Abstract
Purpose
New evidence is available regarding the utility of the 21-gene recurrence score assay in guiding chemotherapy use for node-negative, estrogen receptor-positive breast cancer. We applied this evidence in a decision-analytic model to re-evaluate the cost-effectiveness of the assay.Methods
We cross-classified patients by clinicopathologic characteristics from the Adjuvant! risk index and by recurrence score risk group. For non-recurrence score-guided treatment, we assumed patients receiving hormonal therapy alone had low-risk characteristics and patients receiving chemotherapy and hormonal therapy had higher-risk characteristics. For recurrence score-guided treatment, we assigned chemotherapy probabilities conditional on recurrence score risk group and clinicopathologic characteristics.Results
An estimated 40.4% of patients in the recurrence score-guided strategy and 47.3% in the non-recurrence score-guided strategy were expected to receive chemotherapy. The incremental gain in quality-adjusted life-years was 0.16 (95% confidence interval, 0.08-0.28) with the recurrence score-guided strategy. Lifetime medical costs to the health system were $2,692 ($1,546-$3,821) higher with the recurrence score-guided strategy, for an incremental cost-effectiveness ratio of $16,677/quality-adjusted life-year ($7,613-$37,219). From a societal perspective, the incremental cost-effectiveness was $10,788/quality-adjusted life-year ($6,840-$30,265).Conclusion
The findings provide supportive evidence for the economic value of the 21-gene recurrence score assay in node-negative, estrogen receptor-positive breast cancer.Free full text

Cost-Effectiveness of the 21-Gene Recurrence Score Assay in the Context of Multifactorial Decision Making to Guide Chemotherapy for Early-Stage Breast Cancer
Abstract
Purpose
New evidence is available regarding the utility of the 21-gene Recurrence Score (RS) assay in guiding chemotherapy use for node-negative, estrogen receptor–positive breast cancer. We applied this evidence in a decision-analytic model to reevaluate the cost-effectiveness of the assay.
Methods
We cross-classified patients by clinicopathologic characteristics from the Adjuvant! risk index and by RS risk group. For non–RS-guided treatment, we assumed patients receiving hormonal therapy alone had low-risk characteristics and patients receiving chemotherapy and hormonal therapy had higher-risk characteristics. For RS-guided treatment, we assigned chemotherapy probabilities conditional on RS risk group and clinicopathologic characteristics.
Results
An estimated 40.4% of patients in the RS-guided strategy and 47.3% in the non–RS-guided strategy were expected to receive chemotherapy. The incremental gain in quality-adjusted life-years (QALYs) was 0.16 (95% CI, 0.08 to 0.28) with the RS-guided strategy. Lifetime medical costs to the health system were $2692 ($1546 to $3821) higher with the RS-guided strategy, for an incremental cost-effectiveness ratio of $16,677/QALY ($7613 to $37,219). From a societal perspective, the incremental cost-effectiveness was $10,788/QALY ($6840 to $30,265).
Conclusion
The findings provide supportive evidence for the economic value of the 21-gene RS assay in node-negative, estrogen receptor–positive breast cancer.
INTRODUCTION
The Oncotype DX 21-gene Recurrence Score (RS) assay (Genomic Health, Inc; Redwood City, California, USA) is the most widely used gene signature for guiding the treatment of patients with early-stage, estrogen receptor–positive breast cancer. Using data from 2 major clinical trials—National Surgical Adjuvant Breast and Bowel Project (NSABP) trials B-14 and B-201,2—the RS assay has been validated as a method for distinguishing among patients with higher and lower risks of distant recurrence,3–5 and it has predictive validity in identifying which patients will benefit most from chemotherapy.4,5
Previous economic evaluations predicted cost savings with the use of gene expression assays in early-stage breast cancer.6–8 These studies relied on normative assumptions about the use of chemotherapy on the basis of the genetic assay results. The researchers assumed that patients at low risk of recurrence (according to assay results) would forgo chemotherapy, whereas all other patients would receive chemotherapy regardless of their other clinical and tumor risk factors. Comparison groups representing “standard practice” in these studies varied. In some studies, all patients were assumed to receive chemotherapy or tamoxifen7; in others, the proportions of patients receiving chemotherapy were based on older guidelines that recommended chemotherapy for more than 90% of patients.6,8 Since the publication of these studies, use of gene expression profiling in early-stage breast cancer has expanded to include one-third of eligible patients at some centers.9 Nevertheless, concerns persist about the cost-effectiveness of the RS assay,10,11 considering that physicians routinely personalize recommendations for chemotherapy according to the patient’s pathological and clinical characteristics and independently account for these factors when results of gene expression profiling are available.
Findings from 2 recent studies provide an opportunity to reexamine the cost-effectiveness of the RS assay. In one study, Lo et al12 reported a prospective, multisite study designed to evaluate treatment recommendations before and after receipt of results from the RS assay. Incorporating the results of this study in a cost-effectiveness model is important to approximate expected costs and outcomes in a real-world setting where recommendations for chemotherapy depend not only on RS assay results, but also on other clinical and pathological risk factors (eg, pre-assay recommendations). In the other study, Tang et al5 analyzed patient-level data from NSABP trials B-14 and B-20 to compare the prognostic and predictive validity of the RS assay and Adjuvant!, a decision aid that incorporates information on patients’ clinical and tumor characteristics, such as age, tumor size, node involvement, and hormone and human epidermal growth factor receptor (HER-2) status, with regard to distant recurrence. Both the RS assay and Adjuvant! were strong prognostic indicators of distant recurrence; however, only the RS assay was a significant predictor of benefit from chemotherapy.
Although many physicians do not use Adjuvant! to guide treatment recommendations, the tool incorporates many of the same clinicopathologic factors that are most influential in treatment recommendations13 and broadly agrees with recommendations from multidisciplinary teams.14 Therefore, we sought to incorporate new evidence from Lo et al12 and Tang et al5 to reevaluate the cost-effectiveness of a strategy in which RS assay results are available along with other clinicopathologic characteristics (ie, the RS-guided strategy) compared with a strategy limited to clinicopathologic characteristics (ie, the non–RS-guided strategy) to guide the use of chemotherapy for node-negative, estrogen receptor–positive breast cancer in the United States.
MATERIALS AND METHODS
Model Structure
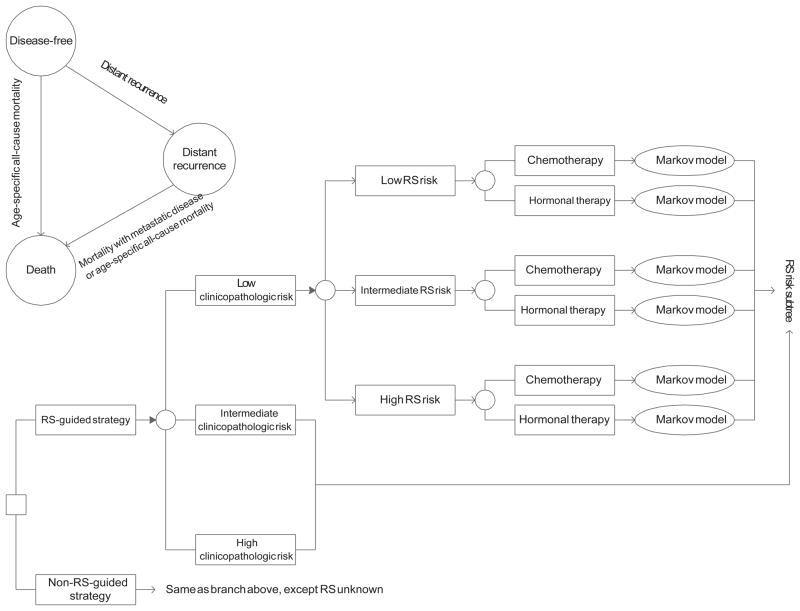
We developed a decision-analytic model to estimate costs, survival, and quality-adjusted survival for RS-guided and non–RS-guided strategies. The model categorized patients according to the clinicopathologic characteristics in the Adjuvant! risk index using cut points reported by Tang et al5 (ie, low risk, ≤ 5.5; intermediate risk, > 5.5 and ≤ 11.9; and high risk, > 11.9; Figure). Using conditional probabilities, we further stratified patients according to previously defined RS risk groups to allow for a fair comparison by ensuring that underlying risk profiles with both treatment strategies were the same.3,4 The impact of the RS-guided strategy was to selectively guide the use of chemotherapy beyond the risk information conveyed by clinicopathologic characteristics. Thus, only the probabilities corresponding to chemotherapy and hormonal therapy differed between the strategies (Table 1).
TABLE 1
Model Parameters in the Base-Case Analysis
| Parameter | Mean (SE) | Source |
|---|---|---|
| Proportion of patients by clinicopathologic risk groupa | Tang et al5 | |
 Low Low | 0.527 (0.020) | |
 Intermediate Intermediate | 0.186 (0.007) | |
 High High | 0.287 (0.011) | |
| Proportion of patients by RS risk group and clinicopathologic risk groupa | ||
 Low clinicopathologic risk Low clinicopathologic risk | Tang et al5 | |
  RS low risk RS low risk | 0.614 (0.033) | |
  RS intermediate risk RS intermediate risk | 0.239 (0.013) | |
  RS high risk RS high risk | 0.148 (0.008) | |
 Intermediate clinicopathologic riska Intermediate clinicopathologic riska | Tang et al5 | |
  RS low risk RS low risk | 0.460 (0.041) | |
  RS intermediate risk RS intermediate risk | 0.194 (0.017) | |
  RS high risk RS high risk | 0.347 (0.031) | |
 High clinicopathologic riska High clinicopathologic riska | Tang et al5 | |
  RS low risk RS low risk | 0.339 (0.024) | |
  RS intermediate risk RS intermediate risk | 0.214 (0.015) | |
  RS high risk RS high risk | 0.448 (0.032) | |
| Proportion of patients receiving chemotherapy by clinicopathologic risk groupa | assumption | |
 Low clinicopathologic risk Low clinicopathologic risk | 0 | |
 Intermediate clinicopathologic risk Intermediate clinicopathologic risk | 1.0 | |
 High clinicopathologic risk High clinicopathologic risk | 1.0 | |
| Proportion of patients receiving chemotherapy by RS risk group and clinicopathologic risk groupa,b | ||
 Low clinicopathologic riskc Low clinicopathologic riskc | Lo et al12 | |
  RS low risk RS low risk | 0.045 (0.044) | |
  RS intermediate risk RS intermediate risk | 0.095 (0.064) | |
  RS high risk RS high risk | 1.0 (0) | |
 Intermediate or high clinicopathologic riskd Intermediate or high clinicopathologic riskd | Lo et al12 | |
  RS low risk RS low risk | 0.250 (0.108) | |
  RS intermediate risk RS intermediate risk | 0.619 (0.106) | |
  RS high risk RS high risk | 1.0 (0) | |
| 10-year distant recurrence-free with hormone therapy | Paik et al4 | |
  RS low risk RS low risk | 0.968 (0.016) | |
  RS intermediate risk RS intermediate risk | 0.909 (0.043) | |
  RS high risk RS high risk | 0.605 (0.073) | |
| Relative risk with chemotherapy on distant recurrence | Paik et al4 | |
 RS low risk RS low risk | 1.31 (0.57e) | |
 RS intermediate risk RS intermediate risk | 0.61 (0.56e) | |
 RS high risk RS high risk | 0.26 (0.31e) | |
| 5-year mortality after distant recurrence | 0.766 (0.01) | SEER Cancer Statistics Review15 |
| Discount rate for costs and QALYs | 3% per year | US Public Health Service Panel39 |
| Health state utilities | Schleinitz et al23 | |
 Chemotherapy in the first year Chemotherapy in the first year | 0.48 (0.06) | |
 Hormonal therapy Hormonal therapy | 0.68 (0.06) | |
 Remission Remission | 0.68 (0.06) | |
 Distant recurrence Distant recurrence | 0.42 (0.06) | |
| Direct medical costs, $ | ||
 21-Gene Recurrence Score Assay 21-Gene Recurrence Score Assay | 4075 | Genomic Health 2010 Annual Report |
 Chemotherapy, first year Chemotherapy, first year | 16,947a (1655) | Oestreicher et al17 |
 Hormonal therapy, annually for 5 years Hormonal therapy, annually for 5 years | 105 | Tamoxifen 10 mg, Drugstore.com, July 2011 |
 Monitoring and follow-up during remission, annually for up to 10 years Monitoring and follow-up during remission, annually for up to 10 years | 1108f (61) | Hensley et al18 |
 Distant recurrence, one-time costf Distant recurrence, one-time costf | 17,478f (2444) | Stokes et al19 |
| Indirect costs, $ | ||
 Absence from work attributable to chemotherapy Absence from work attributable to chemotherapy | 12,686 | Drolet et al20; Bureau of Labor Statistics, National Compensation Survey |
 Patient time during last year of life with metastatic breast cancer Patient time during last year of life with metastatic breast cancer | 3902 | Yabroff et al21; Bureau of Labor Statistics, National Compensation Survey |
Abbreviations: QALY, quality-adjusted life-year; and RS, 21-Gene Recurrence Score Assay.
In the base-case analysis for the non–RS-guided strategy, we assumed that patients categorized as being at intermediate or high risk according to clinicopathologic characteristics (ie, Adjuvant!) would receive chemotherapy followed by hormonal therapy and patients with low-risk clinicopathologic characteristics would receive hormonal therapy alone. For the RS-guided strategy, we incorporated evidence from Lo et al12 indicating that a physician’s recommendation for chemotherapy depends on both the patient’s RS risk group and whether the physician had recommended chemotherapy based on clinicopathologic characteristics before receiving RS assay results. The treatment effect of adjuvant chemotherapy on distant recurrence was conditional on the RS risk classification.4
After stratification by risk and treatment, hypothetical patients cycled through a Markov model representing the incidence of distant recurrence, death from breast cancer, and death from other causes (Figure).3 The cycle length was 6 months. Progression from distant recurrence to death was based on data from a Surveillance, Epidemiology and End Results (SEER) cohort.15 We modeled the probability of death not attributable to breast cancer using age-specific annual mortality rates for women in the United States.16 In the base-case analysis, we assumed that patients were aged 55 years at the time of diagnosis.12
Costs and Utility Weights
We performed the analysis both from the US health system perspective inclusive of all direct medical costs and from the societal perspective inclusive of all direct medical costs plus patients’ time costs.
We assigned costs attributable to chemotherapy to the first cycle in the Markov model, and we assigned biannual costs of hormonal therapy beginning in the third 6-month cycle (Table 1).17 For hormonal therapy, we assigned costs for tamoxifen across 5 years. We assigned medical costs associated with monitoring and follow-up for up to 10 years or until the diagnosis of distant recurrence.18 Upon the development of distant recurrence, we assigned attributable costs estimated from SEER-Medicare data.19
We calculated patient time costs associated with chemotherapy from a study of the cumulative time lost from work over 3 years among women who received adjuvant chemotherapy (9.5 months) compared with women who did not (5.4 months).20 Time associated with distant recurrence was based on the time that patients with breast cancer spent in their last year of life receiving medical care.21 We valued patient time on the basis of wage rates for US civilian workers.22
To account for differential health-related quality of life, we assigned utility weights reported by Schleinitz et al,23 representing different stages of and treatments for breast cancer.
Sensitivity Analyses
To perform a probabilistic sensitivity analysis, we assigned distributions to model parameters to represent the uncertainty associated with the point estimates. As recommended for modeling second-order uncertainty, we used Dirichlet distributions to model multinomial parameters, beta distributions to model probabilities and utility weights, and normal distributions to model costs and log-transformed relative risks.24 We applied a Monte Carlo simulation to generate 1000 runs and identified the 25th and 975th ranks as the corresponding 95% confidence intervals (CIs).
We also performed 1-way sensitivity analyses. We varied the age at diagnosis and evaluated the impact of changes to assumptions regarding treatment decisions and the target population. We also extended the time period during which patients were at risk for recurrence, varied the discount rate, applied utility weights from other sources,25,26 applied wages of government workers to value patient time,22 doubled the cost assigned for chemotherapy, assigned costs for aromatase inhibitors instead of tamoxifen, and applied higher costs for distant recurrence.19,27
RESULTS
For an estimated 27.9% of patients, treatment recommendations changed after the incorporation of RS information. An estimated 40.4% of patients in the RS-guided strategy and 47.3% in the non–RS-guided strategy were expected to receive adjuvant chemotherapy, a 15% relative reduction (Table 2). During the first year, total direct medical costs in the RS-guided strategy were an estimated $11,632, compared with $8735 in the non–RS-guided strategy, a $2897 increase.
TABLE 2
Treatment Probabilities Cross-Classified by RS-Guided and Non–RS-Guided Strategies
| Non–RS-Guided Strategy | RS-Guided Strategy | ||
|---|---|---|---|
| Hormonal Therapy | Chemotherapy | Total | |
| Hormonal therapy, % | 42.2 | 10.5 | 52.7 |
| Chemotherapy, % | 17.4 | 29.9 | 47.3 |
| Total, % | 59.6 | 40.4 | 100.0 |
Abbreviation: RS, Recurrence Score.
Estimated rates of recurrence at 10 years were 6.8% with the RS-guided strategy and 8.9% with the non-RS guided strategy. Targeted use of chemotherapy in the RS-guided strategy was associated with expected gains of 0.19 life-years and 0.16 quality-adjusted life-years (QALYs) (Table 3). From a health system perspective, lifetime direct medical costs were an estimated $2692 higher with the RS-guided strategy, resulting in incremental cost-effectiveness ratios (ICERs) of $14,059 per life-year saved (95% CI, $6840–$28,912) and $16,677 per QALY (95% CI, $7613–$37,219). From a societal perspective that incorporated lower patient time costs of $950 per patient, the ICERs were $9095 per life-year saved (95% CI, dominant-$23,397) and $10,788 per QALY (95% CI, $6840–$30,265). Probabilistic sensitivity analysis indicated that more than 99% of the ICERs generated in Monte Carlo simulations were less than $50,000 per life-year saved and per QALY (Supplemental Figure), consistent with the corresponding 95% CIs.
TABLE 3
Results of the Base-Case Analysis
| Variable | RS-Guided Strategy | Non–RS-Guided Strategy | Difference |
|---|---|---|---|
| Life-years, discounted (95% CI) | 15.02 (14.66 to 15.24) | 14.82 (14.46 to 15.07) | 0.19 (0.09 to 0.32) |
| Quality-adjusted life-years, discounted, (95% CI) | 10.09 (8.240 to 11.79) | 9.93 (8.12 to 11.60) | 0.16 (0.08 to 0.28) |
| Direct costs, discounted (95% CI), $ | 21,090 (19,306 to 23,139) | 18,398 (16,535 to 20,448) | 2692 (1546 to 3821) |
| Indirect costs, discounted (95% CI), $ | 5307 (4615 to 6178) | 6257 (5794 to 6745) | −950 (−1732 to −111) |
| Total costs, discounted (95% CI), $ | 26,397 (24,073 to 28,957) | 24,656 (22,599 to 26,887) | 1741 (−85 to 3710) |
Abbreviations: CI, confidence interval; RS, Recurrence Score.
In sensitivity analyses, the results were relatively unaffected by changes in individual model assumptions and inputs (Table 4). Changes to assumptions necessary to apply the findings from Lo et al9 on the use of chemotherapy had little impact. However, when we limited the target population to patients with intermediate- or high-risk clinical characteristics, 100% of patients in the non–RS-guided strategy were assumed to receive chemotherapy. In this scenario, there was a 37% absolute reduction in the use of chemotherapy, resulting in lower direct medical costs with the RS-guided strategy as compared with the non–RS-guided strategy ($24,857 vs $27,121). When we assumed that all patients across clinicopathologic risk groups in the non–RS-guided strategy would receive chemotherapy, expected savings with the RS-guided strategy were $5945 from the health system perspective and $7526 per patient from the societal perspective.
TABLE 4
Results of Sensitivity Analyses
| Scenario | Differencea | Cost per QALY, $ | |||
|---|---|---|---|---|---|
| Direct Medical Costs | Indirect Costs | QALYs | Health System Perspective | Societal Perspective | |
| Base-case analysis | $2692 | $−950 | 0.161 | $16,677 | $10,788 |
| Use of chemotherapy | |||||
 ”Equipoise” in Lo et al9 represents hormonal therapy instead of chemotherapy ”Equipoise” in Lo et al9 represents hormonal therapy instead of chemotherapy | $2225 | $−1300 | 0.167 | $13,288 | $5522 |
 Treatment probabilities in RS-guided strategy are not conditional on initial recommendations from Lo et al12,b Treatment probabilities in RS-guided strategy are not conditional on initial recommendations from Lo et al12,b | $2919 | $−779 | 0.194 | $18,220 | $13,361 |
 All patients in non–RS-guided strategy receive chemotherapy All patients in non–RS-guided strategy receive chemotherapy | $−5945 | $−7526 | 0.098 | Economically dominant | Economically dominant |
 50% of patients with intermediate-risk clinicopathologic characteristics receive chemotherapy in non–RS-guided strategy 50% of patients with intermediate-risk clinicopathologic characteristics receive chemotherapy in non–RS-guided strategy | $4061 | $191 | 0.208 | $19,566 | $20,485 |
| Target population and use of chemotherapy | |||||
 Target population restricted to patients with low- or intermediate-risk clinicopathologic characteristicsc Target population restricted to patients with low- or intermediate-risk clinicopathologic characteristicsc | $2017 | $−1460 | 0.163 | $12,381 | $3423 |
 Target population restricted to patients with intermediate- or high-risk clinicopathologic characteristicsd Target population restricted to patients with intermediate- or high-risk clinicopathologic characteristicsd | $−2264 | −4758 | 0.067 | Economically dominant | Economically dominant |
| Utility weights | |||||
 Utility weights from Lidgren et al26,e Utility weights from Lidgren et al26,e | $2692 | $−950 | 0.166 | $16,177 | $10,465 |
 Community utility weights from Peasgood et al25,f Community utility weights from Peasgood et al25,f | $2692 | $−950 | 0.161 | $16,757 | $10,840 |
 Patient utility weights from Peasgood et al25,g Patient utility weights from Peasgood et al25,g | $2692 | $−950 | 0.198 | $13,570 | $8778 |
| Cost estimates | |||||
 Double attributable cost of chemotherapy to $33,893 Double attributable cost of chemotherapy to $33,893 | $1516 | $−950 | 0.161 | $9,391 | $3502 |
 Wages for state/local government workers instead of civilian workers Wages for state/local government workers instead of civilian workers | $2692 | $−1193 | 0.161 | $16,677 | $9285 |
 Annual cost of letrozole ($5904) instead of tamoxifen for hormonal therapy Annual cost of letrozole ($5904) instead of tamoxifen for hormonal therapy | $2946 | $−950 | 0.161 | $18,250 | $12,361 |
 Cumulative 10-year cost estimates from Stokes et al19 ($68,559) instead of attributable costs for distant recurrence Cumulative 10-year cost estimates from Stokes et al19 ($68,559) instead of attributable costs for distant recurrence | $1757 | $−950 | 0.161 | $10,889 | $5001 |
 Estimated cost of recurrence from Hornberger et al6 ($104,000) Estimated cost of recurrence from Hornberger et al6 ($104,000) | $1109 | $−950 | 0.161 | $6873 | $985 |
  Triple valuation of patient time spent receiving medical care for treatment of distant recurrence Triple valuation of patient time spent receiving medical care for treatment of distant recurrence | $2692 | $−1091 | 0.161 | $16,677 | $9920 |
| Other assumptions | |||||
 Age 45 years at diagnosis instead of 55 years in base-case Age 45 years at diagnosis instead of 55 years in base-case | $2685 | $−954 | 0.214 | $12,541 | $8084 |
 Age 65 years at diagnosis instead of 55 years in base-case Age 65 years at diagnosis instead of 55 years in base-case | $2,709 | $−941 | 0.107 | $25,352 | $16,548 |
 Duration of 15 years at risk for distant recurrence and monitoring costs instead of 10 years Duration of 15 years at risk for distant recurrence and monitoring costs instead of 10 years | $2707 | $−964 | 0.182 | $14,841 | $9558 |
 Reduce monitoring from 10 years to 5 years Reduce monitoring from 10 years to 5 years | $2620 | $−950 | 0.161 | $16,231 | $10,343 |
 Apply upper limit of 95% CI for recurrence for RS low risk and lower limits of 95% CI for recurrence for RS intermediate risk and RS high risk Apply upper limit of 95% CI for recurrence for RS low risk and lower limits of 95% CI for recurrence for RS intermediate risk and RS high risk | $2749 | $−930 | 0.118 | $23,321 | $15,430 |
 Discount rate of 5% per year instead of 3% Discount rate of 5% per year instead of 3% | $2703 | $−928 | 0.126 | $21,443 | $14,079 |
Abbreviation: QALY, quality-adjusted life-year.
As expected, assigning higher costs to chemotherapy and distant recurrence improved the cost-effectiveness of the RS-guided strategy. To reach cost-neutrality, costs associated with distant recurrence would have to approximate $165,000 per case from the health system perspective or $113,000 from the societal perspective.
DISCUSSION
Our findings provide evidence regarding the economic value of the 21-gene RS assay in the setting of estrogen receptor–positive, node-negative breast cancer. In the base-case analysis, an estimated 47.3% of patients in the non–RS-guided strategy and 40.4% in the RS-guided strategy would receive adjuvant chemotherapy. This 15% reduction generated approximately $1200 in savings in direct medical costs, which offset approximately one-quarter of the cost of the RS assay ($4075), a net increase of approximately $2900. From the patient perspective, indirect costs were approximately $950 lower with the RS-guided strategy. When we combined estimated cost increases with expected gains in quality-adjusted survival with the RS-guided strategy, the incremental cost-effectiveness was approximately $17,000 per QALY from the health system perspective and $11,000 per QALY from the societal perspective.
When we modeled the non–RS-guided strategy in the base-case analysis, we assumed that none of the patients with low-risk clinicopathologic characteristics would receive chemotherapy, consistent with recommendations for patients with Adjuvant! risk index ≤ 5.5. This assumption favored the non–RS-guided strategy on two accounts. First the availability of RS information in the RS-guided strategy could only increase (from zero) the proportion of patients receiving chemotherapy. Second, among the low RS risk group, the receipt of chemotherapy led to lower quality-adjusted survival because of the greater hazard of distant recurrence reported for this risk group.4 An equally important assumption for the non–RS-guided strategy was that all patients with intermediate- or high-risk clinicopathologic characteristics (ie, Adjuvant! risk index > 5.5) would receive chemotherapy. Thus, the addition of RS information could only lead to a reduction in the use of chemotherapy for these patients, improving the cost-effectiveness of the RS-guided strategy through lower chemotherapy costs.
The extent to which these 2 countervailing effects changed the overall number of patients receiving chemotherapy was a function of the distribution of patients across risk groups. Thus, an important consideration is whether patients studied by Tang et al5 are representative of patients who receive the RS assay in practice. The proportions of patients with a low RS in NSABP B-14 (51%) and in observational studies are similar (Supplemental Table).12,27–32 However, approximately one-quarter of patients in NSABP B-14 had a high-risk RS, a larger representation as compared with patients receiving the RS assay in practice (Supplemental Table).12,27–32 This finding may be attributable to physicians having less uncertainty about the use of chemotherapy when a patient presents with several high-risk characteristics. Nevertheless, our sensitivity analyses revealed that reducing the proportion of patients in the high-risk RS group had relatively little impact on estimates of cost-effectiveness.
The study by Lo et al12 allowed us to model treatment recommendations in the setting of knowledge about clinicopathologic risk characteristics alone and with the addition of genetic risk information. Thus, we believe the analysis is representative of real-world decision making. Observational studies have shown that treatment recommendations based on clinical judgment (ie, before RS information) influence treatment recommendations after RS information is provided.12,28,30 For example, among patients with intermediate RS, chemotherapy was recommended for 10% when the initial recommendation was for hormonal therapy, compared to 62% when the initial recommendation was for chemotherapy.12
Although our assumption that patients with low-risk clinicopathologic characteristics forgo chemotherapy while patients at higher risk receive chemotherapy in the non–RS-guided strategy is open to argument, our overall estimate of chemotherapy in 47.3% of patients is similar to rates reported for patients without RS information (eg, 48.3%,12 46.7%,28 48.5%30). Nevertheless, it is important to acknowledge the influence of assumptions about the use of chemotherapy in the non–RS-guided strategy. When we assumed universal chemotherapy with the non–RS-guided strategy, use of the RS assay led to expected savings of $6000 per patient with gains in quality-adjusted survival. When we assumed that only 50% of patients in the low-risk group would receive chemotherapy with the non–RS-guided strategy, the RS assay led to cost savings of more than $2200 per patient with gains in quality-adjusted survival. The ideal data source would have provided treatment recommendations stratified on the basis of Adjuvant! before and after the availability of RS information. However, we identified only one such study of 29 patients.33 Other studies that examined cross-classification by Adjuvant! and RS did not provide information on treatment recommendations before the receipt of RS information.31,32
Early economic evaluations of the RS assay reported cost savings and gains in QALYs.6–8 These studies projected greater cost savings with the RS assay than did our study because of assumptions that the use of chemotherapy without RS risk information (ie, standard care) ranged from 92% to 100% and that the use of chemotherapy was entirely governed according to RS risk categories with 0% use in RS low risk groups and 100% use in RS intermediate- and RS high-risk groups.6,7 These studies also did not model the differential treatment effects of chemotherapy across RS risk groups that were later reported by Paik et al.4
More recent studies portraying real-world decision making had more variable results.27,29,34 Our findings are consistent with a study in Israel ($10,770 per QALY)29 and a study in Japan ($3848 per QALY)35 but more optimistic than a study in Canada ($63,000 per QALY in 2008 Canadian dollars),34 though differences in costs and practice patterns limit the validity of cross-country comparisons.36 Our findings are less optimistic than those from a study in a US managed care population in which cost savings and QALY gains were reported.27 In that analysis, 50% to 60% of patients were expected to receive chemotherapy without RS information. With a 27% reduction in chemotherapy with the RS assay, chemotherapy was expected in about 37%, 40%, and 44% in the low-, intermediate, and high-risk RS groups, respectively. In our model for the RS-guided strategy, chemotherapy was used in 12%, 32%, and 100% of the respective RS risk groups, rates that appear to be consistent with observational studies (Supplemental Table).30–32,37
Another methodological difference is the cost of distant recurrence. Whereas the managed care analysis assigned a cost of $104,000 per case,27 we applied an estimate of approximately $17,500, the difference in 10-year discounted costs between patients who experienced distant recurrence compared with patients who did not.19 Although this cost estimate may appear to be low, the estimate is net of background medical costs and was developed specifically for application in cost-effectiveness analyses.38 The estimate is not ideal, because it relied on 1991–2002 SEER-Medicare data and may not reflect current treatment patterns. However, our threshold analyses showed that costs associated with distant recurrence would have to surpass $165,000 to offset the cost of the RS assay.
Other limitations may also influence the real-world cost-effectiveness of the RS assay. First, patients often do not follow their physicians’ treatment recommendations.12 Also, much of the variability in the results across probabilistic sensitivity analyses stemmed from the wide confidence intervals representing the impact of chemotherapy on distant recurrence in the low RS and intermediate RS groups.4 Results from the Trial Assigning Individualized Options for Treatment (TAILORx) will provide more precise estimates of treatment effect for patients with intermediate RS. In addition, the model does not allow for direct variation of measures of the assay’s diagnostic accuracy, such as positive predictive value. However, the model allows for representations of both accurate and inaccurate predictions of recurrence.
In conclusion, we estimate that use of the RS assay will reduce the use of chemotherapy from 47.3% to 40.4%. Although this reduction is conservative, targeted use of chemotherapy with the RS assay is associated with cost-effectiveness ratios of approximately $17,000 per QALY from the health system perspective and $11,000 per QALY from the societal perspective, both well below commonly cited thresholds of $50,000 to $100,000 per QALY used for gauging the cost-effectiveness of health technologies.
Acknowledgments
Funding/Support: This work was supported by a research agreement between Genomic Health, Inc, and Duke University.
Footnotes
Additional Contributions: Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the work was conducted.
Financial Disclosures: Dr Lyman reported receiving previous research support from Genomic Health. No other disclosures were reported. Drs Reed and Schulman have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/about-us/conflict-of-interest/).
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/gim.2012.119
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/gim2012119.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/gim.2012.119
Article citations
Clinicopathological Factors Associated with Oncotype DX Risk Group in Patients with ER+/HER2- Breast Cancer.
Cancers (Basel), 15(18):4451, 07 Sep 2023
Cited by: 1 article | PMID: 37760420 | PMCID: PMC10527468
Deep learning from HE slides predicts the clinical benefit from adjuvant chemotherapy in hormone receptor-positive breast cancer patients.
Sci Rep, 11(1):17363, 30 Aug 2021
Cited by: 10 articles | PMID: 34462515 | PMCID: PMC8405682
Genetic Landscape and Emerging Therapies in Uveal Melanoma.
Cancers (Basel), 13(21):5503, 02 Nov 2021
Cited by: 18 articles | PMID: 34771666 | PMCID: PMC8582814
Review Free full text in Europe PMC
The concordance of treatment decision guided by OncotypeDX and the PREDICT tool in real-world early-stage breast cancer.
Cancer Med, 9(13):4603-4612, 06 May 2020
Cited by: 4 articles | PMID: 32372569 | PMCID: PMC7333833
Gene Expression Profiling Tests for Early-Stage Invasive Breast Cancer: A Health Technology Assessment.
Ont Health Technol Assess Ser, 20(10):1-234, 06 Mar 2020
Cited by: 17 articles | PMID: 32284770 | PMCID: PMC7143374
Go to all (28) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: a cost-effectiveness evaluation in the German setting.
J Med Econ, 16(1):30-40, 11 Sep 2012
Cited by: 29 articles | PMID: 22966753
Cost effectiveness of a 21-gene recurrence score assay versus Canadian clinical practice in post-menopausal women with early-stage estrogen or progesterone-receptor-positive, axillary lymph-node positive breast cancer.
Pharmacoeconomics, 32(2):135-147, 01 Feb 2014
Cited by: 10 articles | PMID: 24288208
Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer.
J Natl Cancer Inst, 104(1):56-66, 02 Dec 2011
Cited by: 47 articles | PMID: 22138097
Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis.
Health Technol Assess, 17(44):1-302, 01 Oct 2013
Cited by: 54 articles | PMID: 24088296
ReviewBooks & documents Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: UC2 CA148041
Grant ID: 5UC2CA148041-02