Abstract
Free full text

SRM Targeted Proteomics in Search for Biomarkers of HCV-Induced Progression of Fibrosis to Cirrhosis in HALT-C Patients
Abstract
The current gold standard for diagnosis of hepatic fibrosis and cirrhosis is the traditional invasive liver biopsy. It is desirable to assess hepatic fibrosis with noninvasive means. Targeted proteomic techniques allow an unbiased assessment of proteins and might be useful to identify proteins related to hepatic fibrosis. We utilized Selected Reaction Monitoring (SRM) targeted proteomics combined with an organ-specific blood protein strategy to identify and quantify 38 liver-specific proteins. A combination of protein C and retinol binding protein 4 in serum gave promising preliminary results as candidate biomarkers to distinguish patients at different stages of hepatic fibrosis due to chronic infection with hepatitis C virus (HCV). Also, alpha-1-B glycoprotein, complement factor H and insulin-like growth factor binding protein acid labile subunit performed well in distinguishing patients from healthy controls.
1 Introduction
Human liver is an important target for damage by hepatitis viruses, chemicals, and drugs. Liver biopsy is recommended in the management of patients with chronic hepatitis C (CHC) to provide important information about fibrosis stage and disease prognosis [1]. As an invasive procedure, liver biopsy is frequently accompanied by transient pain and may occasionally be associated with serious complications. The accuracy of liver biopsy in staging liver disease is limited by the size and quality of the samples and sampling error [2].
In recent years, intensive research in the field of noninvasive tests of liver fibrosis has yielded a few laboratory markers, which enabled the assessment of some aspects of the severity of hepatitis C virus (HCV)-induced liver disease. For example, the FibroTest, combines six serum markers (Alpha-2-macroglobulin, Haptoglobin, Apolipoprotein A1, Gamma-glutamyl transpeptidase, Alanine transaminase and total bilirubin) with the age and gender of the patient to generate a score that correlates with stage of fibrosis in patients with a variety of liver diseases [3]. Platelet counts [4], AST/ALT ratio [5], and AST-platelet ratio index (APRI)[6] have been reported as predictors of degree of fibrosis in CHC patients. In the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial, a model based on a combination of standard laboratory tests comprising platelet count, AST/ALT ratio, and INR (international normalized ratio of prothrombin time) predicted histological cirrhosis with high accuracy in 50% of patients with CHC [7].
Molecular signatures specific for the liver and/or specific for particular causes of liver damage would be very useful for experimental, clinical, and epidemiology studies of liver diseases. Through development of targeted proteomic assays utilizing SRM incorporating heavy-isotope doping of labeled matched peptides [8, 9], and use of the Human SRMAtlas with optimized transitions associated with typically six different peptides for nearly all of the 20,300 human protein-coding genes (U. Kusebauch, manuscript in preparation), accurate quantitation of target proteins can be achieved for most human proteins present at levels that can be detected by targeted mass spectrometry. Here we report application of SRM proteomics to identify many liver-specific proteins and to characterize the critical progression of fibrosis of the liver to cirrhosis in CHC patients. This objective is quite challenging in the population we studied as liver biopsies used to stage fibrosis may not be accurate for all the patients and the states being compared represent a continuum of liver damage.
2. Materials and methods
2.1 Serum samples
Serum samples were obtained from patients who participated in the HALT-C Trial [10]. Typical clotting time is 30 minutes to 2 hours. This trial enrolled patients with CHC who had liver biopsies showing Ishak stages 2-6 (range 0-6) fibrosis at enrollment. Blood samples at enrollment were studied. Patient information at enrollment is listed in Supplemental Table 1. Control sera from normal female and male donors ages 30-50 years were collected at FDA-regulated blood facilities with a clotting time of about 60 minutes and were non-reactive for HCV antibody (ProMedDx). Pooled plasma from 10 normal donors was obtained using EDTA as anticoagulant from Innovative (Novi, Michigan). Collection and use of control and patient samples were approved by institutional review boards. Samples were stored at −80°C.
2.2 Sample preparation for SRM
To reduce the complexity of samples, the top 14 highly abundant proteins were depleted using an AKTA FPLC system (GE Healthcare, USA) coupled with a Seppro® IgY14 human LC2 depletion column (Sigma-Aldrich, USA). We observed significant sample-to-sample variations with the Seppro® IgY14 spin column. In contrast, the LC system coupled with an IgY14 human LC2 depletion column dramatically improved the reproducibility (supplemental figure 1). All 40 HALT-C and 17 normal serum samples were processed similarly; about 95% of the total protein was depleted. Proteins in depleted sera were denatured in 50% (v/v) 2, 2, 2-trifluoroethanol (TFE, J.T. Baker, Philipsburg, NJ) for 30 min at 55 °C, then cysteine residues were reduced and alkylated. Sample were diluted ten-fold with 100mM NH4HCO3 (pH 8.3) before adding trypsin (1:25 trypsin vs. serum protein) for overnight digestion at 37°C. Peptides were desalted with Oasis MCX cartridges (Waters, Milford, MA).
2.3 Building the liver-specific and liver-enriched proteins list
We used a targeted approach focusing on organ-specific proteins to increase the likelihood of identifying protein biomarkers in blood that may reflect pathology of a particular organ. Our list of liver-specific or liver-enriched proteins (liver proteins) was created by mining multi-organ transcriptomic data generated through Massively Parallel Signature Sequencing (MPSS). The MPSS dataset contains transcriptomes of 34 pooled normal (Caucasian) human tissues [11]. Signatures with their expression levels in liver either 5-fold higher than any other organs or 2-fold greater than the sum of all other organs were selected as liver protein candidates. We also performed organ-specific protein search with Gene Atlas Interface analysis. The databases searched against were 3 datasets from NCBI-GEO (Gene Expression Omnibus) with a total of 180 human tissues from multiple donors [12-14]. We included 21 enzymes and other proteins used in clinical practice or previously reported as liver biomarker candidates.
2.4 Peptide selection from the liver protein list
Two to three peptides were selected for each liver protein based on the sequence of individual liver proteins that were previously detected by mass spectrometry. Peptide selection criteria are as follows [15]: 1) length 8-20 amino acid residues; 2) no chemically unstable residues (single letter notation; M, NG, DG, QG, N-terminal N, and N-terminal Q); 3) LC-compatible; 4) avoid cysteine residue if possible; and 5) sequence specific for the target protein (e.g. proteotypic peptides). Peptides previously identified in PeptideAtlas [16] were preferentially chosen. All peptides used in this study were checked by BLAT at http://genome.ucsc.edu/ and Protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searches to ensure that they are unique to the target protein at both proteomic and genomic levels. Finally, the uniqueness of every Q1/Q3 pair from the target peptides was confirmed by an SRM theoretical collision calculator tool (http://proteomicsresource.washington.edu/cgi-bin/srmcalc.cgi). Mass tolerances for searching both Q1 and Q3 were ± 0.35 Da, which matches the Agilent QQQ settings for SRM.
2.5 Mass spectrometry and HPLC
All SRM analyses were performed on an Agilent 6460A triple quadrupole (QQQ) mass spectrometer with a ChipCube nanoelectrospray ionization source coupled with an Agilent 1200 nanoFlow HPLC system. Serum samples were eluted over a 60-minute gradient with 0.66% per minute acetonitrile slope in the presence of 0.1% formic acid using a large capacity Agilent HPLC chip (Cat # G4240-62101, 160 nL trap, 150 mm C18 column). Spray voltage was set at 1900 V. The scheduled SRM were performed with 5 min retention time windows and an instrument cycle time of 2000±500 ms. Dwell times varied depending on the number of concurrent transitions; in all cases they were at least 10 ms.
2.6 Monitoring liver-specific proteins in blood by SRM
Crude unpurified peptide standards that correspond to the detected natural counterparts (light peptides) were synthesized with heavy isotopic Lysine (13C615N2) or Arginine (13C615N4) at the C-termini (heavy peptides) (Thermo-Fisher Scientific, Germany or Sigma-Aldrich, USA). Collision energies (CE) were determined using the default formula from Agilent (0.036 × precursor mass m/z − 4.80) and then optimized with 4 additional CE steps (±5V, ±10V). The best 4 transitions were selected. Detected heavy peptides were titrated at 6 concentrations in a normal human serum background to build a titration curve and to determine the proper amount of each peptide standard to spike-in. (Supplemental Figure 2).
2.7 SRM data analysis
All SRM data were processed using the Skyline Targeted Proteomics Environment (v1.1) [17]. The setting of 0.055 Th match tolerance m/z was used. The default peak integration and Savitzky-Golay smoothing algorithm were applied. All data were manually inspected to ensure correct peak detection and accurate integration. Peptides with at least 3-fold signal-to-noise ratio were considered detectable. The total peak area and Light/ Heavy ratio of each peptide were exported for statistical analysis.
2.8 Statistic analysis
The exported SRM results were analyzed using R scripts generated for this data set, using standard methods of supervised analysis from R Bioconductor [18]. The key challenge for the present analysis is a high dimension of the feature vector (large number of potentially predictive proteins). Our pre-processing selection included proteins that have no more than 30% missing data. A similar criterion was applied to samples. Missing data were handled using k-nearest neighbor imputation algorithms (k=10) [19]. Repeated (duplicate) measurements for the same protein-peptide-m/z combination were averaged. Platelet level and gender were included as a clinical predictor of liver damage. Regularization methods based on logistic regression were used to reduce overfitting: LASSO and Elastic Nets penalty [20] were applied. The choice of the optimal regularization parameter was done using the Area Under the Receiver-Operating-Characteristic (AUROC) curve as a criterion. Such regularized regression methods make it possible to handle high dimension within the model-based (regression) classification framework and to improve the multivariate correlation of the panel under design with the disease risk. In order to obtain an approximately unbiased assessment of the performance of predictive signatures, ten-fold cross validation was used to correct for potentially over-optimistic model building and signature optimization bias. An average over cross-validation runs is reported as the final optimal AUC characterizing the predicted performance of the biomarker signature. LASSO penalty was preferred for its ability to drop non-essential biomarkers from the signature by explicitly assigning them zero weights. All other analyses including calculation and graphics were generated by Prism 5 (GraphPad software, La Jolla, CA, USA).
3 Results
We adopted an organ-specific protein based strategy for blood biomarker discovery. The approach is centered on the idea that concentration of organ-specific proteins in the blood can be used to monitor the health status of a specific organ because changes in blood concentrations reflect the normal as opposed to disease-perturbed status of their cognate biological networks. We mined comprehensive transcriptomic databases to identify organ-specific proteins. To demonstrate the power of this approach, we undertook a study to identify biomarkers related to HCV infection. We have found several liver proteins that can be used to separate HCV-infected patients from healthy individuals. In addition, the levels of two proteins showed strong correlations with progressive stages of the disease.
3.1 The identification of liver proteins
Using strategies described in Methods 2.3, we identified 109 liver proteins that passed GeneCards verification. GeneCards summarized each gene’s expression in normal and diseased human tissues by three categories: 1) mRNA expression data from GeneNote and GNF BioGPS, 2) UniGene electronic Northern, and 3) SAGE (Serial Analysis of Gene Expression). In combination with the 21 proteins used in clinic practice or reported as liver biomarker candidates, a list composed of 130 proteins was created.
3.2 Proteins detected by SRM
After suitable peptides and transitions for each liver protein were selected, we used control plasma and serum to determine how many liver proteins can be detected by SRM. From the 89 liver proteins previously observed in tandem MS/MS experiments, we detected 100 peptides derived from 54 proteins in pooled control plasma (Supplemental Table 2). However, the HALT-C samples were in the form of serum, which is not ideal for mass spectrometry-based blood protein measurements due to variation in proteolysis derived from the coagulation cascade, resulting in decreased concentrations of proteins compared to plasma[21]. In order to determine how many of our proteins detected in plasma can be detected in serum; we performed the same SRM analyses against pooled healthy human serum samples. Altogether 38 proteins (represented by 65 peptides) were detected in sera (Supplemental Table 3). All SRM parameters and results are deposited in the SRM chromatographic repository at ISB and are publicly available (www.srmatlas.org and www.peptideatlas.org/passel/). Typical SRM chromatograms of 5 target peptides are presented in supplementary Figure 3.
3.3 Consistency and accuracy of SRM data
3.3.1 Duplicate SRM runs are well correlated
Duplicate runs were performed for each sample; technical variations between the two runs were generally small (Fig 1A). Pearson tests showed good correlations between runs (≥ 0.9 in most cases; for example: PROC=0.90, RBP4=0.90, CFH=0.95, A1BG=0.94). Samples from two HALT-C patients, DA000739 and DJ000004 were eliminated from the analysis due to sample degradation.
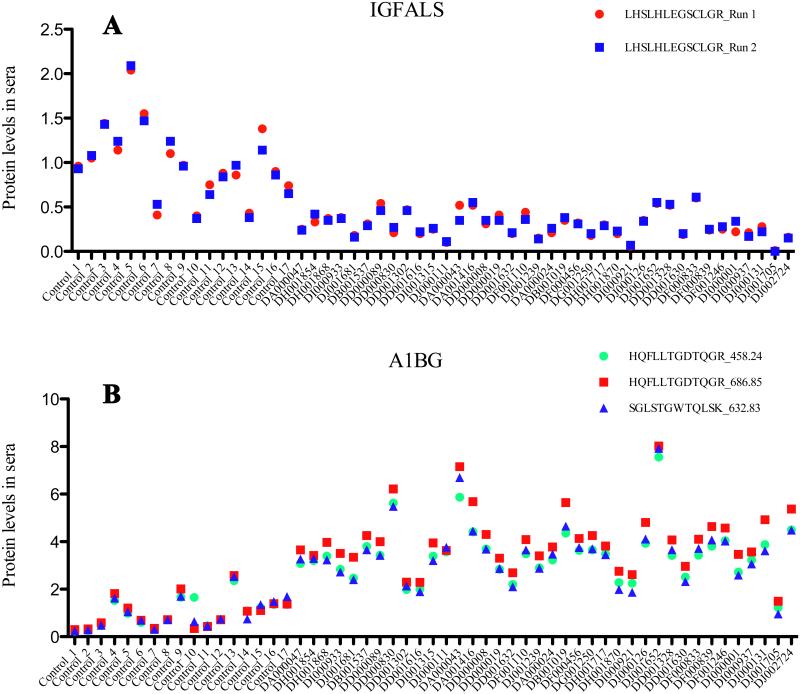
Variations between technical replicates were generally small with a Pearson correlation value >0.9. Between duplicate runs, serum protein levels of IGFALS measured by peptide LHSLHLEGSCLGR were consistent. 1B. Multiple peptides (or features) derived from the same protein performed consistently in most SRM tests. As shown here, A1BG serum levels measured by the three features SGLSTGWTQLSK and HQFLLTGDTQGR precursor charge 2 and 3 were in close agreement. Relative protein levels in blood are indicated by light/heavy peptide ratios.
3.3.2 Protein levels measured by multiple features are consistent
When a protein level is measured by more than one feature (i.e., multiple peptides or same peptide with differently charged precursor ions), close agreement in quantification was observed; an example is the set of three features for the protein of A1BG (Fig 1B). This observation gave us reasonable confidence that protein levels in samples estimated even from a single peptide can be reliable.
3.3.3 Absolute protein levels in sera measured by SRM are similar to prior report
In this study, we did not aim to quantify absolute protein concentrations. The crude heavy peptides synthesized by rapid process peptide synthesis were not appropriate for absolute quantitation of protein levels in specimen due to the wide range of purity (~50% to 80%). In addition, the immunodepletion procedure during sample preparation induces additional variation in protein concentrations. Nevertheless, as summarized in Table 1, levels of five informative proteins in depleted control sera measured by SRM are close to concentrations reported in published literatures. The only exception is RBP4; the concentration in our SRM study is 10-fold lower than the studies of Gahne et al and Polanski et al [20,21] and 100-fold lower than the study of Farrah et al [22].
Table 1
Five informative protein concentrations in control sera measured by crude heavy peptides in SRM. Assuming purity of crude peptide is 80% for 10mers and 70% for 15mers, respectively.
| NCBI Official Symbol | Peptide Sequence | Average conc. in MRM assay (pg/ml) | Conc. range in MRM assay (pg/ml) | Ref_1 conc. (pg/ml) [22, 23] | Ref_2 conc. pg/ml [24] |
|---|---|---|---|---|---|
| A1BG | SGLSTGWTQLSK | 5.53E+07 | 1.23E+07 to 1.62E+08 | 2.20E+8 | 5.00E+07 |
| CFH | CTSTGWIPAPR | 5.51E+07 | 2.35E+07 to 1.07E+08 | 5.70E+07 | |
| IGFALS | VAGLLEDTFPGLLGLR | 6.52E+05 | 1.64E+05 to1.36E+06 | 1.50E+06 | |
| PROC | TFVLNFIK | 1.00E+05 | 2.61E+04 to 1.72E+05 | 3.70E+06 | 6.50E+04 |
| RBP4 | YWGVASFLQK | 3.22E+06 | 1.72E+06 to 5.79E+06 | 3.17E+07 | 5.80E+08 |
3.4 Five informative proteins were found to distinguish normal controls and HCV-infected patients or CHC patients at different stages of fibrosis
We identified five proteins, namely A1BG, CFH, IGFALS, PROC and RBP4, that are able to separate HCV-infected patients from healthy individuals or distinguish CHC patients at different stages of fibrosis.
3.4.1 A1BG, CFH and IGFALS can distinguish controls from patients
As shown in Figure 2A, the average A1BG level in sera of all HCV patients in this study was significantly elevated compared with controls (P=7.4E-15), while CFH and IGFALS levels were significantly decreased in patients versus in controls (P=4.5E-08 and 5.7E-12, respectively). AUROC scores for controls vs. HALT-C patients were 0.99, 0.99, and 0.96 for A1BG, CFH, and IGFALS, respectively.
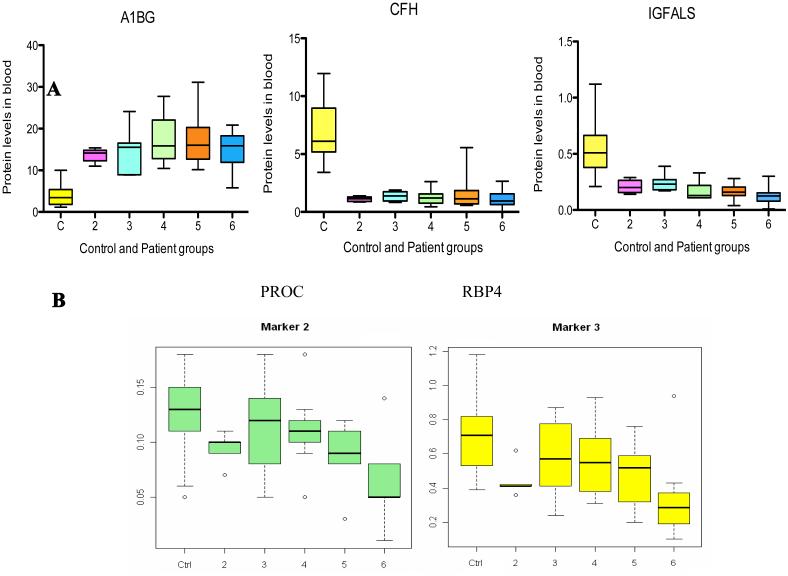
Box plot analysis shows significant differences when comparing serum protein levels of A1BG, CFH and IGFALS in patients from Ishak fibrosis 2-6 against controls (C). The combined serum A1BG level of all patients is significantly elevated compared to the controls (P=7.4E-15), while the combined serum CFH and IGFALS levels are significantly decreased in patients versus controls (P=4.5E-08 and 5.7E-12, respectively) (2A). PROC and RBP4 levels in serum classify HCV patients in different stages of fibrosis. Box plot analysis shows strong evidence that the median serum levels of PROC and RBP4 in every Ishak fibrosis group decreased with the progression of the disease (2B). Each box represents the range between upper (75th) and lower (25th) quartiles with the median level shown as an intersect line. The minimum and maximum values (whiskers) within 1.5 IQR (the interquartile range).”
3.4.2 PROC and RBP4 levels in serum can further classify HCV patients
The concentrations of two proteins--PROC and RBP4--showed good correlation with disease severity. Serum concentrations of PROC and RBP4 decreased as liver disease progressed. Box-and-whisker plots and Student’s t-test showed that each protein can distinguish different disease stages (Figure 2B).
With Student’s t-test, the difference in serum concentrations of PROC or RBP4 between patients with earlier stages of fibrosis (Ishak 2-4) and patients with cirrhosis (Ishak 5-6) is significant. PROC appears to be a good marker to distinguish cirrhosis patients from those with fibrosis (p = 0.004). RBP4 levels showed a similar decrease from normal control to Ishak 5-6 but the difference between Ishak 2-4 and 5-6 was not statistically significant--0.07 with outliers (0.02 without outliers) (Supplemental Figure 4). AUROC scores for PROC were 0.77 for controls vs. patients, 0.75 for Ishak <5 vs. Ishak >5, and 0.83 for Ishak <5 vs. Ishak 6. AUROC scores for RBP4 were 0.79 for controls vs. patients, 0.68 for Ishak <5 vs. Ishak >5, and 0.80 for Ishak <5 vs. Ishak 6.
3.5 Multivariate analysis
Multivariate analysis of Ishak 2-4 vs. 5-6 patients using PROC and RBP4 proteins, gender, and platelets gave a cross-validated AUROC=0.89. PROC, RBP4, and platelets distinguish advanced stages of fibrosis patients (Ishak 5-6) from patients in earlier stages with an impressive sensitivity of 95% and specificity of 84% (Fig 3). A minor improvement (AUROC=0.72 vs. 0.69) was observed with platelets excluded in the analysis based on all available vs. only PROC and RBP4 proteins.
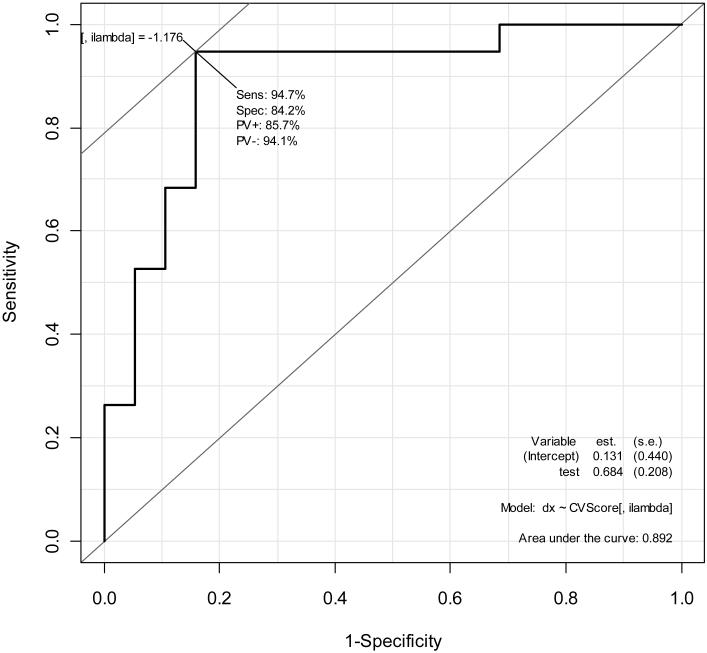
Protein markers PROC and RBP4 and platelets distinguish patients with cirrhosis (Ishak 5-6) from patients in other stages with sensitivity of 95% and specificity of 84%. Inclusion of additional proteins in this study did not result in meaningful further improvement.
Classifying stages of liver fibrosis by biopsy has an accuracy of about 80% [25]. Other studies have suggested that there can be up to a 33% error in the diagnosis of cirrhosis by biopsy [26]. When the value of biomarkers is validated against biopsy, it is improbable to have a discrimination power that exceeds biopsy. In fact, reported noninvasive methods intended for discriminating hepatic fibrosis rarely have an AUROC (accuracy) exceeding 0.8 to 0.9 [27].
4. Discussion
As shown by multivariate analyses, people with the disease and patients at different disease stages may be classified better by employing a combination of new markers with existing markers to increase the sensitivity and specificity. In this study, a 3-protein panel of A1BG, CFH and IGFLAS performed well to distinguish healthy controls from HCV-infected patients. An integrated analysis of A1BG, CFH and IGFALS or A1BG with either of the other two markers resulted in an AUROC score of 1.0 for discriminating healthy controls and HCV-infected patients (Fig 4A and B). We have observed from this and an independent study of chemical hepatotoxicity that sets of markers for discriminating acute and chronic liver diseases may be very different (unpublished observation). The conventional liver markers AST and ALT may be better markers for acute liver injuries but not as good for chronic liver diseases. This 3-protein panel of A1BG, CFH, and IGFALS may potentially prove useful for chronic liver diseases. Apparently these proteins have not previously been reported as liver disease markers. Evaluation will require sampling a larger patient population. Although sera obtained from ProMedDx and HALT-C trial were prepared under similar conditions, these specimens were obtained from separate sources. Thus, the risk of batch effects generated from sera preparation and storage on the differences for these three proteins between controls and patients cannot be totally excluded.
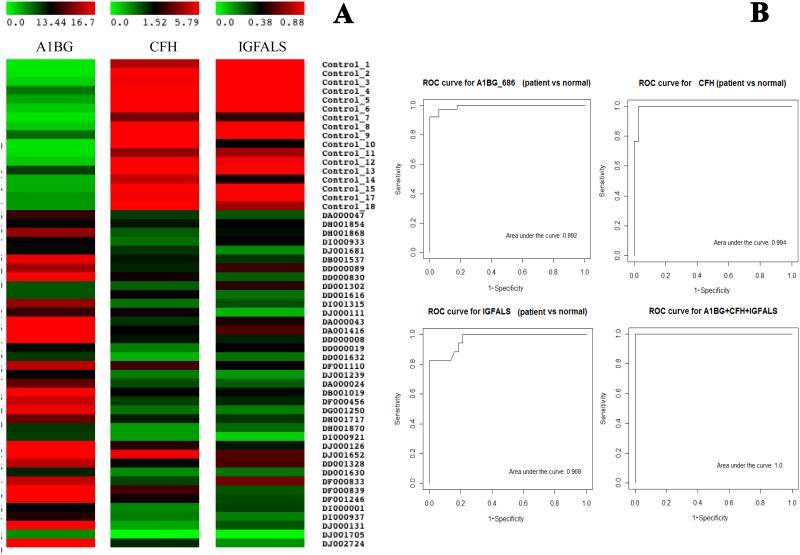
SRM data of serum A1BG and CFH levels in both patients DJ001705 and DJ001652 (arrows) are of good quality; their relative protein levels are probably truly different from other patients. However, this discrepancy can be resolved by examining simultaneously A1BG and CFH or A1BG and IGFALS levels in these patients (4A). A combination of A1BG, CFH and IGFALS is able to discriminate healthy controls from HCV patients from Ishak scores 2 to 6 with predicted high sensitivity and specificity (4B).
PROC and RBP4 have been related to liver diseases. Kłoczko et al. reported that the plasma PROC level of patients with liver damage due to chronic alcohol consumption was decreased and correlated with clinical performance of the patients [26]. Our results with different methods confirmed that serum or plasma PROC levels might constitute a useful marker of advanced liver disease. Romanowska et al. reported serum concentrations of RBP-4 were significantly higher in obese children with non-alcoholic fatty liver disease (NAFLD) compared to controls and proposed RBP4 as a serum marker of intrahepatic lipid content in obese children[28]. A contradictory report showed serum RBP4 levels were not different between the steatosis group and controls as well as between subgroups with high and normal ALT, indicating that serum RBP4 may not be a predictive factor in NAFLD [29]. In this study, we showed a significant decrease of serum RBP4 patients with advanced stages of disease due to HCV infection. Our findings are the first to show PROC and RBP4 may be used for discriminating fibrosis vs. cirrhosis in these patients.
Correct classification of different stages of liver fibrosis needs a multiparameter test. Our preliminary results related to staging the progression of fibrosis do not constitute a new biomarker panel, but do provide additional markers to strengthen the existing noninvasive methods, including platelet counts. There are many other causes of low blood platelet levels. Addition of liver-specific proteins to a diagnostic test panel can increase the diagnostic relevance. Moreover, protein analytes can be good targets for developing antibodies or synthetic capture agents that can be integrated into microfluidic chips (Integrated Blood-Barcode Chip) —devices that have the potential to analyze large numbers of patient samples rapidly (in a few minutes), inexpensively, and in a highly multiplexed format (100s or even 1000s of different assays investigating many different diseases) employing blood from a pinprick [30]. Such microfluidic devices are likely to constitute an important foundation for P4 (Predictive, Preventive, Personalized, and Participatory) Medicine with Point-of-Care Diagnosis [31].
Summary
By employing a liver-specific protein strategy and targeted quantitative SRM proteomics technology, we have analyzed 38 liver-specific protein levels in sera of 18 healthy controls and of 38 HCV patients at Ishak fibrosis stages from 2 to 6. In this pilot study, we found that the measured levels of two proleotypic peptides derived from Protein C and retinol binding protein 4, were present at lower levels in patients than in controls. With Area Under the Curve statistical analyses, these two proteins represented by their corresponding peptides together appear promising for distinguishing fibrosis vs. cirrhosis among HALT-C patients. We also report three proteins, A1BG, CFH and IGFALS, as measured with their unique peptides, that performed well in distinguishing HCV-infected patients from healthy controls, with an individual AUROC score >0.96 for each marker. These five proteins may contribute to multiparameter panels that will have useful diagnostic potential for CHC and other liver diseases.
Supplementary Material
01
Supplemental Figure 1. A typical titration curve (peptide VANYVDWINDR) shows the correct amount of heavy peptide needed to be spiked-in to human blood samples (100 femtomole in this case). X-axis: peptide amount in femtomole, y-axis: total peak AUC (area under transition curve). Synthetic heavy-labeled peptide is in red and endogenous light peptide is in blue.
Supplemental Figure 2. A typical titration curve (peptide VANYVDWINDR) shows the correct amount of heavy peptide needed to be spiked-in to human blood samples (100 femtomole in this case). X-axis: peptide amount in femtomole, y-axis: total AUC (area under transition curve). Synthetic heavy-labeled peptide is in red and endogenous light peptide is in blue.
Supplementary Figure 3. Typical chromatograms of 5 targeted peptides.
Supplemental Figure 4. The mean of serum protein levels among control, Ishak 2-4 and Ishak 5-6 patient samples. There is a significant decrease of PROC levels in Ishak 5-6 patients as compared to Ishak 2-4 patients (P=0.004). PROC levels in Ishak 5-6 patients are also significantly lower than controls (P=0.00005). Both Ishak 2-4 and patients 5-6 patients’ RBP4 levels are significantly lower than controls (P=0.01 and P=0.0001 with or without outliers, respectively). Comparison of RBP4 levels in Ishak 5-6 patients to Ishak 2-4 patients shows a P value of 0.07 with outliers and P value of 0.02 without outliers.
Supp. Table 1. Patient information on age, gender, platelet counts, AST, ALT, and alkaline phosphatase levels in blood and Ishak scores when enrolled in the HALT-C trial.
Supp Table 2. 87 proteins (shown with NCBI office symbol) previously detected by tandem mass spectrometry and tested by SRM with light peptides
Supplemental Table 3, SRM monitoring conditions of 38 proteins that detectable in control sera. Many proteins are represented by two or more peptides. K: 13C615N2 isotopic-labeled lysine, R: 13C615N4 isotopic-labeled arginine
Acknowledgments
The authors would like to thank Drs. Gustavo Glusman and Juan Caballero for their contributions in generating the liver-specific protein list. The authors are grateful to Dr. Kai Wang for valuable discussions throughout this project. This study was partially supported by the Luxembourg Centre for Systems Biomedicine and the University of Luxembourg for Leroy Hood, federal funds from the National Institutes of Health-National Human Genome Research Institute (HG005805 for Robert Moritz) and the NIH National Center for Integrative Biomedical Informatics (U54 DA021519, U54 ES017885 for Gilbert Omenn).
Abbreviations
| PROC | protein C |
| RBP4 | retinol binding protein 4 |
| A1BG | alpha-1-B glycoprotein |
| CFH | complement factor H |
| IGFALS | insulin-like growth factor binding protein acid labile subunit |
| CHC | chronic hepatitis C |
| HCV | hepatitis C virus |
| HALT-C | Hepatitis C Antiviral Long-term Treatment against Cirrhosis |
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/pmic.201100601
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3766736?pdf=render
Citations & impact
Impact metrics
Article citations
Integrated liver and serum proteomics uncover sexual dimorphism and alteration of several immune response proteins in an aging Werner syndrome mouse model.
Aging (Albany NY), 16(10):8417-8445, 24 May 2024
Cited by: 0 articles | PMID: 38795389
Plasma ALS and Gal-3BP differentiate early from advanced liver fibrosis in MASLD patients.
Biomark Res, 12(1):44, 29 Apr 2024
Cited by: 0 articles | PMID: 38679739 | PMCID: PMC11057169
Changes in proteomic cargo of circulating extracellular vesicles in response to lifestyle intervention in adolescents with hepatic steatosis.
Clin Nutr ESPEN, 60:333-342, 23 Feb 2024
Cited by: 1 article | PMID: 38479932
Implicating the cholecystokinin B receptor in liver stem cell oncogenesis.
Am J Physiol Gastrointest Liver Physiol, 326(3):G291-G309, 22 Jan 2024
Cited by: 0 articles | PMID: 38252699 | PMCID: PMC11211039
Altering retinol binding protein 4 levels in hepatitis C: Inflammation and steatosis matter.
Virulence, 11(1):1501-1511, 01 Dec 2020
Cited by: 3 articles | PMID: 33135589 | PMCID: PMC7605351
Go to all (27) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Novel serum biomarker candidates for liver fibrosis in hepatitis C patients.
Clin Chem, 53(10):1792-1799, 16 Aug 2007
Cited by: 106 articles | PMID: 17702858
Novel biomarker candidates to predict hepatic fibrosis in hepatitis C identified by serum proteomics.
Dig Dis Sci, 56(11):3305-3315, 17 May 2011
Cited by: 29 articles | PMID: 21590334 | PMCID: PMC3181275
Analysis of disease-associated protein expression using quantitative proteomics—fibulin-5 is expressed in association with hepatic fibrosis.
J Proteome Res, 14(5):2278-2286, 06 Apr 2015
Cited by: 42 articles | PMID: 25807371
Noninvasive diagnosis of liver fibrosis in patients with chronic hepatitis C.
MedGenMed, 7(4):39, 09 Nov 2005
Cited by: 11 articles | PMID: 16614661
Review
Funding
Funders who supported this work.
Luxembourg Centre for Systems Biomedicine and the University of Luxembourg for Leroy Hood
NCRR NIH HHS (2)
Grant ID: UL1 RR024986
Grant ID: M01 RR000042
NHGRI NIH HHS (2)
Grant ID: RC2 HG005805
Grant ID: HG005805
NIDA NIH HHS (1)
Grant ID: U54 DA021519
NIEHS NIH HHS (2)
Grant ID: P30 ES017885
Grant ID: U54 ES017885
NIH National Center for Integrative Biomedical Informatics (2)
Grant ID: U54 ES017885
Grant ID: U54 DA021519
National Institutes of Health-National Human Genome Research Institute (1)
Grant ID: HG005805





