Abstract
Free full text

Acute Gastroenteritis Surveillance through the National Outbreak Reporting System, United States
Abstract
Implemented in 2009, the National Outbreak Reporting System provides surveillance for acute gastroenteritis outbreaks in the United States resulting from any transmission mode. Data from the first 2 years of surveillance highlight the predominant role of norovirus. The pathogen-specific transmission pathways and exposure settings identified can help inform prevention efforts.
Acute gastroenteritis (AGE; defined as diarrhea or vomiting) is a major cause of illness in the United States; an estimated 179 million episodes occur annually (1). AGE is caused by a variety of viral, bacterial, and parasitic pathogens and by toxins, chemicals, and other noninfectious causes. Noroviruses are the leading cause of epidemic gastroenteritis, detected in ≈50% of AGE outbreaks across Europe and the United States (2,3). However, until 2009, national surveillance for AGE outbreaks in the United States had been limited to foodborne or waterborne disease outbreaks because no national surveillance existed for AGE outbreaks spread by other transmission modes.
To better understand and guide appropriate interventions to prevent epidemic gastroenteritis, the Centers for Disease Control and Prevention (CDC) launched a novel national surveillance system in 2009—the National Outbreak Reporting System (NORS). This system enhanced and expanded upon 2 existing surveillance systems, the Foodborne Disease Outbreak Surveillance System and the Waterborne Disease and Outbreak Surveillance System. NORS is an Internet-based system for local, state, and territorial health departments to report all outbreaks of foodborne and waterborne disease; AGE outbreaks caused by contact with infected persons, animals, or environmental sources; and AGE outbreaks caused by other or unknown modes of transmission (4). As such, NORS provides a national surveillance system for all pathways of AGE outbreaks in the United States. To assess the roles of specific pathogens, temporal trends, and exposure pathways, we summarized AGE outbreak data submitted through NORS during the first 2 years after implementation of the system.
The Study
In the United States, outbreaks (defined as >2 cases of a similar illness epidemiologically linked to a common exposure, e.g., setting or food) can be reported through NORS by all 50 US states, the District of Columbia, US territories (American Samoa, Guam, Commonwealth of the Northern Mariana Islands, Puerto Rico, and the United States Virgin Islands), and Freely Associated States (Federated States of Micronesia, Republic of Marshall Islands, and Republic of Palau). NORS was launched in February 2009, but sites were encouraged to report outbreaks that occurred since January 1, 2009.
For analysis, we extracted data reported through NORS for AGE outbreaks in which the symptom onset date for the first reported illness was during January 1, 2009–December 31, 2010. Outbreaks of diseases that do not typically cause AGE (e.g., listeriosis, legionellosis, hepatitis A) were excluded from analysis (1). We analyzed various outbreak characteristics: date of first illness onset, primary transmission mode, confirmed or suspected etiology (5), exposure setting, and number of outbreak-associated illnesses, hospitalizations, and deaths. Primary mode of transmission is determined by each reporting site on the basis of the local public health investigation and CDC guidance documents (6).
Of 4,455 outbreaks reported through NORS during 2009–2010, a total of 4,376 (98%) were AGE outbreaks (1,883 in 2009, 2,493 in 2010) (Table 1), associated with 122,488 reported illnesses, 2,952 hospitalizations, and 168 deaths. A single suspected or confirmed etiology was implicated in 2,819 (64%) outbreaks, associated with 88,958 (73%) illnesses, 2,381 (81%) hospitalizations, and 146 (87%) deaths. Norovirus, the leading cause of single-etiology outbreaks, was responsible for 1,908 (68%) outbreaks, associated with 69,145 (78%) illnesses, 1,093 (46%) hospitalizations, and 125 (86%) deaths. Salmonella spp., Shigella spp., and Shiga toxin–producing Escherichia coli (STEC), the next most frequently reported etiologic agents, were responsible for 355 (13%), 109 (4%), and 101 (4%) outbreaks, respectively. Salmonella spp. were the second most frequent cause of outbreak-associated hospitalizations (773 [32%]), and STEC was the second most frequent cause of outbreak-associated deaths (9 [6%]).
Table 1
| Outbreak etiology | No. (%) outbreaks | No. (%) outbreak-associated outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Confirmed | Suspected | Total | Illnesses | Hospitalizations | Deaths | |||
| Single agent† | ||||||||
| Norovirus‡ | 1,355 (64.2) | 553 (78.1) | 1,908 (67.7) | 69,145 (77.7) | 1,093 (45.9) | 125 (85.6) | ||
| Salmonella spp. | 344 (16.3) | 11 (1.6) | 355 (12.6) | 8,590 (9.7) | 773 (32.5) | 6 (4.1) | ||
| Shigella spp.§ | 99 (4.7) | 10 (1.4) | 109 (3.9) | 2,135 (2.4) | 115 (4.8) | 1 (0.7) | ||
| STEC | 88 (4.2) | 13 (1.8) | 101 (3.6) | 1,091 (1.2) | 250 (10.5) | 9 (6.2) | ||
| Campylobacter spp.¶ | 56 (2.7) | 13 (1.8) | 69 (2.4) | 1,550 (1.7) | 52 (2.2) | 0 | ||
| Clostridium spp.# | 41 (1.9) | 21 (3.0) | 62 (2.2) | 3,242 (3.6) | 16 (0.7) | 3 (2.1) | ||
| Cryptosporidium spp.** | 17 (0.8) | 30 (4.2) | 47 (1.7) | 598 (0.7) | 21 (0.9) | 1 (0.7) | ||
| Bacillus spp.†† | 13 (0.6) | 12 (1.7) | 25 (0.9) | 522 (0.6) | 3 (0.1) | 0 | ||
| Staphylococcus aureus | 11 (0.5) | 11 (1.6) | 22 (0.8) | 263 (0.3) | 0 | 0 | ||
| Giardia intestinalis | 13 (0.6) | 6 (0.8) | 19 (0.7) | 121 (0.1) | 5 (0.2) | 0 | ||
| Sc ombroid toxin/histamine | 18 (0.9) | 0 | 18 (0.6) | 76 (0.1) | 0 | 0 | ||
| Ciguatoxin | 14 (0.7) | 0 | 14 (0.5) | 59 (0.1) | 6 (0.3) | 0 | ||
| Rotavirus | 9 (0.4) | 5 (0.7) | 14 (0.5) | 372 (0.4) | 9 (0.4) | 0 | ||
| Other‡‡ | 33 (1.6) | 23 (3.2) | 56 (2.0) |
| 1,194 (1.3) | 38 (1.6) | 1 (0.7) | |
| All single-agent etiologies | 2,111 (98.9) | 708 (31.6) | 2,819 (64.4) | 88,958 (72.6) | 2,381 (80.7) | 146 (86.9) | ||
| Multiple agents | 24 (1.1) | 9 (0.4) | 33 (0.8) | 1,236 (1.0) | 61 (2.1) | 2 (1.2) | ||
| Unknown agent | 0 | 1,524 (68.0) | 1,524 (34.8) | 32,294 (26.4) | 510 (17.3) | 20 (11.9) | ||
| All outbreaks | 2,135 (100.0) | 2,241 (100.0) | 4,376 (100.0) | 122,488 (100.0) | 2,952 (100.0) | 168 (100.0) | ||
*STEC, Shiga toxin–producing Escherichia coli.
†Percentages for specific single agents are those among all single-agent etiology outbreaks (N = 2,819).
‡A norovirus genogroup was provided for 1,160 outbreaks: 150 GI, 1,003 GII, and 7 GI/GII.
§S. sonnei (95 confirmed and 8 suspected outbreaks), S. flexneri (5 confirmed outbreaks), Shigella sp. not known (1 confirmed outbreak).
¶C. jejuni (55 confirmed and 4 suspected outbreaks), Campylobacter sp. not known (8 confirmed and 2 suspected outbreaks).
#C. perfringens (37 confirmed and 20 suspected outbreaks), Clostridium sp. not known (4 confirmed and 1 suspected outbreak).
**C. parvum (10 confirmed and 1 suspected outbreak), C. hominis (6 confirmed outbreaks), Cryptosporidium sp. not known (30 confirmed outbreaks).
††B. cereus (13 confirmed and 11 suspected outbreaks), Bacilllus sp. not known (1 suspected outbreak).
‡‡Includes Vibrio sp. (8 outbreaks), cyanobacterial toxins (6 outbreaks), enterotoxigenic and enteropathogenic E. coli (4 outbreaks), Enterococcus spp. (3 outbreaks), mycotoxins (3 outbreaks), Cyclospora spp. (2 outbreaks), pesticides (2 outbreaks), sapovirus (2 outbreaks), paralytic shellfish poison (1 outbreak), Pseudomonas sp. (1 outbreak), sodium hydroxide (1 outbreak), Yersinia sp. (1 outbreak), and other unspecified etiologies (22 outbreaks).
AGE outbreaks were reported by the District of Columbia, Puerto Rico, and all states except Delaware (Figure 1). A median of 42 outbreaks (range 2–331) was reported by each site, and the median rate was 7.3 outbreaks/1 million person-years (range 0.9–44.8). Overall, AGE outbreaks exhibited winter seasonality: 2,972 (68%) of the 4,376 outbreaks occurred during November–April (Figure 2). This trend was driven largely by outbreaks caused by norovirus and by unknown etiologies, of which 1,530 (80% of 1,908 total) and 1,086 (71% of 1,524 total), respectively, occurred during November–April. In contrast, 62% of outbreaks caused by other etiologies, primarily bacteria, occurred during May–October.
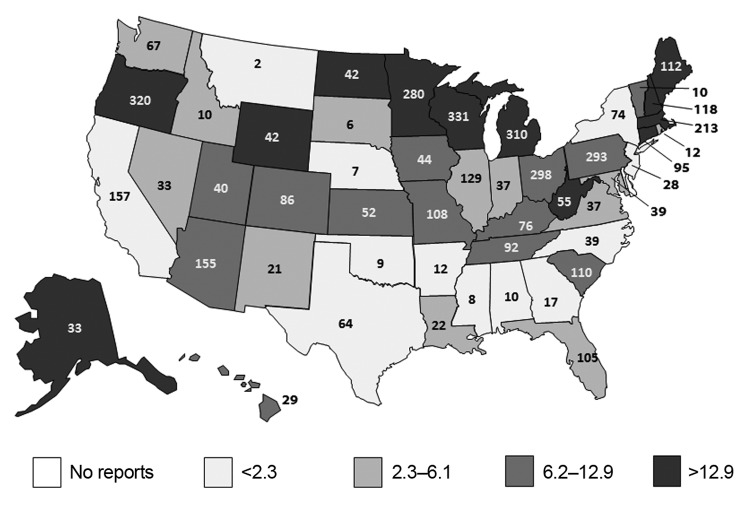
Total number and annual rate of reported acute gastroenteritis outbreaks per 1 million population by reporting state, National Outbreak Reporting System, United States, 2009–2010. The number given in each state indicates the total number of outbreaks over the 2-year study period; the shading denoted by the legend indicates the reporting rate by quartiles. Multistate outbreaks (n = 48) and those reported by Puerto Rico (n = 15) and the District of Columbia (n = 24) are not shown.
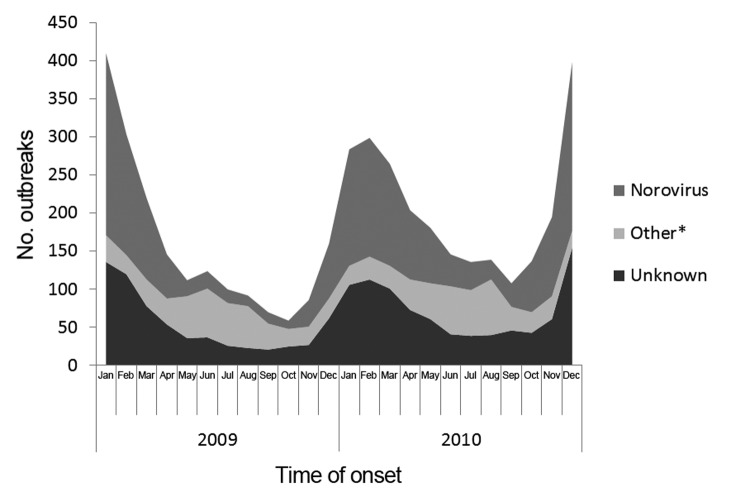
Number of reported acute gastroenteritis outbreaks by month of first illness onset and etiology, National Outbreak Reporting System, United States, 2009–2010. *Includes outbreaks caused by a single etiologic agent other than norovirus or multiple etiologies.
The primary reported mode of transmission in most AGE outbreaks was person to person (2,271 [52%]), followed by foodborne (1,513 [35%]), waterborne (65 [2%]), animal contact (44, 1%), and environmental contamination (9, 0.2%); the transmission mode was unknown in 474 (10%) outbreaks (Table 2). Person-to-person transmission was implicated in most outbreaks caused by norovirus (1,261 [66%]) and Shigella spp. (86 [79%]), whereas foodborne transmission was implicated in most outbreaks caused by Salmonella spp. (254 [72%]) and STEC (64 [63%]). Among the 3,052 (70%) AGE outbreaks for which a single exposure setting was reported, health care facilities, primarily nursing homes, were the most frequent settings (1,499 [49%]), followed by restaurants or banquet facilities (657 [22%]), schools or day-care facilities (290 [10%]), and private residences (227 [7%]). Most norovirus outbreaks (64%) occurred in health care facilities, whereas shigellosis outbreaks (74%) occurred predominantly in schools or day-care facilities. Private residences and restaurants/banquet facilities were the most frequent exposure settings for outbreaks caused by Salmonella spp. (32% and 36%, respectively) and STEC (46% and 20%, respectively).
Table 2
| Outbreak characteristic | No. (%) outbreaks | ||||||
|---|---|---|---|---|---|---|---|
| Norovirus, n = 1,908 | Salmonella spp., n = 355 | Shigella spp., n = 109 | STEC, n = 101 | Other, n = 379† | Unknown, n = 1,524 | Total, N = 4,376 | |
| Primary transmission mode | |||||||
| Person to person | 1,261 (66.1) | 17 (4.8) | 86 (78.9) | 11 (10.9) | 47 (12.4) | 849 (55.7) | 2,271 (51.9) |
| Foodborne | 494 (25.9) | 254 (71.5) | 8 (7.3) | 64 (63.4) | 220 (58.0) | 473 (31.0) | 1,513 (34.6) |
| Waterborne | 4 (0.2) | 0 | 2 (1.8) | 6 (5.9) | 51 (13.5) | 2 (0.1) | 65 (1.5) |
| Animal contact | 0 | 26 (7.3) | 0 | 5 (5.0) | 12 (3.2) | 1 (0.1) | 44 (1.0) |
| Environmental contamination | 5 (0.3) | 2 (0.6) | 1 (0.9) | 0 | 0 | 1 (0.1) | 9 (0.2) |
| Unknown | 144 (7.5) | 56 (15.8) | 12 (11.0) | 15 (14.9) | 49 (12.9) | 198 (13.0) | 474 (10.8) |
| Exposure setting‡ | |||||||
| Health care facility | 932 (48.8) | 5 (1.4) | 0 | 0 | 25 (6.6) | 537 (35.2) | 1,499 (34.3) |
| Restaurant or banquet facility | 287 (15.0) | 69 (19.4) | 5 (4.6) | 12 (11.9) | 77 (20.3) | 207 (13.6) | 657 (15.0) |
| School or day-care facility | 98 (5.1) | 14 (3.9) | 50 (45.9) | 6 (5.9) | 15 (4.0) | 107 (7.0) | 290 (6.6) |
| Private residence | 31 (1.6) | 62 (17.5) | 4 (3.7) | 28 (27.7) | 60 (15.8) | 42 (2.8) | 227 (5.2) |
| Other single setting | 114 (6.0) | 42 (11.8) | 9 (8.3) | 15 (14.9) | 101 (26.6) | 98 (6.4) | 379 (8.7) |
| Multiple | 33 (1.7) | 19 (5.4) | 13 (11.9) | 10 (9.9) | 10 (2.6) | 21 (1.4) | 106 (2.4) |
| Not reported | 264 (13.8) | 86 (24.2) | 15 (13.8) | 15 (14.9) | 42 (11.8) | 313 (20.5) | 735 (16.8) |
| Not collected§ | 149 (7.8) | 58 (16.3) | 13 (11.9) | 15 (14.9) | 49 (12.9) | 199 (13.1) | 483 (11.0) |
*Data include both suspected and confirmed etiologies. STEC, Shiga toxin-producing Escherichia coli.
†Includes outbreaks caused by a single etiologic agent other than norovirus, Salmonella spp., Shigella spp., and STEC or by multiple etiologic agents, as listed in Table 1.
‡Data on specific settings are restricted to outbreaks with a single exposure setting; for foodborne outbreaks, setting refers to the setting where implicated food was consumed.
§The setting was systematically not collected for outbreaks caused by environmental contamination or unknown transmission mode.
Conclusions
As the national surveillance system for US AGE outbreaks, NORS provides valuable insights into the epidemiology of the pathogens most often involved. Building upon previous surveillance systems and analyses focused on specific transmission modes (7–10), NORS provides a more complete characterization of AGE outbreaks, particularly the relative importance of specific transmission modes and settings for the key pathogens. This analysis highlights norovirus as not only the leading cause of reported AGE outbreaks but also the leading cause of AGE outbreak–associated hospitalizations and deaths. Although norovirus usually causes self-limiting disease, it can cause severe outcomes when outbreaks occur among vulnerable populations, such as nursing-home residents (11). Salmonella spp., Shigella spp., and STEC are also key contributors to AGE outbreaks. Expanded surveillance through NORS revealed that 28%, 91%, and 31%, respectively, of outbreaks caused by these 3 bacteria result from routes other than contaminated food or water. In addition, NORS provides information on non-AGE outbreaks transmitted by food or water. For example, ≈25% of waterborne disease outbreaks are caused by Legionella spp. (8,9), and among foodborne disease outbreaks, listeriosis is a major cause of outbreak-related hospitalizations and deaths (10).
As a passive reporting system, NORS is subject to variability in reporting practices between states and among outbreaks associated with different transmission modes and exposure settings. Reporting rates and data completeness may be improved through ongoing NORS enhancements, including direct data upload functionality and all-mode collection of setting information. For 35% of outbreaks, no suspected or confirmed etiology was identified, primarily because diagnostic specimens were not collected. However, outbreaks of unknown etiology exhibited similar temporal trends and epidemiologic characteristics as norovirus outbreaks, suggesting that many of these may have been caused by norovirus. NORS does not include AGE outbreaks on international cruise ships; however, if combined with the outbreaks reported to NORS, these cruise-ship outbreaks would represent <1% of all reported outbreaks attributed to norovirus and all-cause AGE (12).
Although a small minority of AGE cases in the United States are associated with reported outbreaks (≈1 in 3,000), outbreak surveillance provides unique insights that can inform prevention efforts. Norovirus control through hand hygiene, environmental disinfection, and isolation of ill persons should remain a priority and likely affords protection against other AGE agents (13). Ongoing surveillance through NORS will help further elucidate trends, identify gaps, and assess the effects of future interventions on reducing epidemic gastroenteritis.
Acknowledgments
We gratefully acknowledge state, local, and territorial health departments for contributing data to NORS for this analysis. We also thank Karen Herman, Amie Nisler, Kelly Walsh, Elizabeth Mungai, and Shacara Johnson for assistance in administration of NORS and Rickey Zachary, Franky Maslim, and Don Wade for information technology support.
Biography
Dr Hall is an epidemiologist with the Viral Gastroenteritis Team in the Division of Viral Diseases of the National Center for Immunization and Respiratory Diseases, CDC. His research interests focus on all aspects of the epidemiology of noroviruses and other agents of viral gastroenteritis.
Footnotes
Suggested citation for this article: Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg Infect Dis. 2013 Aug [date cited]. http://dx.doi.org/10.3201/eid1908.130482
References
Articles from Emerging Infectious Diseases are provided here courtesy of Centers for Disease Control and Prevention
Full text links
Read article at publisher's site: https://doi.org/10.3201/eid1908.130482
Read article for free, from open access legal sources, via Unpaywall:
https://wwwnc.cdc.gov/eid/article/19/8/pdfs/13-0482.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3201/eid1908.130482
Article citations
Measures for preventing norovirus outbreaks on campus in economically underdeveloped areas and countries: evidence from rural areas in Western China.
Front Public Health, 12:1406133, 04 Jun 2024
Cited by: 0 articles | PMID: 38894991 | PMCID: PMC11183813
Sapovirus: an emerging pathogen in kidney transplant recipients?
Infection, 52(5):1831-1838, 09 Apr 2024
Cited by: 1 article | PMID: 38592660 | PMCID: PMC11499398
Simultaneous detection of human norovirus GI, GII and SARS-CoV-2 by a quantitative one-step triplex RT-qPCR.
Front Microbiol, 14:1269275, 08 Jan 2024
Cited by: 1 article | PMID: 38260899 | PMCID: PMC10800780
Common and Potential Emerging Foodborne Viruses: A Comprehensive Review.
Life (Basel), 14(2):190, 28 Jan 2024
Cited by: 2 articles | PMID: 38398699 | PMCID: PMC10890126
Review Free full text in Europe PMC
Multicenter evaluation of BioCode GPP for syndromic molecular detection of gastrointestinal pathogens from stool specimens.
J Clin Microbiol, 62(3):e0154523, 08 Feb 2024
Cited by: 2 articles | PMID: 38329337
Go to all (119) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Outbreaks of acute gastroenteritis transmitted by person-to-person contact--United States, 2009-2010.
MMWR Surveill Summ, 61(9):1-12, 01 Dec 2012
Cited by: 66 articles | PMID: 23235338
Epidemiology of Norovirus Outbreaks Reported to the Public Health Emergency Event Surveillance System, China, 2014⁻2017.
Viruses, 11(4):E342, 11 Apr 2019
Cited by: 25 articles | PMID: 30979014 | PMCID: PMC6520956
Norovirus: a growing cause of gastroenteritis in catalonia (Spain)?
J Food Prot, 76(10):1810-1816, 01 Oct 2013
Cited by: 4 articles | PMID: 24112586
Noroviruses: agents in outbreaks of acute gastroenteritis.
Disaster Manag Response, 2(1):4-9, 01 Jan 2004
Cited by: 18 articles | PMID: 14760287
Review






