According to this model, the distal mesodermal progenitors that express Twist2 (also known as Dermo1), Foxf1, Tbx4, and Pdgfra are multipotent. As development proceeds they are exposed to a series of inductive signals that regulate their commitment to different lineages. Those committed to become airway SM move proximally and envelop the endodermal tubes. By contrast, vascular SM progenitors become incorporated into vessels. Some progress has been made in studying gene function in the early mesoderm through the development of a new transgenic line based on the Twist2/Dermo1 gene. This has been used to target cre to the lung mesoderm as early as E10.5 and to demonstrate a cell-autonomous role for Wnt/beta-catenin signaling in the growth and survival of early lung mesoderm (De Langhe et al., 2008; Yin et al., 2008). However, Twist2 is expressed quite broadly. In the future more specific cre lines will be needed to test the role of genes in specific cell types. Single-cell lineage tracing is also necessary for rigorous testing of the models of mesoderm development discussed here.
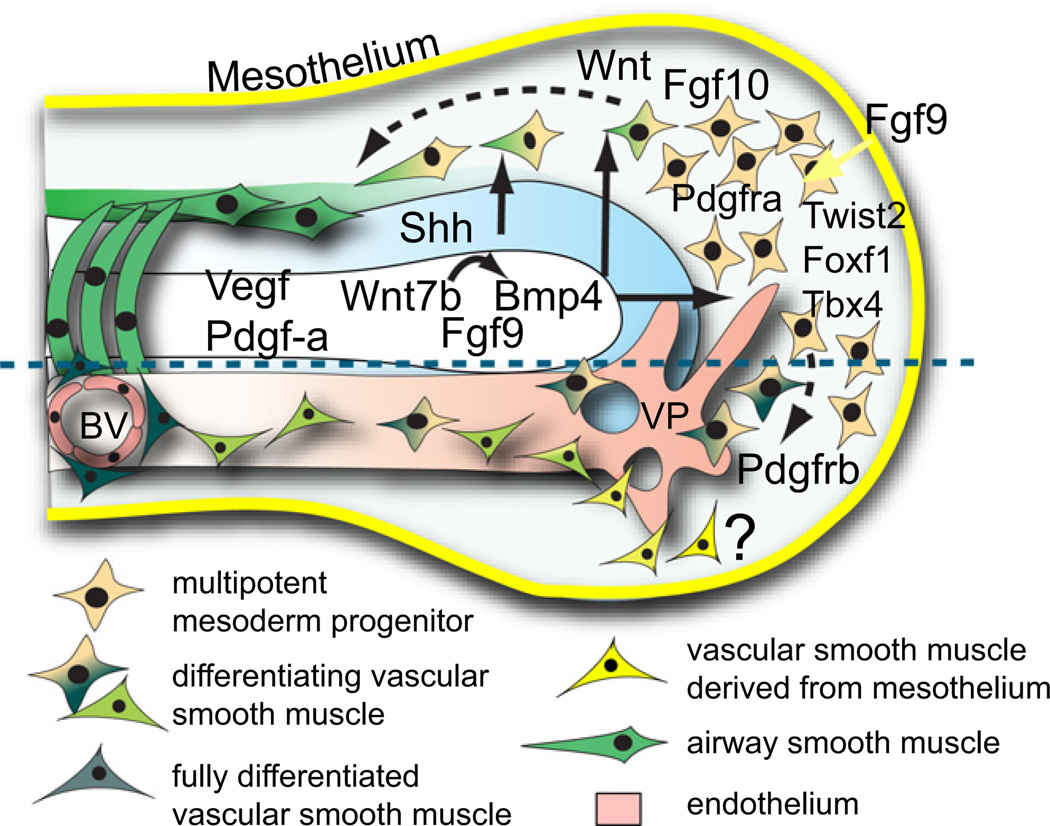
Undifferentiated multipotential progenitors in the distal tip express Twist2, Foxf1, and Tbx4. As shown above the dashed line, they include precursors of bronchiolar smooth muscle (Pdgfra+) that translocate proximally and associate with the endoderm. As shown below the dashed line, other progenitors give rise to pericytes (Pdgfrb+) that migrate around blood vessels. Some pericytes and interstitial cells may arise from the mesothelium (yellow). Blood vessels (BV) likely arise by sprouting angiogenesis and remodeling of the vascular plexus (VP) surrounding the distal tip that is in continuity with the embryonic circulation. There is some evidence for distal vasculogenesis (see text). The growth and differentiation of BVs and smooth muscle is regulated by signals from the endoderm (Shh, Wnt7b, Vegf, and Fgf9) and mesothelium (Fgf9). It has been proposed that early mesoderm is subdivided into submesothelial and subepithelial domains that differ in their response to signaling factors (White et al., 2007).
Development of the Pulmonary Vasculature
There is evidence, sometimes contradictory, for both vasculogenic and angiogenic processes in the development of pulmonary blood vessels. Given that early defects in vascular development are likely to impact late events, including, as described earlier, the critical process of alveolization, more research is clearly needed. Early models argued that the capillary plexus around the distal endoderm is generated from endothelial Flk1/Kdr+ precursors within the lung mesoderm by vasculogenesis. This plexus later connects up with vessels that have developed by angiogenesis from the aortic sac. Evidence supporting this model comes from studies suggesting direct communication between the proximal and distal vasculature only after E13.5 (deMello et al., 1997; Stenmark and Mecham, 1997). Moreover, in embryonic lungs grafted under the renal capsule, vessels develop from endogenous precursors and also connect up with the vasculature of the host (Vu et al., 2003). By contrast, later studies have found evidence for blood flow within the capillary plexus around the lung from as early as E11.5 in the mouse (Parera et al., 2005; Schwarz et al., 2009). This and other data support a model in which distal sprouting angiogenesis and remodeling of the capillary plexus occur without vascular leakage (Figure 5). In either case, the early blood vessels in the lung are rather undifferentiated in their arterial versus venous lineage. Expression of markers such as ephrin B2 and ephrin B4 is not restricted to arterial versus venous vessels up to the pseudoglandular stage (Gale et al., 2001; Schwarz et al., 2009). Only in the canalicular stage does expression of ephrin B2 become restricted to arterial endothelial and vascular SM, and ephrin B4, to venous endothelium (Gale et al., 2001; Schwarz et al., 2009).
As might be expected there is growing evidence for crosstalk between the developing vasculature and the developing airways. The endoderm expresses Vegfa. A variety of studies suggest that the correct isoform, dose, and timing of Vegfa expression all play critical roles in blood vessel development. For example, conditional downregulation of Vegfa in the endoderm leads to a failure of blood vessel formation around the distal airways (primary septae) at the saccular stage. Moreover, HGF made by the endothelial cells at this time positively regulates the proliferation of the endoderm (Yamamoto et al., 2007). Vegfa is also made in the mesoderm early in development. Its expression, as judged by the activity of a VegfalacZ reporter, is coordinately regulated by several factors, including Shh and Fgf9 made in the epithelium and mesothelium (White et al., 2007).
In other tissues, sprouting angiogenesis and the remodeling of the vascular plexus involves not only Vegfa/Vegfr2 but a whole host of other signaling pathways including Notch/delta and factors first identified as axon guidance molecules. In the future it will be important to determine the role of these pathways, including those based on semaphorin/plexin and slit/robo, in pulmonary blood vessel development. These factors may also regulate the ingrowth of nerves that occurs alongside the airways early in development (Tollet et al., 2001).
Airway Smooth Muscle Development
There are two basic types of SM within the lung: airway and vascular. Airway SM controls the diameter of the airway tubes, and overproliferation is observed in chronic asthma. Vascular SM controls blood vessel diameter, and hyperproliferation is associated with familial pulmonary hypertension. The relationship and developmental origins of these lineages are poorly understood and, as in all other questions about mesoderm development, appropriate fate mapping analysis is required to resolve this problem. Noggin is specifically expressed on airway SM in the lung (Weaver et al., 2003), suggesting that future genetic tools based on this gene will be useful in targeting this lineage. The current hypothesis is that airway SM develops from lung mesoderm through the sequential action of multiple inductive signals within the lung bud (Figure 5). According to this model, mesoderm cells destined to form proximal lineages receive cues to move in a retrograde fashion toward the airway stalks, surround the epithelium, and differentiate into SM. Shh plays a critical role in airway SM development as demonstrated by the lack of these cells in Shh−/− mutants and by the ability of exogenous Shh to induce SM differentiation in lung explants (Litingtung et al., 1998; Pepicelli et al., 1998; Weaver et al., 2003). Shh expression in the lung endoderm is regulated by Foxa1/2 (Wan et al., 2005). In turn, Shh-regulated expression of the critical mesoderm transcription factor Foxf1 is required for proper SM and cartilage development (Mahlapuu et al., 2001). Wnt/beta-catenin also plays an important role in airway SM development. The BAT-GAL Wnt/beta-catenin reporter line reveals Wnt signaling activity early in the developing lung mesoderm and later in the differentiating airway SM (Cohen et al., 2009; Shu et al., 2005). Deletion of beta-catenin in early lung mesoderm using the Twist2/Dermo1-cre line results in decreased airway SM progenitor expansion due to downregulation of Fgfr2 (Cohen et al., 2009).
Fgf10 expression, as reported by a transgenic insertion of lacZ near the Fgf10 locus, also marks early airway SM progenitors in the developing lung (Mailleux et al., 2005; Ramasamy et al., 2007). As summarized in Figure 2, Fgf10 initiates an important early gene expression program in the distal endoderm, including Bmp4. This, in turn, signals to distal mesoderm to initiate the SM differentiation program (Mailleux et al., 2005). Fgf9 expression (from endoderm and mesothelium) works in concert with Fgf10 to maintain the SM progenitor pool in the distal lung mesenchyme (Colvin et al., 2001; del Moral et al., 2006; Yin et al., 2008). Downregulation of Fgf10 levels is thought to be necessary for differentiation of these progenitors (Cohen et al., 2007).
Lung Vascular Smooth Muscle Development
The developmental origins of the SM pericytes surrounding the larger blood vessels in the lung are poorly understood. The current model suggests that undifferentiated mesoderm cells surrounding the developing vascular endothelial plexus differentiate into vascular SM cells, in response to a combination of paracrine cues from endothelial, epithelial, and mesothelial cells. However, there is no genetic cell lineage mapping data to support this model. Recent evidence has raised the possibility of a mesothelial cell contribution to the vascular SM lineage in the developing lung, similar to what happens in the mesentry and gut (Wilm et al., 2005). Fate-mapping experiments using a Wilms tumor 1 (WT1)-cre transgenic mouse line, which expresses cre primarily in the mesothelial layer, suggests that these cells can contribute significantly to the vascular SM lineage (Que et al., 2008). However, more work needs to be done to verify these observations and to follow in real time the movement of cells from the outer mesothelium layer into the interior of the developing lung.
One of the key regulators of vascular SM function in the lung is Bmpr2. Indeed, mutations in BMPR2 are a leading cause of familial pulmonary hypertension in humans, a disease characterized by excessive SM growth that occludes the lumen of pulmonary blood vessels (Machado et al., 2001; Newman et al., 2001). Bmp4 has been shown to inhibit proliferation of embryonic lung fibroblasts while simultaneously promoting their differentiation into SM (Jeffery et al., 2005). Thus, loss of Bmpr2 may lead to greater vascular SM proliferation due to enhanced Bmp4 signaling. Evidence for a role for Wnt7b in regulating vascular SM development and integrity has also been reported. Wnt7blacZ−/− mutants have hypertrophic and apoptotic vascular SM at birth (Shu et al., 2002). More recent evidence shows that Wnt7b directly regulates the extracellular matrix protein tenascin-C (TnC) in the lung mesoderm, which in turn regulates expression of Pdgfrα/β (Cohen et al., 2009). This Wnt-TnC-Pdgfrα/β pathway is important for proliferation and differentiation of early SM progenitors in the developing lung and is upregulated in a mouse model of asthma as well as in human pulmonary hypertension patients (Cohen et al., 2009).
In addition to Wnt signaling, members of the Fox family of fork-head-domain-containing transcription factors have demonstrated roles in vascular SM development in the lung. Early studies in the mouse established a critical role for Foxf1; about half of Foxf1+/− heterozygous null mutants die perinatally with vascular hemorrhage and defective vessel development (Kalinichenko et al., 2004). These studies have been made particularly relevant by recent findings that heterozygous loss of FOXF1 in humans leads to defective pulmonary vascular development (Congenital Alveolar Capillary Dysplasia), including misalignment and defective patterning of small pulmonary arteries, as well as SM hyperplasia in these arteries (Stankiewicz et al., 2009). This is a solid example of how defects in a gene that acts early in lung development can have an impact on later morphogenesis, including the vascularization of the alveoli.
Differentiation of the Epithelial Cells of the Lung
One of the most important questions that remains underexplored in the lung is how the undifferentiated epithelial cells present in the primary buds give rise to all of the specialized cells types of the postnatal lung. These are the AEC1 and AEC2 cells of the alveoli (Figure 4) and the secretory, multiciliated, and neuroendocrine (NE) cells of the airways (bronchi and bronchioles). Secretory cells, generically called Clara cells, are typically marked by synthesis of the secretoglobin family member Scgb1a1 (also known as CC10 or CCSP), while ciliated cells express Foxj1 and acetylated tubulin IV. NE cells, which are found in small clusters at branchpoints along the airways and in lower numbers distally, express calcitonin (Calca or Cgrp) and Pgp9.5 (Uchl1). The proportion of ciliated to secretory cells is lower near the distal end of the bronchioles but elsewhere is roughly 50:50. Mucus-producing goblet cells that express Muc5ac, Foxa3, and the secreted ligand anterior gradient 2 (Agr2) are also found in the airways. The number of goblet cells is low in the mouse compared with human but increases dramatically in response to airway allergens and certain cytokines (e.g., Il-13) and upon downregulation of Foxa2 (Wan et al., 2004). Recent lineage tracing experiments show that Scgb1a1+ Clara cells can give rise to goblet cells without cell proliferation, by a process that requires the transcription factor Spdef (Chen et al., 2009). Whether other cell types, e.g., ciliated cells, can give rise to goblet cells is not known (Figure 6).

(A) At the pseudoglandular stage (E11–16.5), progenitor cells (P1, red) within the distal tip epithelium both self-renew and generate descendents (green) that exit the tip and populate the conducting airways. At least up to E13.5 the P1 cells are able to generate all of the different airway epithelial cell types, strongly suggesting multipotency. The pool is maintained by multiple factors (Figure 2 and text). P1 cells persist to the canalicular stage when they generate cells (P2, orange) that populate the future alveoli. It is not clear if this switch from the production of bronchiolar to alveolar descendents is intrinsic or imposed by extrinsic factors. However, there is evidence that Wnt and Notch signaling regulate this change in behavior.
(B) Putative airway progenitors (green) express Sox2. One model for their differentiation is that the first bronchiolar lineage decision is mediated by Notch signaling via Hes1 and is between an NE (blue) and putative non-NE fate (light green). Mash1 expression is required for NE cell commitment and differentiation. The non-NE cells subsequently undergo a second Notch-mediated decision to commit to the secretory cell (yellow, Scgb1a1hi) or ciliated cell (purple, FoxJ1+, β-tubulin IV+) lineages. However, other models are equally possible, including all three lineages sorting out at once. There is good lineage tracing evidence that once Scgb1a1+ cells are formed, they are able, as a population, to self-renew over the long term and to give rise to ciliated cells in the postnatal lung (Rawlins et al., 2009b). Mucus-producing cells can be derived from Clara cells directly (Chen et al., 2009), but other origins are possible.
(C) Similarly to the bronchioles, there is no direct evidence that a common alveolar epithelial progenitor exists. However, the evidence is consistent either with an alveolar progenitor giving rise to AEC1 (Aqp5+, T1alpha+) and AEC2 (Sftpc+) cells, or AEC2 cells themselves generating AEC1. It is not clear what genetic mechanisms regulate these decisions.
The current model of epithelial development is that during the pseudoglandular stage the cells in the tips of the buds constitute a pool of highly proliferative multipotent progenitor cells. These are maintained by the coordinated activity of signaling factors including Wnts and Fgf10 (Figure 2). Progeny left behind in the stalks by the outgrowth of the epithelium continue to divide and give rise to all of the different cell types of the future bronchi and bronchioles. Cells in the tips also continue to divide and populate the distal tubules at the canalicular and saccular stages. These late progenitors give rise to the AEC1 and AEC2 cells, either because their intrinsic development potential has changed or because their environment now only supports alveolar development. Evidence for this general model has come from recent cell lineages studies using an Id2-CreER knockin allele (Rawlins et al., 2009a). Id2 (also known as Idb2) encodes a transcription factor that is specifically expressed in the distal epithelial cell population of the lung throughout prenatal development but is largely switched off after birth. By using the Id2-CreER allele to conditionally activate reporter genes, the fate of cells present in the distal epithelium has been followed at different times. These experiments show that between E11.5 and E13.5, distal tip cells, as a population, give rise to Clara, ciliated, and NE cells as well as to AEC1 and AEC2. However, cells labeled at E17.5 only populate the alveoli. Though these studies have clarified our understanding of epithelial development, many important questions remain. First, do all the cells in the distal tips have the same developmental potential or is there a mixture of subpopulations with more restricted fates, a question that can only be addressed by following the fates of single cells? Using this approach, Rawlins et al. (2009a) have provided good evidence that some individual cells in the early tips are multipotent and can give rise to daughters in both the airways and alveoli. Another question is whether cells in the late tips could give rise to proximal airway cells if provided with the right environment, or whether these progenitors are irreversibly restricted in a temporal manner. This could be tested by combining them with embryonic mesenchyme and grafting them to ectopic sites.
Even when these issues are resolved, we still need to understand the mechanisms that regulate the number and developmental potential of the distal progenitor populations at different stages and what controls the allocation of their descendants to different lineages. A full understanding will require, among other things, a catalog of all the genes expressed in the various regions of the developing lung (tip versus stalks, for example) at different times. Although such a custom database does not yet exist, valuable information about the spatial distribution of transcripts of hundreds of genes expressed in the embryonic lung at E14.5 is available from www.genepaint.org and www.eurexpress.org.
Several studies have cataloged genes encoding transcription factors specifically expressed in the early distal epithelial population that are downregulated proximally as development proceeds, and that are absent from all or most cells of the adult lung. These are candidates for regulating the phenotype and developmental potential of the early multipotent progenitors and their immediate descendants. However, the roles of most of these genes are still unclear. In addition to Id2, these include Sox9, Etv5 (Ets variant 5, or Erm), Foxp1/p2, Nmyc, and various members of the Iroquox gene family including Irx1, 2, and 3 (Cardoso and Lu¨, 2006; Shu et al., 2007). Conditional deletion of Nmyc does lead to severe reduction in the progenitor population (Okubo et al., 2005). However, experiments to test the function of other candidates in early development have been inconclusive because such candidates are members of multigene families with overlapping patterns of expression. For example, the lung-endoderm-specific loss of Sox9 does not alter lung development or epithelial differentiation (Perl et al., 2005), suggesting that other family members are compensating for its loss. Likewise, loss of Etv5 may be compensated for by Evt4 (Pea3), which is also expressed in the distal endoderm and upregulated by Fgf signaling (Liu et al., 2003). Loss of Irx2 is also compensated for by other family members (Lebel et al., 2003). Foxp1 and Foxp2 have overlapping patterns of expression in the distal epithelium. Foxp2 null mutants have defects in the differentiation of AEC1 cells in the alveoli and Foxp1−/+; Foxp2−/− mutant embryos have more severe and earlier defects, including loss of Nmyc and Hopx expression (Shu et al., 2007). However, the effects of complete deletion of Foxp1 and Foxp2 as well as the related Foxp4 are not yet known. Further analysis of the function of these and other genes that are members of closely related families will require conditional inactivation of multiple genes in the lung epithelium at different times.
Some transcription factors required for early lung development appear to function not by controlling the behavior and phenotype of distal progenitors per se but by globally regulating whole transcriptional programs in epithelial cells of the respiratory system. Among these global regulators are families of transcriptional factors including Gata6, Nkx2-1, and Foxa1/2. Significantly, Gata and Foxa transcription factor families act as”pioneer” factors to establish a chromatin environment critical for initiation of liver gene transcription (Cirillo et al., 2002). There is good evidence that Gata6 and Nkx2-1 physically interact through their DNA binding domains and are found associated together on promoter/enhancer elements of target genes. A triumvirate of Foxa, Gata6, and Nkx2-1 could provide a permissive environment in endoderm cells for transcription of respiratory-system-specific genes, from the time of trachea specification throughout lung development. A role for Foxa and Gata factors in foregut endoderm development is conserved in the nematode Caenorhabditis elegans, as noted by the importance of the pha-4 gene, a Foxa homolog, and the end-1/elt-2 homologs of vertebrate Gata factors in gut endoderm development (Fukushige et al., 1998; Gaudet and Mango, 2002). In mice, complete loss of either Gata6 or Nkx2-1 results in severe defects in branching morphogenesis and cell lineage differentiation in the lung (Kimura et al., 1996; Zhang et al., 2008). In contrast, Gata6/Nkx2-1 double heterozygous mice exhibit a less severe alveolarization defects (Zhang et al., 2007). This could result from a specific effect on alveolar progenitors. However, defects in alveolarization might also be secondary to the presence of an abnormally small progenitor pool at the time when the switch to this stage of development occurs. Additional experiments using precise temporal inactivation of these critical factors and pathways and comparing early versus late inactivation will be required to resolve this issue.
According to the current model of lung development, the progeny of distal tip progenitor cells that are left behind in the proximal stalks change their pattern of gene expression and begin to differentiate into the various epithelial lineages. For example, cells in the stalks of the advancing buds downregulate the expression of Sox9 characteristic of the tip cells and upregulate Sox2. Both loss- and gain-of-function studies support a role for Sox2 in regulating the differentiation of the proximal progenitors (Que et al., 2009). The role of other members of the Sox family expressed in the lung still remains to be determined (Sock et al., 2004).
Differentiation of epithelial cells in the airways proceeds in a proximal to distal direction, with the first evidence from E14.5. At this time small groups of Dll1lacZ +ve cells are seen in the developing airways, marking future NE cells (Post et al., 2000). Ciliated cells, marked by the expression of a Foxj1-GFP trans-gene, are also detected in the proximal epithelium in a “salt and pepper” pattern (Rawlins et al., 2007). A critical role for Foxj1 in the development of the ciliated lineage is demonstrated by the lack of ciliated epithelium in Foxj1−/− mice (Chen et al., 1998). Moreover, expression of Foxj1 in distal endoderm of the developing lung results in ectopic development of ciliated epithelium in distal airways (Tichelaar et al., 1999). Cells that express Scgb1a1 are detected around E15.5, in a similar pattern to Foxj1 as revealed by either antibody staining or recombination of reporter alleles driven by a Scgb1a1-CreER knockin allele (Rawlins et al., 2007). The salt and pepper expression pattern suggests that Notch-mediated lateral inhibition or related mechanisms are active in early specification of the epithelial lineages (Kageyama et al., 2008). Evidence in support of this idea comes from analysis of mouse mutants lacking the Notch target genes Hes1 or Mash1 (Ascl1) that have increased or decreased numbers of NE cells, respectively.
Recently, further evidence that Notch signaling plays a role in establishing bronchiolar epithelial cell lineages has come from loss- and gain-of-function studies. Chemical inhibition of Notch signaling very early in lung development leads to expansion of the distal progenitor domain (Tsao et al., 2008). Later, genetic inactivation, either through conditional deletion of the genes encoding Pofut1, an O-fucosyltransferase necessary for Notch signaling, or Rbpjk, a transcriptional effector of Notch, results in a loss of secretory cells with an expansion of ciliated and NE lineages (Tsao et al., 2009). In contrast, increased Notch signaling by expression of an activated Notch1 intracellular domain in lung epithelium results in expansion of secretory lineages including mucus-producing cells at the expense of ciliated cells (Guseh et al., 2009).
Epithelial Differentiation in the Mouse Trachea
It is important to recognize that there are significant differences between the cellular composition and organization of the airway epithelium of mouse and human lungs. Because they differ in the kind of progenitor cells they harbor in the adult, these differences could affect their responses to pathologic conditions such as injury and inflammation (Rawlins and Hogan, 2006). In the mouse, the epithelium of the lung is columnar or cuboidal, and a pseudostratified layer is found only in the trachea and main stem bronchi (Figure 7). By contrast, most of the epithelium of the human lung is pseudostratified and just the smallest, most distal tubes leading into the alveoli are lined by a simple epithelium. In both species the pseudostratified epithelium consists of ciliated and secretory cells and clusters of NE cells. In addition, about 30% of the population is made up of relatively undifferentiated basal cells. Like basal cells in other epithelial tissues such as the skin and esophagus, they are characterized by the expression of the transcription factor Trp63 (p63) and cytokeratins 5 and 14 (Krt5/14). There is good evidence that in the mouse trachea, the basal cells are long term progenitor/stem cells that give rise to Clara and ciliated cells both in steady state and after epithelial injury (Rock et al., 2009). Well-defined basal cells are not seen in the mouse trachea until around the time of birth, and the specific mechanisms responsible for their differentiation are still not entirely worked out (Daniely et al., 2004; Que et al., 2007, 2009). It is assumed that Notch signaling is involved in the differentiation of the daughters of the basal cells into different lineages, because activating this pathway leads to an increase in the proportion of secretory (mucus-producing) cells whereas inhibition leads to more ciliated cells (Guseh et al., 2009). Significantly, the tracheal epithelium of embryos that are homozygous for an inactivating mutation in p63 consists entirely of columnar, ciliated cells (Daniely et al., 2004). Deletion of Sox2 in the early foregut endoderm leads to mucus metaplasia and to a severe decrease in the proportion of basal cells that are defective in self renewal and in supporting epithelial repair after injury (Que et al., 2009).

In the embryonic trachea all the progenitors express Sox2, p63, and Nkx2-1. They give rise to basal cells, Scgb1a1+ secretory cells, ciliated cells, and NE cells, but the precise mechanism is not known. Postnatally, at least some basal cells function as long term self-renewing progenitors, although there is likely to be heterogeneity. Lineage-labeled Scgb1a1+ cells can divide and give rise to ciliated cells but they do not self-renew over the long term. Basal cells can give rise to Scgb1a1+ and ciliated cells but the precise mechanism is not yet known.
Epigenetic Regulation of Lung Epithelial Progenitor Differentiation
Although still poorly understood, there are several examples of epigenetic and posttranscriptional mechanisms that regulate lung epithelial differentiation. The Hopx protein, a homeodo-main-like transcriptional cofactor that does not bind DNA, regulates AEC2 maturation and gene expression programs including expression of surfactant proteins A, B, and C (Yin et al., 2006). Hopx is a target of Nkx2-1 and Gata6, thus providing a potential negative feedback loop to regulate appropriate expression of surfactant protein genes and other potential targets in the lung epithelium. Hopx also interacts with Hdac2, providing a potential bridge between the important transcription factors Nkx2-1 and Gata6 and chromatin remodeling complexes. Further analysis will be required to assess the full contribution of such molecular pathways to lung epithelial development. The miRNA pathway has also been shown to play an important role in lung epithelial differentiation and development. Loss of the critical miRNA processing enzyme Dicer results in increased epithelial apoptosis, defective lung epithelial differentiation, and decreased airway branching (Harris et al., 2006). Several miRNA families have been hypothesized to play important roles in lung development including the miR17–92 family. Loss of miR17–92 expression results in lung hypoplasia, whereas overexpression blocks lung epithelial differentiation, leading to increased numbers of early epithelial progenitors, as noted by an increase in Sox9-, Nmyc-, and CGRP-positive cells (Lu et al., 2007; Ventura et al., 2008). This result is likely due to the increased proliferation of early distal epithelial progenitors upon overexpression of miR17–92, but also suggests that downregulation later in lung development is necessary for proper lung epithelial differentiation.
Summary and Future Directions
Despite recent intensive investigation, we are only beginning to formulate a basic understanding of the cellular and molecular mechanisms controlling the development of the lung. As we have seen, the lung is a complex organ, made up of many closely interwoven and interdependent tissues–the airways and alveoli, the blood vessels, different kinds of SM, myofibroblasts and interstitial cells, lymphatics and outer mesothelium. We need to know much more about the transcription factors and signaling pathways that mediate the reciprocal interactions between the progenitors of these populations as the lung is being built. Mapping the expression patterns of gene transcripts, and cataloging the downstream targets, interacting proteins, and splicing variants and phosphorylation sites of transcription factors active in the lung will be extremely important. However, this information alone is not enough. A consistent theme of this review is that there is a real need for more genetic tools to conditionally manipulate gene expression in specific cell populations. This is particularly important for disentangling the biological function of transcription factors that belong to large families with overlapping expression, and for driving gene expression in similar cell types, for example airway versus vascular SM. We also need many more sophisticated genetic tools for lineage tracing, to improve our still very rudimentary ideas about how the different cell types of the lung epithelium and mesoderm are related to each other. In determining gene function it will be critical to describe mutant phenotypes in terms of underlying cellular processes. To be able to do this requires that the normal morphogenesis of the lung is teased apart into modular units of cell behavior, and described in quantifiable terms such as planes of cell division, alignment, height of basolateral membranes, and adhesion and motility. Once this is done, phenotypic analysis of mutants obtained from genetic screens or from gene targeting experiments should be both easier and more informative.
Finally, how do all of these pathways and morphological processes impact the human lung, both in the neonate and adult? We have argued that defects that occur early in development, for example in the formation of blood vessels, the differentiation of myofibroblasts, or the size of progenitor pools, can have significant consequences for later stages. For example, they may result in defects in the elaboration of the highly vascularized alveoli where gas exchange takes place, leading to the bronchopulmonary dysplasia that may occur in premature babies. There is some evidence that limited alveolar regeneration is possible postnatally, but this is not robust enough to overcome major defects (Bourbon et al., 2009). In the future, a deeper understanding of normal development may enable clinicians to avoid problems in VLBW neonates. This information may also throw light on some of the cellular changes that occur in serious respiratory diseases such as asthma and chronic obstructive pulmonary disease, and provide important molecular pathways that may be of use as therapeutic targets in the future.
ACKNOWLEDGMENTS
We apologize for omitting references due to space restrains. We thank members of our laboratories and David Brass, Department of Pediatrics at Duke, for critical reading of the manuscript; and Ross Metzger, Department of Anatomy at UCSF, for thoughtful discussion and references to clinical papers. Research in the Hogan lab is supported by HL071303, and in the Morrissey lab, by HL064632, HL071589, and HL087825.
REFERENCES
- Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA, et al. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. [Europe PMC free article] [Abstract] [Google Scholar]
- Ackerman KG, Wang J, Luo L, Fujiwara Y, Orkin SH, Beier DR. Gata4 is necessary for normal pulmonary lobar development. Am. J. Respir. Cell Mol. Biol. 2007;36:391–397. [Europe PMC free article] [Abstract] [Google Scholar]
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat. Rev. Genet. 2007;8:663–674. [Abstract] [Google Scholar]
- Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–2064. [Abstract] [Google Scholar]
- Alejandre-Alcázar MA, Shalamanov PD, Amarie OV, Sevilla-Pérez J, Seeger W, Eickelberg O, Morty RE. Temporal and spatial regulation of bone morphogenetic protein signaling in late lung development. Dev. Dyn. 2007;236:2825–2835. [Abstract] [Google Scholar]
- Alon U. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 2007;8:450–461. [Abstract] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell. 2005;8:229–239. [Abstract] [Google Scholar]
- Biesalski HK, Nohr D. Importance of vitamin-A for lung function and development. Mol. Aspects Med. 2003;24:431–440. [Abstract] [Google Scholar]
- Bluemink JG, Van Maurik P, Lawson KA. Intimate cell contacts at the epithelial/mesenchymal interface in embryonic mouse lung. J. Ultra-struct. Res. 1976;55:257–270. [Abstract] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. [Abstract] [Google Scholar]
- Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr. Res. 2005;57:38R–46R. [Abstract] [Google Scholar]
- Bourbon JR, Boucherat O, Boczkowski J, Crestani B, Delacourt C. Bronchopulmonary dysplasia and emphysema: in search of common therapeutic targets. Trends Mol. Med. 2009;15:169–179. [Abstract] [Google Scholar]
- Brunner HG, van Bokhoven H. Genetic players in esophageal atresia and tracheoesophageal fistula. Curr. Opin. Genet. Dev. 2005;15:341–347. [Abstract] [Google Scholar]
- Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. [Abstract] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J. Clin. Invest. 1998;102:1077–1082. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lü J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. [Abstract] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 2009;119:2914–2924. [Europe PMC free article] [Abstract] [Google Scholar]
- Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. [Europe PMC free article] [Abstract] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell. 2002;9:279–289. [Abstract] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J. Clin. Invest. 2007;117:1794–1804. [Abstract] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the lung via a tenascin C/PDGFR pathway. J. Clin. Invest. 2009;119:2538–2549. [Europe PMC free article] [Abstract] [Google Scholar]
- Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. [Abstract] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. [Abstract] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 2004;287:C171–C181. [Abstract] [Google Scholar]
- Das RM. The effects of intermittent starvation on lung development in suckling rats. Am. J. Pathol. 1984;117:326–332. [Europe PMC free article] [Abstract] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS ONE. 2008;3:e1516. [Europe PMC free article] [Abstract] [Google Scholar]
- del Moral PM, De Langhe SP, Sala FG, Veltmaat JM, Tefft D, Wang K, Warburton D, Bellusci S. Differential role of FGF9 on epithelium and mesenchyme in mouse embryonic lung. Dev. Biol. 2006;293:77–89. [Abstract] [Google Scholar]
- deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am. J. Respir. Cell Mol. Biol. 1997;16:568–581. [Abstract] [Google Scholar]
- Dikic I, Giordano S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003;15:128–135. [Abstract] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 2008;14:570–581. [Europe PMC free article] [Abstract] [Google Scholar]
- Fukushige T, Hawkins MG, McGhee JD. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev. Biol. 1998;198:286–302. [Abstract] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 2001;230:151–160. [Abstract] [Google Scholar]
- Gaudet J, Mango SE. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–825. [Abstract] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and β-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell. 2009;17:290–298. [Europe PMC free article] [Abstract] [Google Scholar]
- Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 2007;87:69–98. [Europe PMC free article] [Abstract] [Google Scholar]
- Greenough A. Does low birth weight confer a lifelong respiratory disadvantage? Am. J. Respir. Crit. Care Med. 2009;180:107–108. [Abstract] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. [Europe PMC free article] [Abstract] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:2208–2213. [Europe PMC free article] [Abstract] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA. 2009;106:16287–16292. [Abstract] [Google Scholar]
- Hinck L. The versatile roles of “axon guidance” cues in tissue morphogenesis. Dev. Cell. 2004;7:783–793. [Abstract] [Google Scholar]
- Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. [Europe PMC free article] [Abstract] [Google Scholar]
- Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev. Biol. 2003;258:185–200. [Abstract] [Google Scholar]
- Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L370–L378. [Abstract] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 2008;11:1247–1251. [Abstract] [Google Scholar]
- Kalinichenko VV, Gusarova GA, Kim IM, Shin B, Yoder HM, Clark J, Sapozhnikov AM, Whitsett JA, Costa RH. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L521–L530. [Abstract] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 2009;41:793–799. [Europe PMC free article] [Abstract] [Google Scholar]
- Kauffman SL. Cell proliferation in the mammalian lung. Int. Rev. Exp. Pathol. 1980;22:131–191. [Abstract] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. [Abstract] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–176. [Abstract] [Google Scholar]
- Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasing-ham V, Zhang X, Cohen DR, Husain M, Cheng SH, et al. The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol. Cell. Biol. 2003;23:8216–8225. [Europe PMC free article] [Abstract] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev. Biol. 2002;248:68–81. [Abstract] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev. Biol. 2005;287:86–97. [Abstract] [Google Scholar]
- Li M, Li C, Liu YH, Xing Y, Hu L, Borok Z, Kwong KY, Minoo P. Mesodermal deletion of transforming growth factor-beta receptor II disrupts lung epithelial morphogenesis: cross-talk between TGF-beta and Sonic hedgehog pathways. J. Biol. Chem. 2008a;283:36257–36264. [Europe PMC free article] [Abstract] [Google Scholar]
- Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev. Biol. 2008b;322:145–155. [Europe PMC free article] [Abstract] [Google Scholar]
- Liebeskind A, Srinivasan S, Kaetzel D, Bruce M. Retinoic acid stimulates immature lung fibroblast growth via a PDGF-mediated autocrine mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L81–L90. [Abstract] [Google Scholar]
- Lindahl P, Karlsson L, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. [Abstract] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998;20:58–61. [Abstract] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev. Biol. 2003;261:10–24. [Abstract] [Google Scholar]
- Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. 2004;14:897–905. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu Y, Martinez L, Ebine K, Abe MK. Role for mitogen-activated protein kinase p38 alpha in lung epithelial branching morphogenesis. Dev. Biol. 2008;314:224–235. [Europe PMC free article] [Abstract] [Google Scholar]
- Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 2007;310:442–453. [Europe PMC free article] [Abstract] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, 3rd, Newman J, Williams D, Galié N, et al. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am. J. Hum. Genet. 2001;68:92–102. [Europe PMC free article] [Abstract] [Google Scholar]
- Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol. Rev. 2007;87:219–244. [Abstract] [Google Scholar]
- Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. [Abstract] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. [Abstract] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. [Europe PMC free article] [Abstract] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. [Europe PMC free article] [Abstract] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. [Abstract] [Google Scholar]
- Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, 3rd, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N. Engl. J. Med. 2001;345:319–324. [Abstract] [Google Scholar]
- Nogawa H, Morita K, Cardoso WV. Bud formation precedes the appearance of differential cell proliferation during branching morphogenesis of mouse lung epithelium in vitro. Dev. Dyn. 1998;213:228–235. [Abstract] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. [Abstract] [Google Scholar]
- Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L141–L149. [Abstract] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 1998;8:1083–1086. [Abstract] [Google Scholar]
- Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L299–L308. [Europe PMC free article] [Abstract] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. [Abstract] [Google Scholar]
- Post LC, Ternet M, Hogan BL. Notch/Delta expression in the developing mouse lung. Mech. Dev. 2000;98:95–98. [Abstract] [Google Scholar]
- Prem C, Salvenmoser W, Würtz J, Pelster B. Swim bladder gas gland cells produce surfactant: in vivo and in culture. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R2336–R2343. [Abstract] [Google Scholar]
- Prodhan P, Kinane TB. Developmental paradigms in terminal lung development. Bioessays. 2002;24:1052–1059. [Abstract] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. [Abstract] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. [Europe PMC free article] [Abstract] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. USA. 2008;105:16626–16630. [Abstract] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. [Europe PMC free article] [Abstract] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, Zhou Q, McMahon AP, Melton DA. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev. Biol. 2007;307:237–247. [Europe PMC free article] [Abstract] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. [Abstract] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc. Natl. Acad. Sci. USA. 2007;104:410–417. [Abstract] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009a;136:3741–3745. [Europe PMC free article] [Abstract] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009b;4:525–534. [Europe PMC free article] [Abstract] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev. Biol. 2008;321:141–149. [Abstract] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:12771–12775. [Abstract] [Google Scholar]
- Sakiyama J, Yokouchi Y, Kuroiwa A. Coordinated expression of Hoxb genes and signaling molecules during development of the chick respiratory tract. Dev. Biol. 2000;227:12–27. [Abstract] [Google Scholar]
- Schwarz MA, Caldwell L, Cafasso D, Zheng H. Emerging pulmonary vasculature lacks fate specification. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L71–L81. [Europe PMC free article] [Abstract] [Google Scholar]
- Segalen M, Bellaïche Y. Cell division orientation and planar cell polarity pathways. Semin. Cell Dev. Biol. 2009;20:972–977. [Abstract] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999;21:138–141. [Abstract] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. [Abstract] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. [Abstract] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev. Biol. 2005;283:226–239. [Abstract] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. [Abstract] [Google Scholar]
- Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, Giguere V, McGowan SE. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr. Res. 2005;57:384–391. [Abstract] [Google Scholar]
- Sock E, Rettig SD, Enderich J, Bösl MR, Tamm ER, Wegner M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol. Cell. Biol. 2004;24:6635–6644. [Europe PMC free article] [Abstract] [Google Scholar]
- Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Maisenbacher MK, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am. J. Hum. Genet. 2009;84:780–791. [Europe PMC free article] [Abstract] [Google Scholar]
- Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu. Rev. Physiol. 1997;59:89–144. [Abstract] [Google Scholar]
- Tichelaar JW, Lim L, Costa RH, Whitsett JA. HNF-3/fork-head homologue-4 influences lung morphogenesis and respiratory epithelial cell differentiation in vivo. Dev. Biol. 1999;213:405–417. [Abstract] [Google Scholar]
- Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudo-glandular stage of fetal mouse lung development. Dev. Dyn. 2001;221:48–60. [Abstract] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J. Biol. Chem. 2008;283:29532–29544. [Europe PMC free article] [Abstract] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. [Europe PMC free article] [Abstract] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. [Europe PMC free article] [Abstract] [Google Scholar]
- Verma RP, McCulloch KM, Worrell L, Vidyasagar D. Vitamin A deficiency and severe bronchopulmonary dysplasia in very low birthweight infants. Am. J. Perinatol. 1996;13:389–393. [Abstract] [Google Scholar]
- Vu TH, Alemayehu Y, Werb Z. New insights into saccular development and vascular formation in lung allografts under the renal capsule. Mech. Dev. 2003;120:305–313. [Europe PMC free article] [Abstract] [Google Scholar]
- Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. [Abstract] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J. Biol. Chem. 2005;280:13809–13816. [Abstract] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. [Abstract] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev. Biol. 2003;258:169–184. [Abstract] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. [Abstract] [Google Scholar]
- White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal Vegfa expression and development of the pulmonary capillary network. Development. 2007;134:3743–3752. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilkinson GA, Schittny JC, Reinhardt DP, Klein R. Role for ephrinB2 in postnatal lung alveolar development and elastic matrix integrity. Dev. Dyn. 2008;237:2220–2234. [Abstract] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. [Abstract] [Google Scholar]
- Wilson SM, Olver RE, Walters DV. Developmental regulation of lumenal lung fluid and electrolyte transport. Respir. Physiol. Neurobiol. 2007;159:247–255. [Abstract] [Google Scholar]
- Winata CL, Korzh S, Kondrychyn I, Zheng W, Korzh V, Gong Z. Development of zebrafish swimbladder: The requirement of Hedgehog signaling in specification and organization of the three tissue layers. Dev. Biol. 2009;331:222–236. [Abstract] [Google Scholar]
- Wongtrakool C, Malpel S, Gorenstein J, Sedita J, Ramirez MI, Underhill TM, Cardoso WV. Down-regulation of retinoic acid receptor alpha signaling is required for sacculation and type I cell formation in the developing lung. J. Biol. Chem. 2003;278:46911–46918. [Europe PMC free article] [Abstract] [Google Scholar]
- Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev. Biol. 2007;308:44–53. [Abstract] [Google Scholar]
- Yin Z, Gonzales L, Kolla V, Rath N, Zhang Y, Lu MM, Kimura S, Ballard PL, Beers MF, Epstein JA, Morrisey EE. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L191–L199. [Abstract] [Google Scholar]
- Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesen-chyme development. Dev. Biol. 2008;319:426–436. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. [Europe PMC free article] [Abstract] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J. Clin. Invest. 2009;119:428–437. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–198. [Abstract] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008;40:862–870. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.devcel.2009.12.010
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1534580709005279/pdf
Citations & impact
Impact metrics
Article citations
The Generation of ROS by Exposure to Trihalomethanes Promotes the IκBα/NF-κB/p65 Complex Dissociation in Human Lung Fibroblast.
Biomedicines, 12(10):2399, 20 Oct 2024
Cited by: 0 articles | PMID: 39457711 | PMCID: PMC11505202
Developmental-status-aware transcriptional decomposition establishes a cell state panorama of human cancers.
Genome Med, 16(1):124, 28 Oct 2024
Cited by: 0 articles | PMID: 39468667 | PMCID: PMC11514945
High-parametric protein maps reveal the spatial organization in early-developing human lung.
Nat Commun, 15(1):9381, 30 Oct 2024
Cited by: 0 articles | PMID: 39477961 | PMCID: PMC11525936
Lung epithelial-endothelial-mesenchymal signaling network with hepatocyte growth factor as a hub is involved in bronchopulmonary dysplasia.
Front Cell Dev Biol, 12:1462841, 03 Sep 2024
Cited by: 0 articles | PMID: 39291265 | PMCID: PMC11405311
Review Free full text in Europe PMC
Influence of mesenchymal and biophysical components on distal lung organoid differentiation.
Stem Cell Res Ther, 15(1):273, 02 Sep 2024
Cited by: 0 articles | PMID: 39218985 | PMCID: PMC11367854
Go to all (560) article citations
Other citations
Wikipedia
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development.
Birth Defects Res C Embryo Today, 78(1):80-89, 01 Mar 2006
Cited by: 42 articles | PMID: 16622850
Review
mSmile is necessary for bronchial smooth muscle and alveolar myofibroblast development.
Anat Rec (Hoboken), 295(1):167-176, 28 Sep 2011
Cited by: 9 articles | PMID: 21956870
Generation of lung organoids from human pluripotent stem cells in vitro.
Nat Protoc, 14(2):518-540, 01 Feb 2019
Cited by: 184 articles | PMID: 30664680 | PMCID: PMC6531049
Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung.
Dev Dyn, 231(1):57-71, 01 Sep 2004
Cited by: 109 articles | PMID: 15305287
Funding
Funders who supported this work.
NHLBI NIH HHS (10)
Grant ID: R01 HL064632
Grant ID: R01 HL071589
Grant ID: R37 HL071303
Grant ID: R01 HL087825
Grant ID: HL087825
Grant ID: HL064632
Grant ID: U01 HL100405
Grant ID: HL071589
Grant ID: R01 HL071303
Grant ID: HL071303





